Abstract
The vasopressin-receptor antagonists have received approval for the treatment of hyponatraemia secondary to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). It is therefore necessary that physicians encountering hyponatraemia focus on SIADH. Recent studies show that hyponatraemia is often poorly managed—insufficient diagnostic tests are ordered and patients are undertreated. At the same time, it has become clear that chronic hyponatraemia causes neurological symptoms such as gait disturbances and attention deficits. However, physicians often tolerate chronic hyponatraemia as if it were benign, or as if its treatment would cause significant morbidity. Therefore, physicians must reconsider the diagnostic and therapeutic approaches to hyponatraemia and SIADH.
Keywords: adrenal insufficiency, diagnosis, epidemiology, vasopressin, vasopressin-receptor antagonists
Introduction
The recent introduction of vasopressin-receptor antagonists makes it imperative to revisit hyponatraemia and the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Selective vasopressin V2-receptor antagonists, also called ‘vaptans’, represent the first targeted therapy for SIADH, which is one of the most common causes of hyponatraemia [1]. The various compounds are in different stages of development and include mozavaptan [2] lixivaptan, satavaptan and tolvaptan [1]. Tolvaptan has recently been approved by the European Medicines Agency for the treatment of hyponatraemia secondary to SIADH. When new drugs become available, the excitement about their possibilities sometimes overshadows the disorder for which they will be used. Because hyponatraemia is such a heterogeneous and complex disorder, and because tolvaptan has been approved only for the treatment of hyponatraemia secondary to SIADH, a rational use of vasopressin-receptor antagonists first requires a proper diagnosis of SIADH. Therefore, may we suggest that in addition to reading the package insert of these new drugs, read this article, in which we will review hyponatraemia and SIADH and discuss the potential role of vasopressin-receptor antagonists. Finally, on a more semantic note, it was recently proposed that SIADH should be called the syndrome of inappropriate antidiuresis (SIAD), because vasopressin (antidiuretic hormone) is not always elevated, for example in patients with reset osmostat or with an activating mutation of the vasopressin V2-receptor [1]. However, in this article we will use the traditional term SIADH, because we focus on disorders with elevated vasopressin.
Why does hyponatraemia matter?—The previous concept
The more obvious answers as to why hyponatraemia is clinically relevant used to be because of cerebral oedema and the osmotic demyelination syndrome (ODS). Although relatively rare, these two neurological conditions represent the two most severe complications of hyponatraemia and its treatment. Both can occur with any cause of hyponatraemia, including SIADH. Cerebral oedema usually occurs in acute hyponatraemia of moderate or severe degree (drop in serum sodium [SNa] ≤ 125 mmol/L in ≤ 48 h). In this setting, brain cells do not have time to adapt to extracellular hypotonicity and cell swelling. It is a medical emergency that, if left untreated, can lead to herniation of the brain stem into the foramen magnum and permanent brain damage [3]. ODS is not a consequence of hyponatraemia but of its treatment. The term refers to the neurological sequelae (e.g. sudden para or quadraparesis, dysphagia, dysarthria, diplopia and loss of consciousness) that can develop when chronic hyponatraemia is corrected too fast [4]. Because the brain damage in ODS is often irreversible, iatrogenic and preventable, it remains a concern in the management of hyponatraemia [5]. ODS can usually be prevented, however, by adhering to the recommended maximal correction rates of ≤12 mmol/L/24 h and ≤18 mmol/L/48 h in chronic hyponatraemia [4,6,7]. If chronic hyponatraemia is associated with other risk factors for ODS (hypokalaemia, malnutrition, alcoholism), the limit should be adjusted more towards 8 mmol/L/24 h [8,9]. If the duration of hyponatraemia is in doubt (acute versus chronic), computed tomography or magnetic resonance imaging of the brain may sometimes help to diagnose cerebral oedema, suggesting acute hyponatraemia.
Are there any other reasons why hyponatraemia matters? There are also two less obvious answers to this question. The first is that the current management of patients with hyponatraemia is inadequate. Below, in the section entitled ‘Current management of hyponatraemia is inadequate’, we will review three recent studies in which the clinical reality of the management of hyponatraemia was studied. Euphemistically speaking, it was found that there is considerable room for improvement. For instance, the causes of hyponatraemia were poorly studied, hyponatraemia was undertreated, not treated at all or all of the above. The second less obvious answer to why hyponatraemia matters relates to a landmark study by Renneboog and colleagues [10]. They demonstrated that chronic and apparently asymptomatic mild-to-moderate hyponatraemia is not as innocent as it seems. We shall address these issues later in this article (section ‘Asymptomatic hyponatraemia is not asymptomatic—the new concept’).
