Abstract
Microarray and real-time RT-PCR were used to examine expression changes in primary bone marrow cells and RAW 264.7 cells in response to RANKL. In silico sequence analysis was performed on a novel gene which we designate OC-STAMP. Specific siRNA and antibodies were used to inhibit OC-STAMP RNA and protein, respectively, and TRAP+ multinucleated osteoclasts were counted. Antibodies were used to probe bone tissues and western blots of RAW cell extracts +/− RANKL. cDNA overexpression constructs were transfected into RAW cells and the effect on RANKL-induced differentiation was studied. OC-STAMP was very strongly up-regulated during osteoclast differentiation. Northern blots and sequence analysis revealed 2 transcripts of 2 kb and 3.7 kb differing only in 3'UTR length, consistent with predictions from genome sequence. The mRNA encodes a 498 amino acid, multi-pass transmembrane protein that is highly conserved in mammals. It has little overall homology to other proteins. The carboxy-terminal 193 amino acids, however, are significantly similar to the DC-STAMP family consensus sequence. DC-STAMP is a transmembrane protein required for osteoclast precursor fusion. Knockdown of OC-STAMP mRNA by siRNA and protein inhibition by antibodies significantly suppressed the formation of tartrate-resistant acid phosphatase (TRAP) +, multinucleated cells in differentiating osteoclast cultures, with many TRAP+ mononuclear cells present. Conversely, overexpression of OC-STAMP increased osteoclastic differentiation of RAW 264.7 cells. We conclude that OC-STAMP is a previously unknown, RANKL-induced, multi-pass transmembrane protein that promotes the formation of multinucleated osteoclasts.
INTRODUCTION
Skeletal homeostasis requires coordinated action by bone-forming osteoblasts and bone-resorbing osteoclasts (Marks and Odgren, 2002). Deficient bone resorption leads to sclerotic bone, as seen in osteopetrosis, whereas excessive resorption is central to the pathogenesis of osteoporosis, tumor metastasis to bone, arthritis, periodontal disease, and prosthetic joint implant loosening. Understanding the mechanisms that control the differentiation and activity of osteoclasts is therefore of central importance to many widespread clinical conditions. The differentiation of active, multinucleated osteoclasts from mononuclear hematopoietic precursors is a complex process requiring endocrine signals, local signals from growth factors in the bone environment, and the successful expression of the many gene products needed for the precursors to fuse to form multinucleated cells; for the osteoclast to attach to the bone surface; to secrete protons, ions, and proteases; and to ingest the solubilized bone matrix and transport it through the cell for export (Balemans et al., 2005; Boyle et al., 2003).
Multinucleated osteoclasts are formed by fusion of mononuclear, hematopoietic cells of the monocyte/macrophage lineage (Marks and Walker, 1981; Walker, 1975). Osteoclast precursors respond to signals from colony-stimulating factor-1 (CSF-1; M-CSF) and express RANK, the receptor for the TNF superfamily member RANKL (TRANCE, OPGL, ODF)(Boyle et al., 2003). CSF-1 and RANKL are supplied in the bone environment by osteoblasts. Normally, both CSF-1 and RANKL are required for osteoclast differentiation, although it is possible to circumvent this pathway by means of TNF-α and TGF-β (Kim et al., 2005). To understand better how osteoclasts differentiate and carry out resorptive activity, we have used high-density microarrays to investigate global gene expression changes that accompany osteoclast differentiation (Yang et al., 2006a; Yang et al., 2006b).
In the course of these experiments, we identified a previously uncharacterized gene that is strongly up-regulated during osteoclast differentiation. The gene product is unrelated to other known proteins with the notable exception of a long stretch of its carboxy-terminal region that bears significant similarity to the DC-STAMP protein family consensus. DC-STAMP is a multi-pass transmembrane protein that was originally identified in gene expression screens of dendritic cells (Hartgers et al., 2000). Further work showed that DC-STAMP expression responded strongly to RANKL and played an important role in osteoclast differentiation. Blocking or knocking down DC-STAMP inhibited osteoclast differentiation and overexpressing it increased osteoclast differentiation in response to RANKL (Kukita et al., 2004). In addition, DC-STAMP knockout mice have a unique osteoclast phenotype in that they have large numbers of mononuclear, TRAP-positive osteoclasts that are able to resorb bone, albeit inefficiently, leading to moderate osteopetrosis (Yagi et al., 2005). DC-STAMP-negative cells are unable to initiate cell-cell fusion, but they are able to fuse with DC-STAMP-positive cells, providing the first mechanistic insights into the process of fusion (Vignery, 2005; Yagi et al., 2005). The ligand for DC-STAMP remains unknown. Proteins containing the DC-STAMP family consensus sequence are not limited to vertebrates with mineralized skeletons. The Conserved Domain Database (Marchler-Bauer et al., 2005) lists several dozen DC-STAMP consensus-containing proteins, including mouse, human, and rat DC-STAMP as well as the protein we describe here and its vertebrate orthologues. In addition, there are several other proteins from invertebrates including the nematode Caenorhabditis elegans, and the arthropods Drosophila melanogaster and Anopheles gambii (http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=pfam07782) whose functions remain to be investigated. We report here the characterization of this new protein, which we call osteoclast-stimulatory transmembrane protein (OC-STAMP) and explore functional similarities with DC-STAMP.
