Abstract
We have investigated the expression and function of the Sox15 transcription factor during the development of the external mechanosensory organs of Drosophila. We find that Sox15 is expressed specifically in the socket cell, and have identified the transcriptional cis-regulatory module that controls this activity. We show that Suppressor of Hairless [Su(H)] and the POU-domain factor Ventral veins lacking (Vvl) bind conserved sites in this enhancer and provide critical regulatory input. In particular, we find that Vvl contributes to the activation of the enhancer following relief of Su(H)-mediated default repression by the Notch signaling event that specifies the socket cell fate. Loss of Sox15 gene activity was found to severely impair the electrophysiological function of mechanosensory organs, due to both cell-autonomous and cell-non-autonomous effects on the differentiation of post-mitotic cells in the bristle lineage. Lastly, we find that simultaneous loss of both Sox15 and the autoregulatory activity of Su(H) reveals an important role for these factors in inhibiting transcription of the Pax family gene shaven in the socket cell, which serves to prevent inappropriate expression of the shaft differentiation program. Our results indicate that the later phases of socket cell differentiation are controlled by multiple transcription factors in a collaborative, and not hierarchical, manner.
Keywords: Sox15, Su(H), Vvl, tormogen, socket cell, Notch signaling
1.1.2 Introduction
Metazoan development depends fundamentally on the capacity of a handful of cell-cell signaling pathways to effect an extremely diverse array of conditional cell fate decisions. This capacity is based in turn on the ability of each pathway to activate distinct subsets of its target gene repertoire in different contexts, which is achieved through the integrative properties of the associated transcriptional cis-regulatory modules (Barolo and Posakony, 2002).
The Notch (N) signaling pathway is particularly well suited to binary cell fate choices, in which two possible cell fates are partitioned among two or more adjacent or nearby cells (Lai, 2004; Bray, 2006). The architecture and function of the N pathway have long been studied in the context of mechanosensory organ development in Drosophila, a setting in which the pathway is used repeatedly from the selection of primary precursor cells to the specification of post-mitotic cell fates in the ensuing lineage (Hartenstein and Posakony, 1990; Posakony, 1994). The first step takes place in small groups of cells with neural cell fate potential called proneural clusters (PNCs). Within each PNC, one cell becomes stably committed to the sensory organ precursor (SOP) fate and inhibits all of its neighbors from adopting the same fate, in a N-mediated signaling process called lateral inhibition. The inhibited cells adopt the alternative, epidermal cell fate. The SOP then divides, initiating a fixed cell lineage that ultimately generates the four terminally differentiated cells comprising the mechanosensory organ found in the adult fly: tormogen (socket cell), trichogen (shaft cell), thecogen (sheath cell), and a bipolar neuron (Fig. 1A,B). The socket and shaft cells are sister cells in this lineage (resulting from the division of the pIIa secondary precursor), as are the sheath cell and neuron (progeny of the pIIIb tertiary precursor) (Hartenstein and Posakony, 1989; Gho et al., 1999). When these cell pairs are born, they are each bipotent: both sisters can adopt either of the two possible fates. Asymmetric N signaling is responsible for insuring that the two cells adopt different fates. Thus, one cell in each pair receives and responds to a N-mediated signal from its sister and is accordingly directed to the N-responsive fate (socket or sheath). The remaining sister adopts the N-non-responsive fate (shaft or neuron).