Most physicians have learned to deal with the extremes regarding hyponatraemia. They infuse hypertonic saline in a patient with acute hyponatraemia who has a seizure, and hypotonic saline in a patient with chronic hyponatraemia whose SNa is rising too fast. Therefore, it is probably this much larger group of patients with mild-to-moderate chronic hyponatraemia and subtle neurological symptoms who receive suboptimal care. It appears that they deserve better medical attention and improved care.
Current management of hyponatraemia is inadequate
To talk about improving the management of hyponatraemia, we first need to know where we stand at present. Three recent studies of hyponatraemia, including in patients with SIADH, give a clear albeit worrisome picture. They show that hyponatraemia at present is missed, misdiagnosed or undertreated.
Gill and colleagues conducted a prospective case-control study in 104 patients who had a SNa < 125 mmol/L. They were then compared to random normonatraemic controls [11]. Cases had longer hospital stays (16 ± 12 versus 13 ± 11 days) and a higher mortality rate (28% versus 9%). In terms of underlying diseases, cases more often had pneumonia, alcohol abuse, neurological disease and renal insufficiency, or were on therapies including thiazides, loop diuretics and selective serotonin reuptake inhibitors. Half of the cases had a further drop in SNa in the course of the hospital treatment. These patients with ‘hospital-aggravated’ hyponatraemia more often were on diuretics (18% versus 2%). They also had a higher mortality rate (34% versus 16%) compared to hyponatraemic patients without a further decrease in SNa. In some of these cases, iatrogenic factors (hypotonic intravenous fluids) or missed diagnoses (primary adrenal insufficiency) were identified. In 73% of cases, no plausible explanation for hyponatraemia had been documented in the charts. The study did not report details of any treatment of hyponatraemia, but the average discharge SNa was reported to be 131 ± 7 mmol/L, suggesting that hyponatraemia was not corrected fully in a sizeable portion of patients.
In a second study, Clayton and colleagues performed a retrospective analysis of 108 patients with a SNa ≤ 125 mmol/L [12]. Neurological symptoms, including unsteadiness of gait and falls, were found to be present upon admission in 36% of patients. The majority of patients (75.3%) had a multifactorial cause of hyponatraemia, of which diuretics, congestive heart failure and liver cirrhosis were the most common. The high mortality rate (20% during hospital stay, 45% after a follow-up of ½ years) was mainly ascribed to the severity of the underlying diseases (usually heart and liver failure). Perhaps most revealing were the diagnostic shortcomings. Serum glucose was ordered in 70%, serum osmolality in 61%, urine osmolality in 47%, urine sodium in 40%, thyroid function in 49% and adrenal function in 15%. None of the 48% of patients in whom SIADH was suspected met established diagnostic criteria, usually because insufficient tests were ordered. Why these parameters are important in the diagnosis of hyponatraemia and SIADH will be discussed in more detail in the next section (How to diagnose SIADH reliably?). This study did not report details on the treatment of hyponatraemia.
A third study was published by our own group [13]. It corroborated most of the above findings. Thirty-eight patients who had hyponatraemia on admission (SNa 121 ± 4 mmol/L) were compared to 36 patients who developed hyponatraemia in the hospital (SNa 133 ± 5 to 122 ± 4 mmol/ L in ∼7 days). Neurological symptoms (e.g. sensorium changes, seizures) were frequent in all (in 40% of admission hyponatraemia and in 33% of hospital-acquired hyponatraemia). Similar to the study by Clayton et al., hyponatraemia was often multifactorial. In patients with hospital-acquired hyponatraemia, the number of factors predisposing to hyponatraemia was increased from 1.7 ± 1.5 to 3.3 ± 1.6 during hospitalization, suggesting that these patients were sicker. The factors included thiazides, surgery, hypotonic intravenous fluids and vasopressin-stimulating drugs. The duration of hospitalization was similar to that reported by Gill et al. (18.2 ± 11.5 days), but patients with hospital-acquired hyponatraemia were hospitalized for longer (30.7 ± 23.4 days). It was also found that hyponatraemia was frequently not documented in the charts (in 42% of admission hyponatraemia, and in 69% of hospital-acquired hyponatraemia). In cases where treatment was given for hospital-acquired hyponatraemia, therapy was delayed. Patients who did not receive therapy for hyponatraemia had significantly higher mortality rates (37 versus 13%).