MATERIALS AND METHODS
Unless otherwise noted, reagents were from Sigma Chemical Co. (St Louis, MO). All work requiring animals was done following procedures approved by the UMMS IACUC. Mouse bone marrow mononuclear cells (BMC), obtained from normal littermates of the op/op strain (genotype either +/+ or +/Csf1op), and RAW 264.7 murine pre-osteoclast-like cells were isolated, cultured in multi-well plates, and induced to differentiate with CSF-1 and RANKL (BMC) or RANKL (RAW 264.7) as previously described by us (Yang et al., 2006a; Yang et al., 2006b; Yang and Odgren, 2005). RNA samples were isolated from cell cultures, reverse transcribed, and used for high density microarrays (mouse chipset MOE 430, Affymetrix, Santa Clara, CA) or real-time RT-PCR (Roche Light Cycler, Indianapolis, IN) as described (Yang et al., 2006a; Yang et al., 2006b; Yang and Odgren, 2005). A single gene array was hybridized for each cell type and time point. For BMC, there was a sample treated only with CSF-1 (required for cell survival), and one each treated with RANKL for 2 and 4 days. For RAW 264.7 cells, CSF-1 is not needed, so those cells were tested without treatment and after treatment with RANKL for 2 and 4 days. Resulting data were normalized and analyzed using D-Chip 1.3 software as previously described (Yang et al., 2006a; Yang et al., 2006b). For real-time RT-PCR, a LightCycler System (Roche Diagnostics, Indianapolis, IN) was used according to the manufacturer's instructions. Reactions were set up in micro capillary tubes using 0.5 μL cDNA with 9.5 μL of a LightCycler FastStart DNA Master SYBR Green I mix (Roche Diagnostics) to which gene-specific upstream and downstream PCR primers had been added. The final concentrations of the reaction components were 1.0 mM of each primer and 2.5 mM MgCl2. Conditions were: 95°C preincubation for 10 minutes; 95°C for 5 seconds; 55°C for 10 seconds; and 72°C for 12 seconds; for 45 cycles. Primers for OC-STAMP were, forward: 5'-TGGGCCTCCATATGACCTCGAGTAG-3; reverse: 5'-TCAAAGGCTTGTAAATTGGAGGAGT-3'. Regions amplified were confirmed by sequence analysis and melting curves were used to determine that single amplification products were obtained. Normalization was to GAPDH. For Northern blot analysis, the pCMV SPORT8 full-length (1956 bp) clone described below was linearized and used as template to prepare anti-sense riboprobe transcribed from the T7 site. Digoxigenin-UTP (Roche) was incorporated into the probe using the Ampliscribe in vitro transcription kit (Epicentre Technologies, Madison, WI). Mouse BMC were either untreated or treated with RANKL for 48 hours as described (Yang et al., 2006a; Yang and Odgren, 2005). RNA was isolated and 20 μg per lane was run under standard denaturing conditions in agarose and labeled with ethidium bromide. The blot was pre-hybridized, hybridized, and probed with alkaline phosphatase-conjugated anti-digoxigenin per the manufacturer's instructions (https://www.roche-applied-science.com/fst/publications.jsp?page=/PROD_INF/MANUALS/DIG_MAN/dig_toc.htm). Signal was developed using Attoglow chemiluminescent substrate (Michigan Diagnostics, Royal Oak, MI).
Overexpression and knockdown
Full-length OC-STAMP cDNA of the 1956 nt transcript was obtained by RT-PCR from mouse BMC following 48 hours of RANKL treatment. It was cloned in-frame into the pcDNA 3.1D/V5-His-TOPO over-expression vector (“pTOPO”; Invitrogen, Carlsbad, CA). In addition, a full-length cDNA clone in the pCMV SPORT6 expression vector was obtained from Invitrogen, clone # 3966086. Both constructs use the CMV strong promoter to drive expression. In-frame insertion was verified by DNA sequencing. RAW 264.7 cells were transfected using the siIMPORTER reagent (Upstate, Lake Placid, NY) according to the manufacturer's instructions. Plasmid DNA was added at 50 ng/well in 96-well plates. The corresponding empty vector was used in negative control wells. For siRNA knockdown experiments, a pool of 4 duplex siRNAs was employed (Dharmacon, Lafayette, CO). The sense strand sequences were: 5'-GACCUGCGUUUCGACAAUAUU-3'; 5'-CCUGGUACCUUCAUCGCUAUU-3'; 5'-CGGAACACCUCUUUGGCUUUU-3'; 5'-GUAACGAACUACUGACCCAUU-3'. BLAST analysis confirmed that none of the oligonucleotides had homology to DC-STAMP sequence or to other mRNAs. One day after plating, RAW cells were transfected with siRNA at 2 concentrations, 100 μM and 300 μM, using siIMPORTER according to the manufacturer's specifications, and treated with 100ng/ml RANKL. This treatment was determined experimentally to give minimal non-specific disruption of RAW cell differentiation. Negative control siRNA was obtained from Ambion (Austin, TX; Silencer Negative Control #1 siRNA). Forty-eight hours after transfection, total RNA was extracted from some wells and analyzed for OC-STAMP mRNA using real-time RT-PCR (see above). To rule out cross-reactivity of siRNA with DC-STAMP, two of the control siRNA- and two of the OC-STAMP siRNA-treated RAW 264.7 RNA samples were also subjected to real-time RT-PCR in duplicate using 2 different sets of DC-STAMP-specific primers: pair 1 forward: 5'-CTAGCTGGCTGGACTTCATCC-3'; reverse: 5'-TCATGCTGTCTAGGAGACCTC-3'; pair 2 forward: 5'-TCTCAGTGTGTCTGAGACTTG-3; reverse: 5'-GACTGTGTTGCTCATAGATCATC-3'. Seventy-two hours post-transfection, other wells were stained for TRAP activity (Sigma) and assayed microscopically for the total number of TRAP-positive cells per well with 3 or more nuclei. The counts were done, blind, by two individuals and the results were pooled. Each experimental condition was tested in 6 replicate wells for each siRNA, and experiments were carried out three separate times. Results for each well were expressed as “percent siRNA control”, and the data from the 3 experiments were pooled.