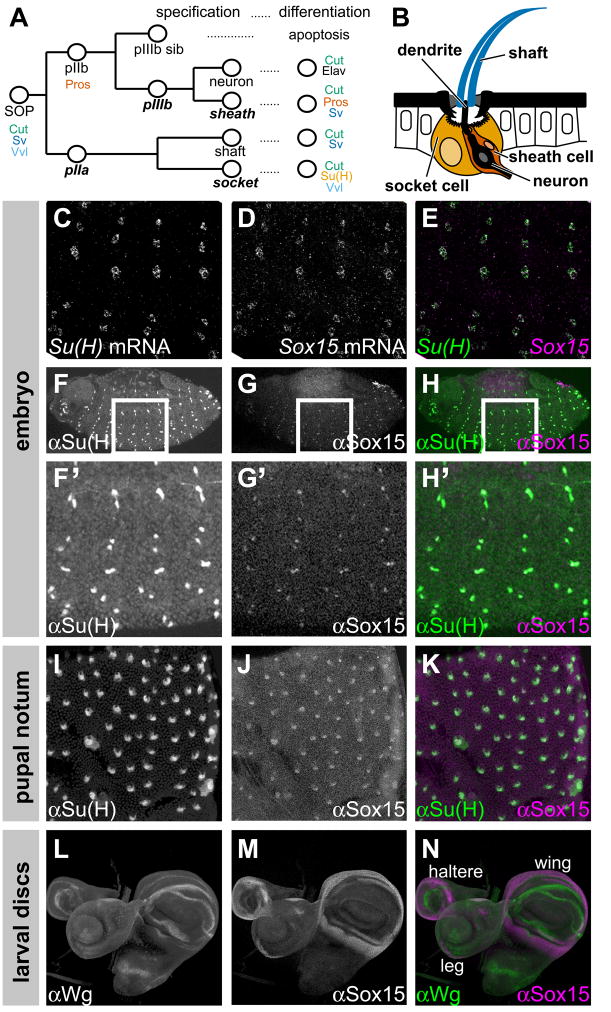
Fig. 1.
Sox15 is expressed specifically in socket cells of external sensory organs. (A) Diagram of the Drosophila mechanosensory bristle lineage, indicating protein expression markers characteristic of specific cells. Expression of the three SOP markers and of the pIIb marker Pros is maintained in all of their progeny cells during the mitotic phase of the lineage. As post-mitotic cells differentiate (24–36 hours APF), protein expression profiles refine to those indicated at right. Notch-dependent cell fates are shown in bold italics. (B) Cross-section diagram of adult mechanosensory bristle organ [adapted from Walker et al. (Walker et al., 2000)], showing the shaft, neuron, sheath, and socket cells. Also indicated is the dendrite of the neuron. (C–E) Su(H) mRNA (C, green in E) and Sox15 mRNA (D, magenta in E) appear in the same cells in the late embryo. (F–N) Pattern of Sox15 protein accumulation (G,G′,J,M, and magenta in H,H′,K,N) compared with that of Su(H) (F,F′,I, and green in H,H′,K) in socket cells of the embryo and the pupal notum, and compared with the pattern of Wg protein (L, green in N) in wing, haltere, and leg imaginal discs of late third-instar larvae.
Following these cell fate specification events, each cell executes a complex program of differentiation that confers the distinctive structural and physiological properties that constitute its unique contribution to the construction of a functional mechanosensory organ (Hartenstein and Posakony, 1989). For example, the trichogen builds the long microtubule-based shaft structure that functions as a very sensitive receptor of mechanical stimuli; this cell degenerates by the adult stage. The tormogen tightly surrounds the other cells with a cytoplasmic sheath, and produces the cuticular socket structure that surrounds the base of the shaft. The socket cell is also responsible for maintaining a distinct (high K+, low Na+) ionic environment in the endolymph, the fluid in the lymph cavity inside the organ. The composition of the endolymph is essential to the proper functioning of the mechanosensitive ion channels in the membrane of the neuron, and thus for mechanotransduction itself (Jarman, 2002).