Together these studies illustrate the current shortcomings in the diagnosis and management of hyponatraemia. The diagnostic work-up for patients with hyponatraemia is often insufficient and risking misdiagnosis and subsequent mismanagement. Patients whose hyponatraemia develops or worsens in hospital have poorer outcomes, and there is a possibility that hyponatraemia contributed to the outcome.
How to diagnose SIADH reliably?
The generally accepted criteria for SIADH include a number of essential and supplemental criteria (Table 1) [14,15]. For SIADH, but also for hyponatraemia in general, it is important to first establish that one is truly dealing with hypotonic hyponatraemia by finding a low serum tonicity. Tonicity or ‘effective osmolality’ is the measured osmolality minus serum urea (and alcohol if present). Pseudohyponatraemia still exists, even after the introduction of ion-selective electrodes and may occur in the setting of high triglycerides, total protein or cholesterol [16]. In pseudohyponatraemia, the measured osmolality will be normal. Hyperglycaemia-induced hyponatraemia (often mistakenly called pseudohyponatraemia) is the other condition to consider (SNa drops ∼3 mmol/L for every 10 mmol/L rise in glycaemia). It would be wise to always order serum osmolality and glucose together in patients with hyponatraemia [12].
Table 1.
Diagnostic criteria for SIADH
| Essential features |
| • Decreased effective serum osmolality (<275 mOsm/kg) |
| • Urinary osmolality >100 mOsm/kg during hypotonicity of the serum |
| • Clinical euvolaemia |
| • Urinary sodium >40 mmol/L with normal dietary salt intake |
| • Normal thyroid and adrenal function |
| • No recent use of diuretics |
| Supplemental features |
| • Serum uric acid <0.24 mmol/L |
| • Serum urea <3.6 mmol/L, low normal serum creatinine |
| • Fractional sodium excretion >1%, fractional urea excretion >55% |
| • Failure to correct hyponatraemia after 0.9% saline infusion |
| • Correction of hyponatraemia through fluid restriction |
| • Abnormal water loading test (excretion <80% of a 20 mL/kg water load in 4 h) |
| • Elevated vasopressin levels despite hypotonicity and clinical euvolaemiaa |
Once hypotonic hyponatraemia has been established and the patient has a relatively high urine sodium (> 40 mmol/L) and osmolality (> 100 mOsm/kg, but usually > serum osmolality) SIADH should be considered (Tables 1 and 2). Euvolaemia is an essential requirement, but the clinical assessment of the extracellular fluid volume has been shown to present frequent difficulty [17,18]. The assessment of fluid volume status is easy in patients who are frankly hyper- or hypovolaemic. Hypovolaemia means extracellular fluid volume contraction and may occur after vomiting, diarrhoea or overly ambitious use of diuretics, for example in essential hypertension. It is characterized by orthostatic hypotension, tachycardia, a flat jugular venous pressure and often a low urinary sodium concentration (< 10– 20 mmol/L in the absence of diuretics). Patients with hypervolaemic hyponatraemia usually present with peripheral oedema or ascites, low normal or low blood pressure, and a low urinary sodium concentration (<10–20 mmol/L in the absence of diuretics) and typically have a compatible history of congestive heart failure or liver cirrhosis. However, in doubtful cases, we believe that the bedside assessment of the extracellular fluid volume should not be a decisive parameter, as is unfortunately still the case in the majority of clinical diagnostic algorithms [19].
Table 2.