Antibody studies
The carboxy-terminal 180 amino acid region of OC-STAMP was expressed in E. coli and used to immunize two rabbits. The resulting antisera, designated Ab1 and Ab2, were used to perform immunohistochemistry on sections of 2-week-old normal rat tibiae, which were wild-type (+/+) littermates of rats of the toothless strain (Van Wesenbeeck et al., 2002). Bones were dissected, paraformaldehyde fixed, demineralized in EDTA, paraffin embedded, and processed for immunohistochemistry as previously described (Yang et al., 2006b). Primary antibody concentration was determined empirically. Photomicrography (Zeiss Axioskop and Zeiss Axiocam HRc; Carl Zeiss, Oberkochen, Germany) was done as described (Yang et al., 2006a; Yang et al., 2006b). For immunoblots, CytoBuster reagent (Novagen, LaJolla, CA) was used to extract proteins from RAW 264.7 cells with and without RANKL treatment for 48 hours. Protein was measured by Bradford assay (BioRad) and equal amounts per lane were separated on 10% SDS-PAGE minigels and blotted onto PVDF membrane (Immobilon P, Millipore, Billerica, MA) using a semi-dry transfer device (Owl Scientific, Cambridge, MA). After blocking in 5% nonfat dry milk in Tris-buffered saline (TBS), rabbit polyclonal anti-OC-STAMP antibody diluted 1:100 in TBS containing 0.1% Tween-20 and 1% milk was added and the blot was incubated for 90 minutes at room temperature. Following washes in TBS, goat anti-rabbit IgG alkaline phosphatase conjugate (BioRad) was added, followed by more TBS washes. The blots were developed with Attoglow chemiluminescent substrate (Michigan Diagnostics). The OC-STAMP antibodies were also used in studies of inhibition of osteoclast differentiation of BMC. These studies were done as previously described for OGR-1 (Yang et al., 2006a). Briefly cells were plated in 96 well plates, and antibody or pre-immune negative control serum were added at 1 μl per 100 μl of medium per well. Multinucleated, TRAP-positive cells were counted after 4-day exposure to RANKL plus either antibody or control serum.
Statistical tests
Experiments were performed at least 3 times, with multiple wells for each condition in each experiment. For overexpression studies, n = 50 for each condition. For antibody inhibition, n = 6 for each condition. For siRNA experiments, n = 18 for each condition. F-tests were performed to determine whether samples had equal or unequal variances before proceeding the appropriate t-test for significance of difference of means. Tests were done using Microsoft Excel 2003. Results are shown as mean ± sem.
RESULTS
Identification, expression and sequence analysis of OC-STAMP
Mouse bone marrow mononuclear cells and RAW 264.7 cells were treated with RANKL to induce osteoclast differentiation for 2 and 4 days, and the resulting RNA was used as template to make cRNA for hybridization to high-density microarrays. A sequence present as an EST on the microarrays, GenBank accession number BC020160, was found to have very low baseline abundance, especially in BMC, and to be strongly up-regulated by RANKL in both cell types and at both time points. We confirmed these results using real-time RT-PCR (Table 1). In BMC, the results were particularly striking, with up-regulation on the order of 50- to 150-fold by microarray and in the thousands by real-time PCR. For the RAW 264.7 pre-osteoclast-like cell line, the up-regulation was also very strong, roughly 8-fold by microarray and over 300-fold by real-time PCR. For reasons described below, we have designated this gene product “osteoclast-stimulatory transmembrane protein,” (OC-STAMP). OC-STAMP mRNA thus rises from extremely low levels, especially in primary cells, to very high levels within 2 days during RANKL-induced osteoclast differentiation.
Table 1. OC-STAMP mRNA induction by RANKL.
BMC and RAW 264.7 cells were cultured with or without RANKL. At the days indicated, RNA was collected and assessed by high-density microarray (Affymetrix Gene Chip) and by real-time RT-PCR. Results are shown in Relative Units. Real-time PCR results were normalized to GAPDH. Both methods found very low baseline levels and strong up-regulation of OC-STAMP by RANKL in both BMC and RAW cells.
| Microarray | ||||
|---|---|---|---|---|
| Days | 2 | 4 | ||
| BMC | RAW | BMC | RAW | |
| − RANKL | 20.7 | 29.3 | 56 | 46.1 |
| + RANKL | 3076.3 | 239.5 | 2859.3 | 336.9 |
| Real-Time RT-PCR | ||||
|---|---|---|---|---|
| − RANKL | 0.007 | 0.064 | 0.089 | 0.079 |
| + RANKL | 125.9 | 23.1 | 65.9 | 25.8 |
The gene encoding this sequence (GenBank NM_029021, chromosome 2H3) has not been previously characterized. It is distinct from the DC-STAMP gene locus, which is on chromosome 15C (NM_029422). The locus (RIKEN: 4833422F24Rik) is predicted to contain a total of 3 exons encoding a 1956 nucleotide (nt) transcript, including a 63 nt 5'-UTR, a 1494 nt coding region encoding a 498 amino acid protein, and the remainder 3'-UTR (http://www.ensembl.org/Mus_musculus/geneview?gene=ENSMUSG00000027670). The Havana Vega prediction algorithm for the same locus shows identical intron-exon organization (RP23-395E18.4-001), plus a longer 3' UTR transcribed from exon 3 due to read-through of the first poly-adenylation site, yielding a 3,657 nt mRNA (http://www.ensembl.org/Mus_musculus/geneview?gene=OTTMUSG00000001116;db=vega). Database searching revealed an EST clone (GenBank AK032330) from mouse olfactory brain cDNA that confirms the existence of the longer form of the transcript. It begins within the OC-STAMP coding region in exon 2 and continues through exon 3 beyond the end of the RIKEN predicted 3'UTR terminus for an additional 1700 nt, ending at the Havana Vega predicted site. The overlapping portions are identical in sequence (not shown). This difference in 3'UTR is discussed further below.