The fundamental role played by N signaling in the development of Drosophila mechanosensory organs is founded on context-dependent transcriptional responses to activation of the N receptor. Suppressor of Hairless [Su(H)], the transducing transcription factor for the N pathway, is by default a repressor (Barolo et al., 2000; Morel and Schweisguth, 2000; Furriols and Bray, 2001). In the absence of signaling, the adaptor protein Hairless (H) binds to Su(H) and recruits the co-repressors Groucho (Gro) and C-terminal Binding Protein (CtBP), thus conferring repressive activity on Su(H) bound to its targets (Morel et al., 2001; Barolo et al., 2002). N activation leads to proteolytic cleavage of the receptor’s intracellular domain (NICD) and its translocation to the nucleus, where it and the co-activator protein Mastermind (Mam) form a complex that replaces the H/Gro/CtBP complex on Su(H), converting Su(H) from a repressor to an activator (Bray, 2006). Which subset of N pathway target genes is activated following any given signaling event is determined by “local activators” — transcription factors that cooperate with Su(H) to activate only those targets with the appropriate binding sites in their N-responsive cis-regulatory modules (Nellesen et al., 1999; Cooper et al., 2000; Barolo and Posakony, 2002). Different local activators are expressed in different developmental contexts. Thus, a full understanding of a particular cell’s distinctive response to N signaling requires knowledge of the full set of pathway target genes activated by the signal in that cell; identification of each target’s responding cis-regulatory module; and identification of the local activators that contribute to the context-specific activation of each module. The goal is to elucidate the cell’s “specification-differentiation interface”; in other words, to understand how the same cell fate specification signal elicits a distinct differentiative outcome in each setting.
Our previous work has characterized one highly specific transcriptional response of the socket cell to the N signaling event that specifies its fate (Barolo et al., 2000). This cell uniquely expresses very high levels of Su(H), far higher than that required to transduce a N signal. This is the result of the cell type-specific activation of the autoregulatory socket enhancer (ASE) downstream of the Su(H) gene. This autoactivation loop, and the elevated Su(H) levels it generates, are not required for specification of the socket cell fate, nor for many aspects of socket cell differentiation, including the normal formation of the socket cuticular structure. Instead, sensory organs in which the socket cell lacks ASE activity exhibit severe deficits in the electrophysiological properties that underlie mechanotransduction. This finding suggested that different components of the socket cell differentiation program are under separate regulatory control.
In the present study we show that the Sox-family transcription factor Sox15 is likewise expressed specifically in the socket cell of external sensory organs and we identify the cis-regulatory module that directs this expression. We provide evidence that this module is a direct target of Su(H) and the N pathway, and that N signaling acts principally to relieve default repression by Su(H), leaving to local activators the task of activating the module in the socket cell. We show that one such local activator is the POU-domain transcription factor Ventral veins lacking (Vvl). By analyzing flies carrying deletion mutations within the Sox15 locus, we define an important role for Sox15 in the socket cell differentiation program. We find that Sox15, like Su(H), is required for the proper electrophysiological function of the sensory organ. Important phenotypic differences between Sox15 and Su(H) ASE mutants, however, imply that the two factors control at least partially non-overlapping components of the socket program. Finally, we demonstrate that Sox15 and Su(H) collaborate to inhibit socket cell expression of shaven (sv), which encodes a Pax family transcription factor that is a high-level regulator of the shaft differentiation program (Kavaler et al., 1999). Thus, actively preventing execution of the alternative (sister cell) program, which helps insure the robustness of cell fate commitment, is a critical response to N signaling in the mechanosensory organ lineage.
1.1.3 Materials and methods
1.2 Fly lines
KG09145 flies were a gift of Hugo Bellen; Ubx-FLP flies were a gift of Y. N. Jan; vvlH599 FRT80B flies were a gift of Adi Salzberg; Ubi-nGFP FRT80B and sequencing strain flies were obtained from the Bloomington Stock Center (Stock #5630 and #2057, respectively); the Su(H)AR9/Su(H)SF8; Su(H)RC-ΔASE stock has been described previously (Barolo et al., 2000).
1.3 Sox15 mutant alleles
New mutant alleles of Sox15 described in this study were created by mobilizing the P transposon insertion KG09145 (Roseman et al., 1995; Bellen et al., 2004) by crossing this strain to flies carrying a transposase source (Cooley et al., 1988). F1 progeny were crossed to a CyO balancer and stocks were generated from y− w−, CyO F2 progeny. Stocks were screened for deletions by PCR, first in pools of ten, and then as individual lines from positive pools. DNA was amplified from individual positive lines, and PCR products were sequenced to determine exact deletion breakpoints.