How to diagnose SIADH?
| • Determine the bare minimum: serum and urine sodium and osmolality |
| • Find a low serum osmolality, high urine sodium (>40 mmol/L) and osmolality (>100 mOsm/kg, often > serum osmolality) |
| • Find serum uric acid, urea and/or creatinine levels that are low or low normal |
| • Assess the extracellular fluid volume, but do not make it a decisive parameter |
| • Exclude diuretic use, hypothyroidism, and adrenal insufficiency (low threshold for performing the Synacthen test) |
| • When unsure, assess the response in serum sodium to intravenous isotonic saline |
According to the criteria (Table 1), diuretic use (especially thiazides), hypothyroidism and adrenal insufficiency should be excluded prior to diagnosing SIADH [14,15]. The first two requirements may be less stringent than previously thought. A recent study indicates that it is possible to diagnose SIADH in patients on diuretics (thiazides or loop diuretics) [20]. In such a setting the use of the fractional uric acid excretion (≥12%) was of particular help to the authors in diagnosing SIADH and this did not apply in the same manner to the serum uric acid [20]. For calculating the fractional uric acid excretion, uric acid and creatinine should be measured in serum and a spot urine sample, both collected at around the same time. The fractional uric acid excretion is then calculated as (urine uric acid × serum creatinine)/(serum uric acid × urine creatinine) × 100.
The relationship between hypothyroidism and hyponatraemia was recently dismissed as an ‘old wives’ tale’ [21]. Although Warner et al. showed a statistical association between hyponatraemia and hypothyroidism (for every 10 mU/L rise in thyroid-stimulating hormone, SNa decreased 0.14 mmol/L) [22], the clinical relevance of this association is obviously minute. If the relationship does exist, it probably applies to severe myxoedema in which a low cardiac output and/or a low glomerular filtration rate may contribute to hyponatraemia [23].
Adrenal insufficiency is probably the most important disorder to exclude before diagnosing SIADH. Although SIADH is more common than adrenal insufficiency, the consequences can be grave when adrenal insufficiency is missed [24]. Adrenal insufficiency can have an atypical presentation and Soule proposed to add Addison's disease to the list of ‘great mimickers in medicine’ [25]. Indeed, we recently reported two patients with primary adrenal insufficiency who had severe hyponatraemia, but no hyperkalaemia, orthostatic hypotension, metabolic acidosis, hypoglycaemia, anaemia or eosinophilia [26]. Both cases were first ‘diagnosed’ as SIADH, and only after fluid restriction and isotonic saline-produced little response, adrenal function tests were carried out. Random cortisol levels between 100 and 500 nmol/L do still not exclude adrenal insufficiency fully [27]. Therefore, the response to a high (250 μg) or low (1 μg/1.73 m2) dose of synthetic adrenocorticotropic hormone (ACTH) is a superior test [28]. There should be a low threshold for performing this test in patients with hyponatraemia and a high urine sodium (>40 mmol/L) and osmolality (>100 mOsm/kg, usually > serum osmolality). The measurement of ACTH is also useful (high in primary adrenal insufficiency, low in secondary or tertiary adrenal insufficiency), but it is usually not available rapidly.
In practice, it is often worthwhile to use the response to ‘therapy’ in the diagnosis of SIADH (Table 1). A common approach is to assess the response in SNa to isotonic saline, which should rise in hypovolaemic hyponatraemia. However, two important caveats should be mentioned. First, isotonic saline may aggravate SIADH, especially when the tonicity of the urine is much larger than the infusate. Secondly, in hypovolaemic hyponatraemia, SNa may rise uncontrollably when the hypovolaemic stimulus for vasopressin release abates [29]. If SIADH is established, but its pathophysiology remains unclear and there is little or no response to fluid restriction, a water-loading test could be of use. Namely, if > 80% of a water load (20 mL/kg) is excreted within 4 h after administration, a reset osmostat may be present [30]. We recently used this approach to diagnose reset osmostat in a patient with chronic hyponatraemia and dementia with Lewy bodies [31]. However, one should be extremely cautious to give a water load in other forms of SIADH, because it may aggravate hyponatraemia.
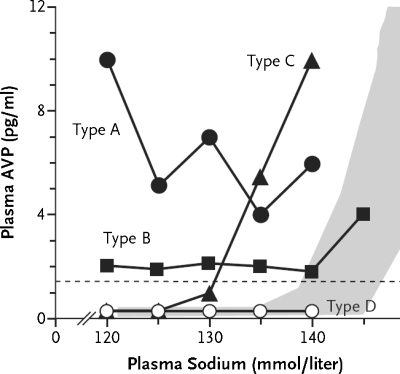
The causes of SIADH are usually classified into five major categories, including pulmonary disorders, malignancy, central nervous system disorders, drugs and a miscellaneous category, which includes idiopathic, transient and hereditary causes (Table 3) [1]. A more physiological classification is based on the level of vasopressin and its response to osmolality (Figure 1) [32].