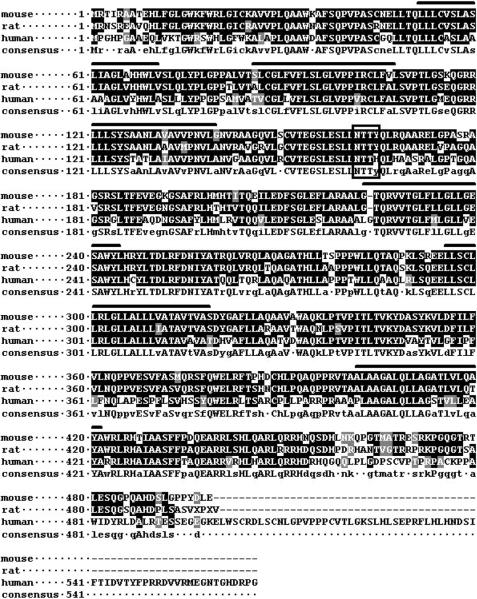
Highly conserved, presumably orthologous proteins are present in all mammals for which sequence is available, and the mouse, rat, and human proteins are shown aligned in Figure 1. The encoded protein is a multiple-pass transmembrane protein. The TMHMM tool (http://www.cbs.dtu.dk/services/TMHMM-2.0/) predicts 6 transmembrane helices, which are indicated in Figure 1. There is a potential N-linked glycosylation site at mouse at aa 162–164, boxed in Figure 1, which resides in a predicted extracellular domain and is conserved in all mammalian sequences, including human, rat, dog, bovine, and macaque. The additional amino acids shown at the C-terminus of the human sequence may or not be genuine, since to date it is only a predicted protein.
Figure 1. Amino acid alignment of mouse, rat, and human OC-STAMP.
CLUSTALW alignment of mouse (NP_083297), rat (XP_230857), and human (XP_943526) OC-STAMP sequences shows the protein is strongly conserved. The conserved, putative N-linked glycosylation site is boxed. Six predicted transmembrane helices are indicated by bars over the mouse sequence. Black indicates identity, gray indicates similarity.
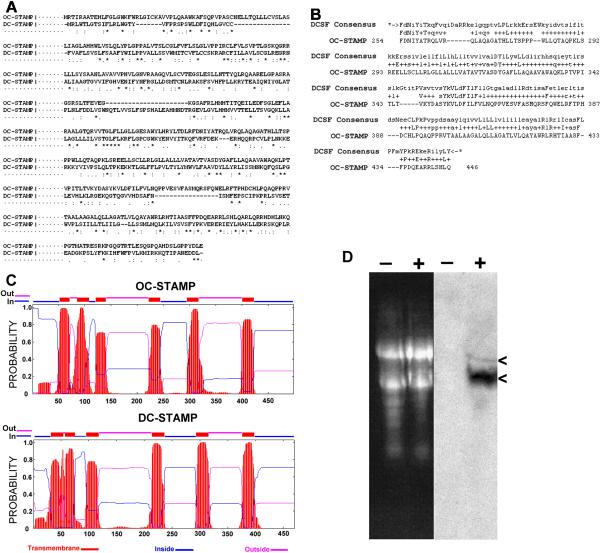
Analysis of the amino acid sequences revealed almost no similarity to other proteins except for the recently-described protein, DC-STAMP (dendritic cell-specific transmembrane protein)(Hartgers et al., 2000; Kukita et al., 2004; Yagi et al., 2005). This similarity is not readily evident when comparing the amino acid sequences directly. For example, CLUSTALW alignment of OC-STAMP and DC-STAMP (Figure 2A) shows only 17% identical amino acids dispersed throughout the protein and several gaps. This level of identity is only slightly higher than that obtained by aligning unrelated protein sequences. A Hidden Markov Model tool for protein family identification (Pfam: http://pfam.wustl.edu/cgi-bin/hmmsearch), however, revealed a large segment (193 aa from 254–446) with highly significant similarity to the DC-STAMP family consensus (P = 1.9 × 10−89), as shown in the alignment in Figure 2B, leading us to designate this protein OC-STAMP. This region of similarity corresponds to amino acids 242–421 of the mouse DC-STAMP sequence (GenBank accession NP_083698).
Figure 2. Comparisons of OC-STAMP and DC-STAMP, and RANKL induction.