1.4 Mosaic analysis
vvl−/− clones were generated utilizing the FLP/FRT system (Golic and Lindquist, 1989; Golic, 1991; Xu and Rubin, 1993). Males of the genotype y w Ubx-FLP/Y; vvlH599 FRT80B/+ were crossed either to y w Ubx-FLP; ASE-nGFP; Ubi-nGFP FRT80B or to y w Ubx-FLP; Sox7.5>nGFP; Ubi-nGFP FRT80B females. Flies were reared at 25°C to induce clones.
1.5 Electrophysiology
Recordings from adult anterior notopleural bristles were performed as described previously (Kernan et al., 1994; Walker et al., 2000). For each genotype, eight bristles were analyzed. Statistical significance was determined using a two-sample t test.
1.6 DNA - binding assays
The vvl-PA coding region was amplified from genomic DNA and cloned into the pGEX-5X-2 vector. GST-Vvl fusion protein was purified as described (Bailey and Posakony, 1995), and 6XHis-Su(H) as described (Janknecht et al., 1991). Electrophoretic mobility shift assays (EMSAs) were performed as described previously (Bailey and Posakony, 1995; Barolo et al., 2000). Wild-type Probe 1 consists of the sequence TTACAAGTAATATTTACATTTTTCCCATGCTAA; the Vvl site mutant version is TTACAAGTAATAGGGACCGTTTTCCCATGCTAA, the Su(H) site mutant version is TTACAAGTAATATTTACATTTTTGCCATGCTAA, and the Vvl/Su(H) double site mutant is TTACAAGTAATAGGGACCGTTTTGCCATGCTAA. Wild-type Probe 2 consists of the sequence GCAGTCGACCATTTACATATTTACGTTTAC; the Vvl site mutant version is GCAGTCGACCAGGGACCGATTTACGTTTAC.
1.7 Reporter transgene constructs
Various regions of the Sox15 locus (see Fig. 2A) were amplified by PCR from genomic DNA using primers with 5′ restriction site sequences added; these were cloned into either the pH-Stinger (nuclear eGFP) or pRed H-Stinger (nuclear DsRed) vectors (Barolo et al., 2000; Barolo et al., 2004). The 7.5-kb enhancer fragment was amplified using the primer sequences GCCGGCGCGCCGATAGCCACCGTGCTCCGGATAATCGCTGC (Asc I site end) and TCCCCGCGGCATATGATCACGAACATCCACATCATCTGC (Sac II site end); the 1.3-kb enhancer fragment was amplified with the primer sequences TAACTCGAGCATATGATCACGAACATCCACATCATCTGC (Xho I site end) and TAAGCATGCTATATGCATTTCCAATCCAGCTTAGTCACG (Sph I site end). Other primer sequences are available upon request. The mutations described above under “DNA-binding assays” were introduced into the 1.3-kb enhancer fragment by overlap extension PCR mutagenesis (Ho et al., 1989). The wild-type version of the 1.3-kb fragment was amplified from the D. melanogaster iso-1 genome sequencing strain (Brizuela et al., 1994); both wild-type and mutant PCR products were sequenced and the results compared to the genome sequence in GenBank by BLAST search (Altschul et al., 1997) to detect unwanted point mutations. Reporter constructs were introduced into the germline of w1118 embryos using standard procedures (Rubin and Spradling, 1982).