Table 3.
Causes of SIADH
| Malignancy | Lung disease | CNS disease | Drugs | Miscellaneous |
|---|---|---|---|---|
| Lung cancer (small | Infections (bacterial, | Infections | Antiepileptics | Idiopathic |
| cell, mesothelioma) | viral, tuberculosis, | (meningitis, | Antidepressants | Transient (nausea, |
| Oropharynx | abscess) | encephalitis, AIDS, | (mainly SSRI's) | pain, stress) |
| GI-tract (stomach, | Cystic fibrosis | abscess) | Antipsychotics | Hereditary |
| duodenum, | Status asthmaticus | Stroke (CVA, | Anaesthetics | Exercise associated |
| pancreas) | subarachnoid, | Chemotherapy | ||
| Genitourinary tract | subdural) | (ifosfamide, | ||
| Endocrine thymoma | Hydrocephalus | cylcofosfamide, | ||
| Lymphomas | Brain tumour | vincristine) | ||
| Sarcomas (Ewing) | Head trauma | AVP analogues | ||
| Multiple sclerosis | MDMA (‘Ecstasy’) | |||
| Guillain–Barré syndrome | ||||
| Shy–Drager syndrome | ||||
| Lewy body dementia |
Adapted from Ellison and Berl [1]. The most common causes are underlined.
AIDS, acquired immunodeficiency syndrome; AVP, arginine vasopressin; CNS, central nervous system; CVA, cerebrovascular accident; GI, gastrointestinal; MDMA, 3,4-methylenedioxymethamphetamine; SSRI, selective serotonin reuptake inhibitors.
Fig. 1.
Four types of SIADH. Type A is characterized by unregulated secretion of vasopressin, type B by elevated basal secretion of vasopressin despite normal regulation by osmolality, type C by a ‘reset osmostat’ and type D by undetectable vasopressin levels (these patients may have a gain of function mutation of the V2-receptor). Especially for types C and D, the term syndrome of inappropriate antidiuresis may be more appropriate. According to present understanding, type C and D patients may not respond to vasopressin-receptor antagonists. The shaded area represents the normal response showing a rise in vasopressin secretion with increasing serum sodium concentrations. Adapted and reprinted from [32], Copyright 2006, with permission from Elsevier.
Asymptomatic hyponatraemia is not asymptomatic—the new concept
In 2006, Renneboog and colleagues published a landmark study on the neurological symptoms in elderly patients with chronic and supposedly asymptomatic hyponatraemia [10]. They conducted a matched case-control study in which 122 patients with chronic hyponatraemia (SNa 126 ± 5 mmol/L) were compared to 244 controls. Patients with congestive heart failure, liver cirrhosis and nephrotic syndrome as well as patients with acute hyponatraemia (polydipsia and/or seizures) were excluded from the study. The pathogenesis of hyponatraemia, as assessed by the response to 2 L of isotonic saline, was SIADH (approximately half of the patients), diuretic-induced hyponatraemia and salt depletion. Hyponatraemic cases had had falls approximately four times more often than controls (21% versus 5%). However, they did not have more acute illnesses, chronic conditions, polypharmacy, central nervous system drugs, or vasodilators. Surprisingly, the frequency of falls was similar in patients with mild hyponatraemia (19% with SNa 130–132 mmol/L) compared to those with more severe hyponatraemia (22% with SNa 115–117 mmol/L). To analyse the mechanism of the falls, 16 similar elderly patients with hyponatraemia (SNa 128 ± 3 mmol/L) were subjected to formal neurocognitive testing (the tandem eyes open test and eight visual and auditory attention tests) during hyponatraemia and normonatraemia. These tests were also performed in healthy volunteers with and without the consumption of alcohol (blood alcohol concentration 0.6 ± 0.2 g/L). The degree of unsteadiness of the gait (as measured by a pressure-sensitive calibrated platform) was significantly greater in patients while hyponatraemic as compared to normonatraemic (Figure 2). With regard to attention tests, both the mean response latency and the total number of errors were significantly greater during hyponatraemia than in normonatraemia. A blood alcohol level of 0.6 g/L induced similar but lesser changes than hyponatraemia. In a follow-up study, it was demonstrated that mild hyponatraemia (SNa 131 ± 3 mmol/L) was also associated with bone fracture in ambulatory elderly [33].