A. CLUSTALW alignment of mouse OC-STAMP vs. DC-STAMP shows low homology at the sequence level, with only 17% identical (*), 17% similar (:), 14% weakly similar (.) amino acids, spread throughout the sequence, and several gaps. B. Searches for potential functions for OC-STAMP revealed a 193 amino acid region from aa 254 through 446 with strong relatedness to the DC-STAMP family consensus (DCSF Consensus). The probability of an unrelated sequence having this degree of similarity is 1.9 × 10−89. C. Transmembrane helix and topology prediction by a TMHMM algorithm for mouse OC-STAMP (NP_083297) (top) shows 6 transmembrane helices with amino- and carboxy-termini on the cytoplasmic side. These are shown schematically along the top and the probabilities for transmembrane helix (red), extracellular domains (pink) and cytoplasmic domains (blue) are plotted below. Lower panel shows the same analysis of mouse DC-STAMP (NP_083698), and reveals strikingly similar organization. D. Northern blot of mouse BMC RNA. Left, ethidium bromide stained gel shows equal loading of RNA from cells without (−) or with (+) RANKL. Right lanes show the blotted RNA hybridized to an OC-STAMP probe. OC-STAMP mRNA is undetected in untreated cells (−) and is strongly induced by RANKL (+). Arrowheads on the right indicate two transcripts, the highly abundant 2 kb transcript and the less abundant 3.7 kb transcript containing a longer 3' UTR.
At the amino acid level, conservation of OC-STAMP between mouse, rat (GenBank XP_230857.4), and human (GenBank AF305068) is strong. Rat OC-STAMP is 84% identical and 92% similar to mouse, and human is 74% identical and 86% similar to mouse. These are very similar to the conservation seen for DC-STAMP. All transmembrane prediction algorithms show that OC-STAMP is a multiple-pass transmembrane protein, although with some differences as to the precise locations and topology. We show in Figure 2C the results of the TMHMM prediction algorithm (http://www.cbs.dtu.dk/services/TMHMM-2.0/) for OC-STAMP and for DC-STAMP. While there are to date no experimental data that directly address the topology of either DC-STAMP or OC-STAMP with respect to the cell membrane, it is clear that the overall organization of the two proteins is highly similar. In summary, OC-STAMP is highly similar structurally to DC-STAMP and contains a region with high homology to the DC-STAMP family consensus sequence, but does not have strong direct sequence similarity to DC-STAMP.
Northern blot of mouse BMC RNA (Figure 2D) shows that OC-STAMP is highly induced by RANKL. Two bands were consistently detected in RANKL-treated BMC and RAW 264.7 cells: the shorter, roughly 2kb band corresponds to the mRNA with the shorter 3'UTR predicted by RIKEN; the longer, roughly 3.7 kb band corresponds with the longer 3'UTR predicted by Havana Vega. Our own sequencing of RT-PCR products confirms this result (not shown). The shorter transcript was by far the predominant transcript. All experimental work with cDNA described below utilized transcript with the shorter 3'UTR.
OC-STAMP protein in cells and bone tissue
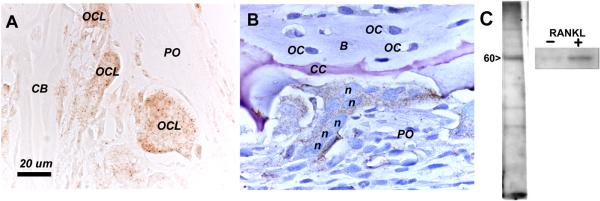
Polyclonal rabbit antibodies were raised against OC-STAMP. The C-terminal 180 amino acids was used as immunogen. Although it contains the bulk of the DC-STAMP family consensus sequence identified by protein family analysis (Figure 2B), it has little direct homology to DC-STAMP at the amino acid level (Figure 2A). The antibodies were used to probe tissue sections of mouse and rat long bones. Results are shown in Figure 3. Anti-OC-STAMP antibody strongly labeled osteoclasts in non-counterstained sections (3A), with little staining of other cell types in the tissue. With toluidine blue counterstain (3B), an osteoclast with many nuclei is seen attached to the periosteal surface of the tibial cortex, and periosteal cells and osteocytes are unlabeled. Similar results were see in mouse long bones (not shown). In 3C, protein extracts tested by western blot from RANKL-minus or −plus RAW 264.7 cells showed a strong band at 60 kDa only after RANKL treatment. The predicted mass of the amino acid sequence is close to 52 kDa, so the 60 kDa electrophoretic mobility may be due to glycosylation at the conserved N-linked glycosylation site.
Figure 3. Antibody detection of OC-STAMP.
A and B, immunohistochemistry of normal, 2-week-old rat proximal tibia, C, western blot of RAW 264.7 cell extracts. A., with no counterstain, several large, OC-STAMP-positive osteoclasts (OCL) can be seen (brownish stain) beneath the periosteum (PO) on the surface of the cortical bone (CB). B, with toluidine blue counterstain, a prominent osteoclast with brownish OC-STAMP signal is seen attached to the bone surface. Several nuclei (n) are visible in the osteoclast. Periosteal cells (PO) are not labeled by the antibody, nor are the numerous osteocytes (OC) inside the bone (B). A cartilage core (CC; pinkish-purple due to toluidine blue metachromasia) within the bone is also seen with this stain. Formalin fixed, demineralized, 5 μm paraffin sections. C. Left, Western blot of total proteins extracted from RANKL-treated RAW cells shows a prominent band at 60 kDa with anti-OC-STAMP antibody. At right, total protein extract from RANKL-minus (−) culture shows only a very faint band compared to RANKL-treated (+) cells.