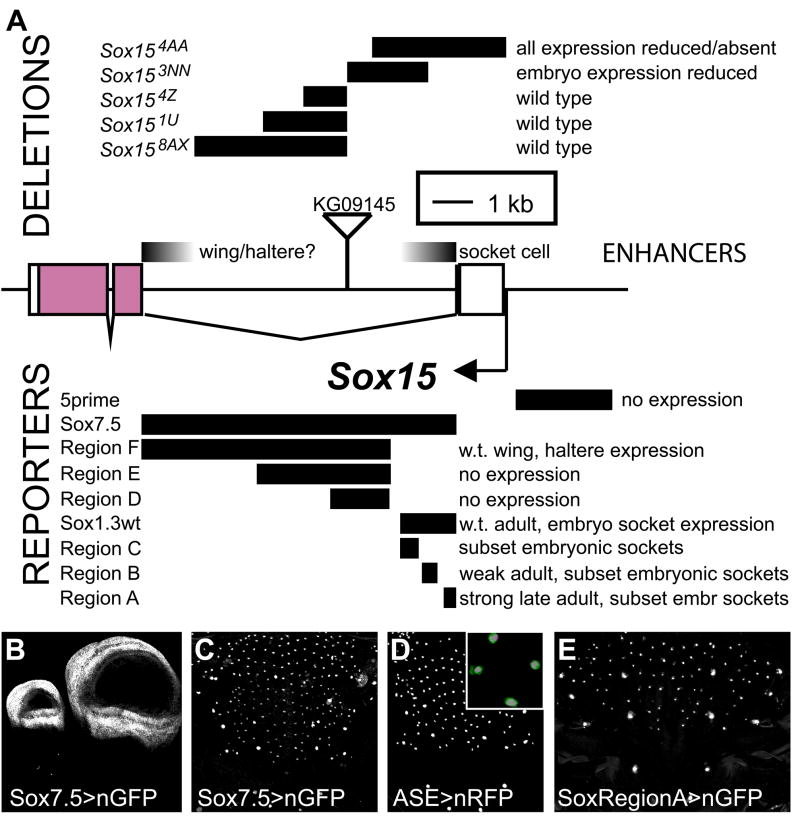
Fig. 2.
Identification of the Sox15 socket enhancer. (A) Schematic of the Sox15 locus. Shown are the intron-exon structure, the location of the KG09145 P-element transposon insertion, the extents and designations of the deletion mutations created by imprecise excision of the KG09145 P element, the mutant phenotypes of the deletion alleles with respect to Sox15 mRNA accumulation, the DNA fragments tested for enhancer activity in vivo, the nGFP expression pattern directed by each fragment, if any, and the deduced locations of the socket cell and wing/haltere enhancers. Note that a 1.3-kb intron fragment immediately adjacent to the first exon (Sox1.3) drives socket cell-specific reporter expression (see Fig. 4A). (B) Haltere (left) and wing (right) imaginal discs from late third-instar larvae, showing nGFP expression directed by the 7.5-kb enhancer fragment (Sox7.5; A); compare with Fig. 5C and 5B, respectively; see also Fig. 1M,N. (C) Pupal notum at 30 hours APF, also showing nGFP expression driven by Sox7.5. (D) 30-hour-APF notum showing nRFP expression driven by the Su(H) ASE. Inset is a high-magnification image of a 30-hour-APF notum from a fly carrying one copy each of Sox7.5>nGFP (green) and ASE>nRFP (magenta), demonstrating activity of both enhancers in the same nuclei. (E) Pharate adult notum showing nGFP expression driven by Region A (see A), a 380-bp fragment sufficient to direct robust reporter activity in socket cells by the time of eclosion.
1.8 Preparation of adult tissues
Wing and hinge tissue was removed from flies under CO2 anesthesia and placed directly onto slides. Permount (Fisher) was then applied to coverslips and these were immediately placed on the tissue. For preparation of notum cuticle, adult flies were dehydrated overnight in methanol and then in ethanol. Flies were then carefully dissected in ethanol and dorsal nota were transferred into fresh ethanol. Ethanol was removed, and 1:2 CMC-10 mounting media (Masters Company, Wood Dale, IL) : lactic acid was added; tissue in this mixture was incubated, covered, overnight at 65°C. Nota in suspension were removed with a pipette and transferred to slides.
1.9 In situ hybridization
A digoxygenin-labeled antisense RNA probe (Tautz and Pfeifle, 1989) to detect Sox15 transcript in situ was made by transcribing with T7 RNA polymerase a linear Sox15 pGEM-T (Promega) cDNA clone containing a Sox15 PCR product from embryonic first-strand cDNA. Su(H) digoxygenin- and biotin-labeled antisense RNA probes (O’Neill and Bier, 1994) were generated from a HindIII-linearized Su(H) cDNA clone in pNB40 (Brown and Kafatos, 1988), transcribed with T7 RNA polymerase. A sv digoxygenin-labeled antisense RNA intron probe was constructed from genomic DNA PCR products covering 3.0 kb from intron 1 and 5.4 kb from intron 4 cloned into pGEM-T, linearized, and transcribed with T7 RNA polymerase. Fluorescent in situ hybridizations to embryos were performed as described (Kosman et al., 2004), using a biotin-labeled Su(H) probe and a digoxygenin-labeled Sox15 probe. Imaginal disc, pupal notum, and adult abdomen in situ hybridizations were performed as described (O’Neill and Bier, 1994; Lai et al., 2000; Reeves and Posakony, 2005).