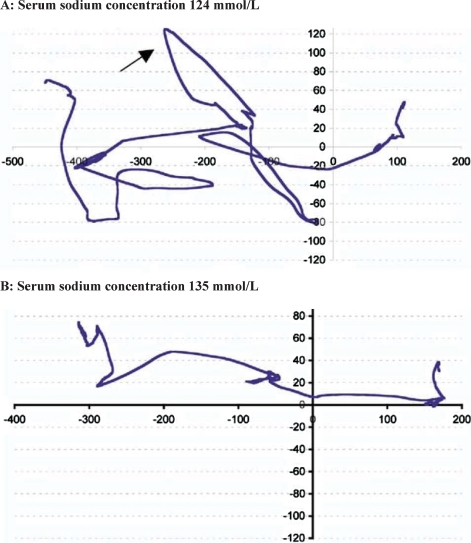
Fig. 2.
Gait pattern in the same patient during and after correction of chronic hyponatraemia. Patient is walking from right to left. The arrow depicts an irregular path of the centre of pressure. Tandem gait parameters were analysed with a pressure-sensitive calibrated platform that evaluates the patient's balance performance on the basis of the recorded displacement of the centre of pressure (i.e. the projection of the centre of gravity on the ground). Adapted and reprinted from [10], Copyright 2006, with permission from Elsevier.
The Renneboog study should change our concept of chronic ‘asymptomatic’ hyponatraemia dismissing it as a benign condition. Currently, chronic hyponatraemia, which is often due to SIADH, is accepted as a possible harmless complication of the underlying disease or treatment. The possibility to relieve subtle but important neurological symptoms by avoidance of hyponatraemia warrants consideration. Falls and fractures are a public health concern, and the potential to prevent these could have a great impact on morbidity and mortality. Similar reasoning would apply to an increased error rate in these patients (e.g. being confused about one's medication, switching the gas off in the kitchen or leaving the door open when going out of the house).
The good news is that the neurological signs in the Renneboog-study were reversible after correction of hyponatraemia [10]. They cite one intriguing case report to explain their findings. This report demonstrated a phenomenon of nerve conduction slowing, which was present during hyponatraemia and resolved thereafter [34]. The authors speculate that the extracellular sodium concentration plays a role in nerve impulse generation. Hyponatraemia would then lead to a lower electrochemical gradient of sodium, and thus to a weaker sodium current during depolarization. If so, nerve conduction slowing represents a new neurological manifestation of hyponatraemia.
How to improve the future management of hyponatraemia
When formulating strategies to improve the management of hyponatraemia, a number of common sense options come to mind first. To name a few: better adherence to guidelines, more precise application of the criteria for SIADH, better analysis which predisposing factors for hyponatraemia are present, earlier recognition of hospital-acquired hyponatraemia, implementation of hospital warning systems and physician education [6,8,11–13,19,35]. A sound diagnostic approach to hyponatraemia in general is important to identify patients with definite SIADH, who may then be suitable for the appropriate treatment. We suggest that currently only SIADH patients with chronic hyponatraemia in the range of 120–132 mmol/L are to be considered potential candidates for vasopressin-receptor antagonists. There is too little experience with vaptans in SNa < 120 mmol/L, whereas SNa between 133 and 135 mmol/L is often not reproducible or represents a lab error.
Will vasopressin-receptor antagonists improve the outcome of SIADH? Mortality will probably be a difficult parameter to use, because the highest mortality rates are seen in patients with hyponatraemia due to advanced heart or liver disease and are usually ascribed to the severity of the disease. A complicating factor is that few studies have looked at SIADH only—the majority studied a mix of hyponatraemic disorders [36]. Other outcome parameters of morbidity may be more achievable, including a reduction in the length of hospital stay, neurological improvement and quality of life once fluid restriction has been lifted. This will probably also lead to a change of perspective for physicians, who often see chronic hyponatraemia as benign. Physicians should begin to envision a hyponatraemic patient to have a mental status that is like that of a normal person who ingested a considerable amount of alcohol. The fact that attention deficits in patients with hyponatraemia were identified only recently suggests that we generally overlook the mental impairments caused by hyponatraemia in our patients, even though they are clearly important in everyday life.