Inhibition of osteoclast differentiation by blocking OC-STAMP
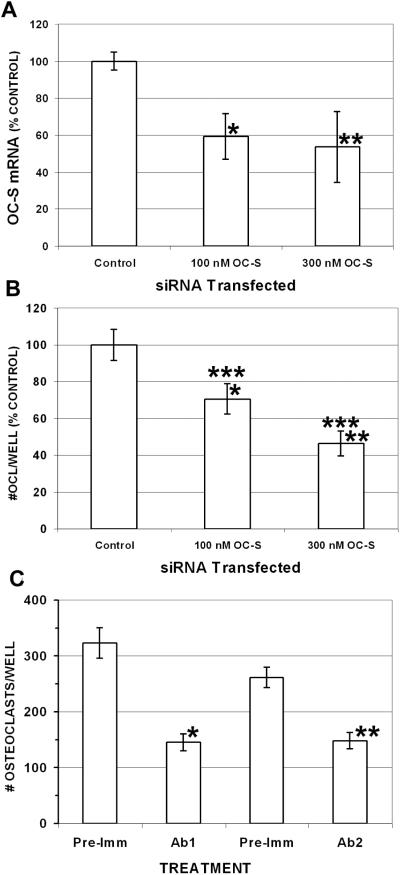
We investigated the effect of inhibiting OC-STAMP with siRNA and antibodies during the RANKL-induced differentiation of RAW 264.7 and mouse BMC cells, respectively. Results are shown in Figure 4. First, we confirmed that the siRNA significantly reduced the OC-STAMP mRNA level in RAW 264.7 cells at both concentrations compared with transfected control siRNA(4A). These treatments also significantly inhibited the differentiation of RAW 264.7 cells into TRAP-positive cells having 3 or more nuclei in a dose-dependent manner (4B). At the 100 nM dose, the osteoclast counts were 70.7% ± 8.4 (mean ± sem., P < 0.01, n = 18) of the number obtained in wells transfected with a control, irrelevant siRNA; and the 300 nM siRNA concentration reduced counts to 46.5% of control ± 6.8 (P < 0.0001, n = 18). The difference between the 100nM and 300 nM siRNA doses was also significant (P < 0.02). Real-time RT-PCR for DC-STAMP mRNA with two different primer pairs confirmed that DC-STAMP mRNA levels, which were strongly induced by RANKL, were not reduced by OC-STAMP siRNA (data not shown). We also investigated the effect of anti-OC-STAMP antibody on CSF-1- and RANKL-induced differentiation of mouse primary BMC. When incubated with either of two polyclonal anti-OC-STAMP antibodies, BMC differentiation was significantly reduced compared with pre-immune sera (4C). Ab1 reduced differentiation from 323 ± 20.4 osteoclasts per well to 145 ± 16.1 per well (P < 0.0005, n = 6). Ab2 had a similar inhibitory effect, reducing the counts from 261 ± 18.9 per well to 148 ± 14.9 (P < 0.001, n = 6).
Figure 4. Inhibiting OC-STAMP inhibits osteoclast differentiation.
siRNA (A, B) or anti-OC-STAMP antibody (C) were added to differentiating cultures and osteoclasts were counted. A. RAW cells were transfected with OC-STAMP siRNA or negative control siRNA at the indicated concentrations and incubated with RANKL, and the OC-STAMP mRNA level was determined by real-time RT-PCR normalized to GAPDH. Both concentrations significantly reduced OC-STAMP mRNA compared with non-specific siRNA control: *P<0.02; **P<0.05. B. OC-STAMP siRNA significantly inhibited formation of TRAP-positive multinucleated cells (OCl) from RAW cells in a dose-dependent manner: *P < 0.01 vs. negative control; **P < 0.001 vs. negative control; ***P < 0.02, 100 nM vs. 300 nM. C. Mouse BMC were incubated with CSF-1 and RANKL, with two different rabbit polyclonal antibodies (Ab 1 and Ab 2) raised against OC-STAMP or with pre-immune serum from the same rabbits (Pre-Imm). Both antibodies had a significant inhibitory effect on the formation of multinucleated, TRAP-positive cells:*P < 0.001; **P < 0.0005.
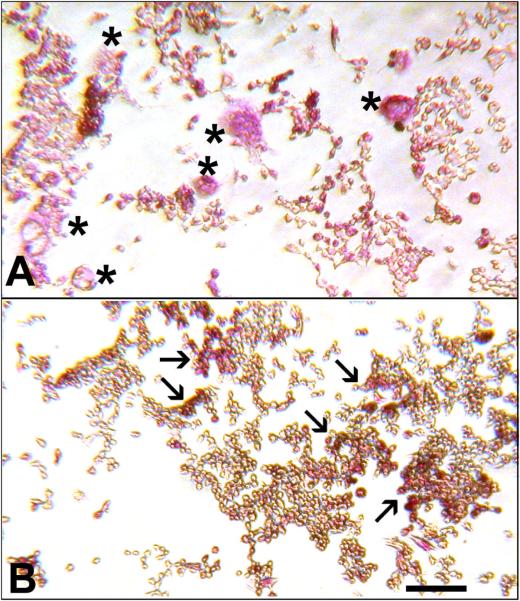
Inhibition of OC-STAMP did not prevent TRAP expression (Figure 5). BMC cultured with CSF-1 and RANKL produced many large, TRAP+ osteoclasts when incubated with pre-immune serum (5A), but when incubated with anti-OC-STAMP, many mononuclear, TRAP+ cells were observed. Although some fusion did occur (as indicated by the graph in 4C), there were also many areas with large numbers of mononuclear cells that stained strongly for TRAP (5B). Similar results were seen in RAW 264.7 cells transfected with OC-STAMP siRNA (not shown). This is consistent with a blockage in fusion of mononuclear cells but not of later steps in osteoclast differentiation.