The question remains whether vasopressin-receptor antagonists are superior to the traditional therapies for SIADH. This may require head-to-head trials (for more details, see the next article by Zietse et al.). Our case studies in the final article of this supplement illustrate some of the current challenges of SIADH (van der Lubbe et al.). Most physicians will agree that fluid restriction, the usual treatment for SIADH, is cumbersome and stressful for both patient and physician. Continuous fluid restriction in patients who have a tendency to develop hyponatraemia due to chronic conditions or lifelong drug therapy is difficult to maintain. In addition, these patients have a permanent risk of worsening hyponatraemia, for example when they do drink more, or when a second insult occurs (vomiting, diarrhoea, new drug, pneumonia). Similarly, as was illustrated by the studies on hyponatraemia, hospitalization introduces many factors (e.g., pain, nausea, drugs) that increase vasopressin, some of which are unavoidable. These situations, in combination with the insight that chronic hyponatraemia is not benign, may suggest more stringent indications for treatment of hyponatraemia, including with vasopressin-receptor antagonists. Now that the vasopressin-receptor antagonists are ‘out there’, it will be interesting to see how popular they will become in the management of hyponatraemia secondary to SIADH.
Acknowledgments
Publication costs for this article were supported by Otsuka Pharmaceutical Europe Ltd. The authors have not received any honorarium or editorial support from Otsuka Pharmaceutical Europe Ltd. in relation to this article. The company has had the opportunity to comment on the medical content and accuracy of the article, however final editorial content resides with the author and NDT Plus. Dr D. J. O’Donoghue serves as guest editor for this supplement and has reviewed this article. The authors would also like to thank Prof. P. Gross for his valuable comments on earlier versions of this article.
Conflict of interest statement. EJH reports having received consulting fees from Otsuka. NL and RZ have no conflicts of interest to declare.
References
- 1.Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- 2.Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624–1632. doi: 10.1016/S0140-6736(08)60695-9. [DOI] [PubMed] [Google Scholar]
- 3.Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314:1529–1535. doi: 10.1056/NEJM198606123142401. [DOI] [PubMed] [Google Scholar]
- 4.Sterns RH, Riggs JE, Schochet SS., Jr Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314:1535–1542. doi: 10.1056/NEJM198606123142402. [DOI] [PubMed] [Google Scholar]
- 5.Hoorn EJ, Zietse R. Tolvaptan for hyponatremia. N Engl J Med. 2007;356:961. Author reply 962–963. [PubMed] [Google Scholar]
- 6.Verbalis JG, Goldsmith SR, Greenberg A, et al. Hyponatremia Treatment Guidelines 2007: Expert Panel Recommendations. Am J Med. 2007;120(11A):S1–S21. doi: 10.1016/j.amjmed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Sterns RH, Cappuccio JD, Silver SM, et al. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994;4:1522–1530. doi: 10.1681/ASN.V481522. [DOI] [PubMed] [Google Scholar]
- 8.Hoorn EJ, Zietse R. Hyponatremia revisited: translating physiology to practice. Nephron Physiol. 2008;108:46–59. doi: 10.1159/000119709. [DOI] [PubMed] [Google Scholar]
- 9.Lohr JW. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med. 1994;96:408–413. doi: 10.1016/0002-9343(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 10.Renneboog B, Musch W, Vandemergel X, et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:e1–e8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Gill G, Huda B, Boyd A, et al. Characteristics and mortality of severe hyponatraemia—a hospital-based study. Clin Endocrinol (Oxf) 2006;65:246–249. doi: 10.1111/j.1365-2265.2006.02583.x. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JA, Le Jeune IR, Hall IP. Severe hyponatraemia in medical in-patients: aetiology, assessment and outcome. Q J Med. 2006;99:505–511. doi: 10.1093/qjmed/hcl071. [DOI] [PubMed] [Google Scholar]
- 13.Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21:70–76. doi: 10.1093/ndt/gfi082. [DOI] [PubMed] [Google Scholar]
- 14.Janicic N, Verbalis JG. Evaluation and management of hypo-osmolality in hospitalized patients. Endocrinol Metab Clin North Am. 2003;32:459–481. doi: 10.1016/s0889-8529(03)00004-5. vii. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz WB, Bennett W, Curelop S, et al. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957;23:529–542. doi: 10.1016/0002-9343(57)90224-3. [DOI] [PubMed] [Google Scholar]
- 16.Turchin A, Seifter JL, Seely EW. Clinical problem-solving. Mind the gap. N Engl J Med. 2003;349:1465–1469. doi: 10.1056/NEJMcps031078. [DOI] [PubMed] [Google Scholar]
- 17.Chung HM, Kluge R, Schrier RW, et al. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83:905–908. doi: 10.1016/0002-9343(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 18.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 19.Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. Q J Med. 2005;98:529–540. doi: 10.1093/qjmed/hci081. [DOI] [PubMed] [Google Scholar]
- 20.Fenske W, Stork S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93:2991–2997. doi: 10.1210/jc.2008-0330. [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick ES. Disorders of sodium balance: hypothyroidism and hyponatraemia: an old wives’ tale? BMJ. 2006;332:854. doi: 10.1136/bmj.332.7545.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner MH, Holding S, Kilpatrick ES. The effect of newly diagnosed hypothyroidism on serum sodium concentrations: a retrospective study. Clin Endocrinol (Oxf) 2006;64:598–599. doi: 10.1111/j.1365-2265.2006.02489.x. [DOI] [PubMed] [Google Scholar]
- 23.Curtis RH. Hyponatremia in primary myxedema. Ann Intern Med. 1956;44:376–385. doi: 10.7326/0003-4819-44-2-376. [DOI] [PubMed] [Google Scholar]
- 24.Small M, MacCuish AC, Thomson JA. Missed Addisonian crisis in surgical wards. Postgrad Med J. 1987;63:367–369. doi: 10.1136/pgmj.63.739.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soule S. Addison's disease in Africa—a teaching hospital experience. Clin Endocrinol (Oxf) 1999;50:115–120. doi: 10.1046/j.1365-2265.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Der Hoek J, Hoorn EJ, de Jong GM, et al. Severe hyponatremia with high urine sodium and osmolality. Clin Chem. 2009 doi: 10.1373/clinchem.2009.125575. [in press] [DOI] [PubMed] [Google Scholar]
- 27.Smith JC, Siddique H, Corrall RJ. Misinterpretation of serum cortisol in a patient with hyponatraemia. BMJ. 2004;328:215–216. doi: 10.1136/bmj.328.7433.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pura M, Kreze A, Jr, Kentos P, et al. The low-dose (1 mug) cosyntropin test (LDT) for primary adrenocortical insufficiency: defining the normal cortisol response and report on first patients with Addison disease confirmed with LDT. Exp Clin Endocrinol Diabetes. 2009 doi: 10.1055/s-0029-1202275. Apr 8 2009 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Liamis G, Kalogirou M, Saugos V, et al. Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant. 2006;21:1564–1569. doi: 10.1093/ndt/gfk090. [DOI] [PubMed] [Google Scholar]
- 30.Saghafi D. Water loading test in the reset osmostat variant of SIADH. Am J Med. 1993;95:343. doi: 10.1016/0002-9343(93)90293-x. [DOI] [PubMed] [Google Scholar]
- 31.Hoorn EJ, Swart RM, Westerink M, et al. Hyponatremia due to reset osmostat in dementia with lewy bodies. J Am Geriatr Soc. 2008;56:567–569. doi: 10.1111/j.1532-5415.2008.01579.x. [DOI] [PubMed] [Google Scholar]
- 32.Robertson GL. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med. 2006;119(7 Suppl 1):S36–S42. doi: 10.1016/j.amjmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Gankam Kengne F, Andres C, Sattar L, et al. Mild hyponatremia and risk of fracture in the ambulatory elderly. Q J Med. 2008;101:583–588. doi: 10.1093/qjmed/hcn061. [DOI] [PubMed] [Google Scholar]
- 34.Aranyi Z, Kovacs T, Szirmai I, et al. Reversible nerve conduction slowing in hyponatremia. J Neurol. 2004;251:1532–1533. doi: 10.1007/s00415-004-0574-1. [DOI] [PubMed] [Google Scholar]
- 35.Adrogue HJ. Consequences of inadequate management of hyponatremia. Am J Nephrol. 2005;25:240–249. doi: 10.1159/000086019. [DOI] [PubMed] [Google Scholar]
- 36.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]