Figure 5. Anti-OC STAMP inhibits multinucleated osteoclast formation.
Mouse BMC were treated with CSF-1 and RANKL plus control pre-immune serum (A) or anti-OC-STAMP antibody (B), and were stained for TRAP enzyme (red color). Control wells contained many large, multinucleated osteoclasts (asterisks). In contrast, mononuclear, TRAP-positive cells dominated the anti-OC-STAMP-treated wells (clusters of them are indicated by arrows).
Overexpression of OC-STAMP promotes osteoclast differentiation
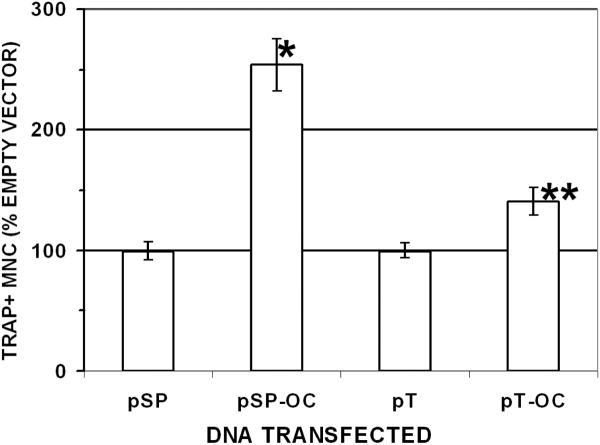
To examine the effects of OC-STAMP overexpression, RAW 264.7 cells were transfected with two different overexpression constructs and cultured in the presence of RANKL, and the number of TRAP-positive cells with 3 or more nuclei were counted (n = 50 for all conditions). The results are shown in Figure 6. After two days of differentiation, pSport OC-STAMP increased the differentiation to 254% (±21.5) of the empty vector control wells (P < 0.0001). The pTOPO clone also increased differentiation to 141% (± 11.5) of controls (P < 0.004).
Figure 6. Overexpression of OC-STAMP increases osteoclast differentiation.
RAW 264.7 cells were transfected with empty vector (pSP = pSport vector; pT = pTOPO vector) or with those vectors containing OC-STAMP full-length cDNA (pSp-OC and pT-OC, respectively). Multinucleated (3 or more nuclei), TRAP-positive cells were counted in multiple wells (6 to 12) in 4 separate experiments by at least 2 individuals, and the results were pooled for analysis. Results were normalized to empty vector counts (100%). When OC-STAMP was expressed from either vector, there was a highly significant increase in osteoclast formation (*P<0.0001; **P<0.002).
DISCUSSION
This report identifies OC-STAMP as a novel, multiple-pass transmembrane protein whose mRNA is very strongly upregulated in primary pre-osteoclasts and in RAW 264.7 cells upon exposure to RANKL, with the shorter 3'UTR-containing transcript being far more abundant than the transcript with the longer 3'UTR. OC-STAMP is present in osteoclasts in bone tissue as well as in cell cultures. Inhibition of OC-STAMP either by siRNA knockdown or by anti-OC-STAMP antibody had strong inhibitory effects on formation of multinucleated, TRAP-positive cells. Conversely, over-expression of OC-STAMP had a stimulatory effect on osteoclast differentiation. The extremely high expression ratios obtained by both gene array and by real-time RT-PCR reflect the very low starting mRNA levels detected by both methods; i.e., OC-STAMP is effectively turned off prior to exposure to RANKL.
Although there have been no previous studies of OC-STAMP, its sequence is present on various gene arrays. We therefore examined its expression via public datasets using its Gene Expression Omnibus number, GSM37205, at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=geo&cmd=search&term=4833422F24. In one experiment by Brooks, at the University of Rochester, OC-STAMP mRNA in mouse femur autografts versus allografts was increased roughly 3-fold. Vascularization and remodeling are more active in autografts due to the greater speed of graft incorporation, suggesting a physiological role for OC-STAMP in skeletal repair. Another experiment by Murray in Brisbane, Australia, showed a roughly 4-fold increase in OC-STAMP within 2 hours when RAW 264.7 cells were treated with the pro-inflammatory lipid lysophosphocholine (LPC), indicating that OC-STAMP responds to inflammatory signals, which are strongly associated with bone loss in various clinical and experimental conditions.
The fact that no other protein similarities were found to OC-STAMP by a variety of algorithms other than the 193 amino acid stretch of the DC-STAMP family consensus is suggestive. DC-STAMP has provided unique insights into mononuclear cell fusion during osteoclast differentiation (Vignery, 2005). It is a multi-pass transmembrane protein originally identified in dendritic cells (Hartgers et al., 2000) which binds an as-yet unidentified ligand. When DC-STAMP activity was blocked, osteoclast differentiation was impaired, and when it was overexpressed, osteoclast differentiation increased (Kukita et al., 2004). Thus, to the extent that we have been able to compare the two, their biological effects are identical.
DC-STAMP knockout mice have moderate osteopetrosis distinguished by numerous TRAP-positive mononuclear osteoclasts that are able to resorb bone, albeit inefficiently. DC-STAMP −/− BMC are unable to fuse when stimulated by CSF-1 and RANKL, instead becoming TRAP-positive mononuclear cells (Yagi et al., 2005). Further insight into fusion was obtained when green fluorescent protein (GFP) was knocked into the DC-STAMP locus. Although DC-STAMP −/−, GFP+ cells were unable to fuse in culture, when they were mixed with DC-STAMP+ cells, osteoclasts with multiple nuclei were formed that incorporated GFP-containing cells (Yagi et al., 2005). This demonstrated that DC-STAMP is required on the “master” precursor cell that initiates fusion, but not on cells which the master cell is engulfing. It also proves that DC-STAMP is not its own ligand. Thus, DC-STAMP is a key factor in the phenomenon of mononuclear cell fusion, about which a great deal remains to be learned.
A similar phenomenon was recently reported when wild-type pre-osteoclasts were mixed with fusion-deficient pre-osteoclasts derived from mice in which the vaculolar ATPase d2 subunit was deleted (Lee et al., 2006). Those knockout animals have a phenotype strikingly similar in many ways to that observed in DC-STAMP knockout mice, i.e., mainly mononuclear, TRAP-positive osteoclasts with mild osteopetrosis. Those authors, investigating potential mechanistic pathways for the ATPase subunit's role in cell fusion, found that it could at partly be rescued by overexpression of members of ADAM (“a disintegrin and metalloproteinase domain”)-family members, ADAM 8 and ADAM 12. A functional relationship between ATPase subunit d2 and DC-STAMP has not been established to date.
Based on the results presented here, it is reasonable to hypothesize a role for OC-STAMP in pre-osteoclast fusion. First is the DC-STAMP family consensus domain. Second, the predicted secondary structures and membrane topology of OC- and DC-STAMP are remarkably similar. Third, both are strongly induced by RANKL. Fourth, both inhibit multinucleated osteoclast formation when blocked by siRNA or by antibody, but leave later steps, like TRAP expression, unaffected. Finally, both promote multinucleated osteoclast differentiation when overexpressed. It therefore seems likely that OC-STAMP, like DC-STAMP, is a membrane receptor with a role in fusion. It is clear that OC-STAMP cannot compensate for the loss of DC-STAMP since DC-STAMP −/− mice have only mononuclear osteoclasts. Investigations into the ligands for these putative receptors will undoubtedly give further insights into the regulation of the important biological process of osteoclast precursor fusion. Three possibilities are, 1) that DC-STAMP and OC-STAMP are reciprocal ligands for one another, 2) that each has a distinct ligand on the surface of the interacting cell, in which case it may reflect a need for positive reciprocal signals on both cells for fusion to proceed, or 3), OC-STAMP and DC-STAMP may interact with one another in a dimer or oligomer on the cell surface as part of a receptor complex. Genetic models, e.g., knockout and/or transgenic mice, combined with further biochemical analyses, will be essential to investigating these possibilities.
Acknowledgments
This work was supported by grant DE07444 to PRO from the US NIDCR, National Institutes of Health and by core facility support from the Diabetes Endocrinology Research Center Grant DK32520 from NIDDKD, National Institutes of Health. The work is solely the responsibility of the authors and does not necessarily reflect the views of the NIH.
Supported by grant DE 07444 from the US National Institute of Dental and Craniofacial Research to PRO
Footnotes
Authors have no conflicts of interest
REFERENCES
- Balemans W, Van Wesenbeeck L, Van Hul W. A clinical and molecular overview of the human osteopetroses. Calcif Tissue Int. 2005;77(5):263–274. doi: 10.1007/s00223-005-0027-6. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Hartgers FC, Vissers JL, Looman MW, van Zoelen C, Huffine C, Figdor CG, Adema GJ. DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur J Immunol. 2000;30(12):3585–3590. doi: 10.1002/1521-4141(200012)30:12<3585::AID-IMMU3585>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202(5):589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200(7):941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, Pignolo RJ, Koczon-Jaremko B, Lorenzo J, Choi Y. v-ATPase V(0) subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12(12):1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33(Database issue):D192–196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SC, Jr., Odgren PR. The structure and development of the skeleton. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd ed. Academic Press; New York: 2002. pp. 3–15. [Google Scholar]
- Marks SC, Jr., Walker DG. The hematogenous origin of osteoclasts: experimental evidence from osteopetrotic (microphthalmic) mice treated with spleen cells from beige mouse donors. Am J Anat. 1981;161(1):1–10. doi: 10.1002/aja.1001610102. [DOI] [PubMed] [Google Scholar]
- Van Wesenbeeck L, Odgren PR, MacKay CA, D'Angelo M, Safadi FF, Popoff SN, Van Hul W, Marks SC., Jr. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: Evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci U S A. 2002;99(22):14303–14308. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignery A. Macrophage fusion: the making of osteoclasts and giant cells. J Exp Med. 2005;202(3):337–340. doi: 10.1084/jem.20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG. Spleen cells transmit osteopetrosis in mice. Science. 1975;190(4216):785–787. doi: 10.1126/science.1198094. [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202(3):345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Mailhot G, Birnbaum MJ, MacKay CA, Mason-Savas A, Odgren PR. Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J Biol Chem. 2006a;281(33):23598–23605. doi: 10.1074/jbc.M602191200. [DOI] [PubMed] [Google Scholar]
- Yang M, Mailhot G, MacKay CA, Mason-Savas A, Aubin J, Odgren PR. Chemokine and chemokine receptor expression during colony stimulating factor-1-induced osteoclast differentiation in the toothless osteopetrotic rat: a key role for CCL9 (MIP-1γ) in osteoclastogenesis in vivo and in vitro. Blood. 2006b;107(6):2262–2270. doi: 10.1182/blood-2005-08-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Odgren PR. Molecular cloning and characterization of rat CCL9 (MIP-1gamma), the ortholog of mouse CCL9. Cytokine. 2005;31(2):94–102. doi: 10.1016/j.cyto.2005.04.001. [DOI] [PubMed] [Google Scholar]