1.10 Antibody production
The Sox15 protein coding sequence was amplified from embryo first-strand cDNA and cloned into pGEX-5X-2. The optimum conditions for purification were determined as described (Mercado-Pimentel et al., 2002). Since these consistently yielded some non-specific bands, purified protein was electrophoresed on a 10 cm X 10 cm SDS-PAGE gel and the location of the majority of the protein was determined by Coomassie staining of a thin lengthwise slice. A slice was then cut from the gel at this latitude and sent to Pocono Rabbit Farm and Laboratory for immunization of two guinea pigs. Upon receipt, antisera were preabsorbed against fixed embryos and tested for the anticipated staining pattern.
1.11 Immunohistochemistry
White pre-pupae were collected and aged to the appropriate developmental time at 25°C. Late third-instar larvae or aged pupae were dissected in 1X PBS, fixed for 30 minutes in 1X PBS with 0.3% Triton X-100, and washed 5X for 10 minutes each in 1X PBS with 0.1% Triton X-100 (PBT). Tissue was then incubated with primary antibody overnight at 4°C, followed by five washes in PBT, a 1-hour incubation with fluorescent secondary antibody, and five further PBT washes. After the last PBT wash, samples were suspended in 2.5% w/v DABCO, 50 mM Tris-HCl pH 8.0, 90% glycerol (Kosman et al., 2004) for fluorescence microscopy, or in 80% glycerol, 100 mM Tris-HCl pH 8.0 for light microscopy. Any tissue containing fluorescent reporters was kept in the dark from the point of fixation onward as much as possible.
Embryos were fixed and prepared for antibody staining as previously described (Kosman et al., 2004; Reeves and Posakony, 2005). They were then brought into 1X PBT and stained as described above for larval and pupal tissues.
Primary antibodies included mouse anti-Cut hybridoma supernatant (Developmental Studies Hybridoma Bank, University of Iowa), diluted 1:500; mouse anti-Prospero hybridoma supernatant (DSHB), diluted 1:20; rat anti-Elav hybridoma supernatant (DSHB), diluted 1:200; mouse anti-Wg hybridoma supernatant (DSHB), diluted 1:200; and rabbit anti-Su(H) (Santa Cruz Biotechnology), diluted 1:1000. Guinea pig anti-Sox15 antiserum was preabsorbed against embryos and used at either a 1:500 or 1:1000 dilution. All secondary antibodies were used at a 1:1000 dilution and included anti-mouse-HRP conjugate (Jackson Laboratories), anti-rat-Alexa647 conjugate, and anti-mouse-Alexa555 conjugate (Molecular Probes). HRP was detected with DAB as described (Goldstein and Fyrberg, 1994).
1.12 Microscopy
Fluorescence microscopy was performed on a Leica TCS SP2 confocal microscope equipped with Leica Confocal Software v2.5 (Leica Microsystems). Figure panels are composed of maximum projections of stacks taken along the apical-basal axis at 2 μm increments. Fluorophores were excited separately at 488 nm (GFP), 543 nm (RFP, Alexa 555), or 633 nm (Alexa 647).
Samples for scanning electron microscopy were collected and dehydrated overnight in isoamyl acetate as described (Bang et al., 1991). Imaging was performed at the Scripps Institution of Oceanography Unified Laboratory Facility on an FEI Quanta 600 instrument.
1.13 Gene diagrams
Gene diagrams in Figs. 2–4 and Fig. S4 were created using GenePalette (Rebeiz and Posakony, 2004) and edited in Adobe Illustrator.
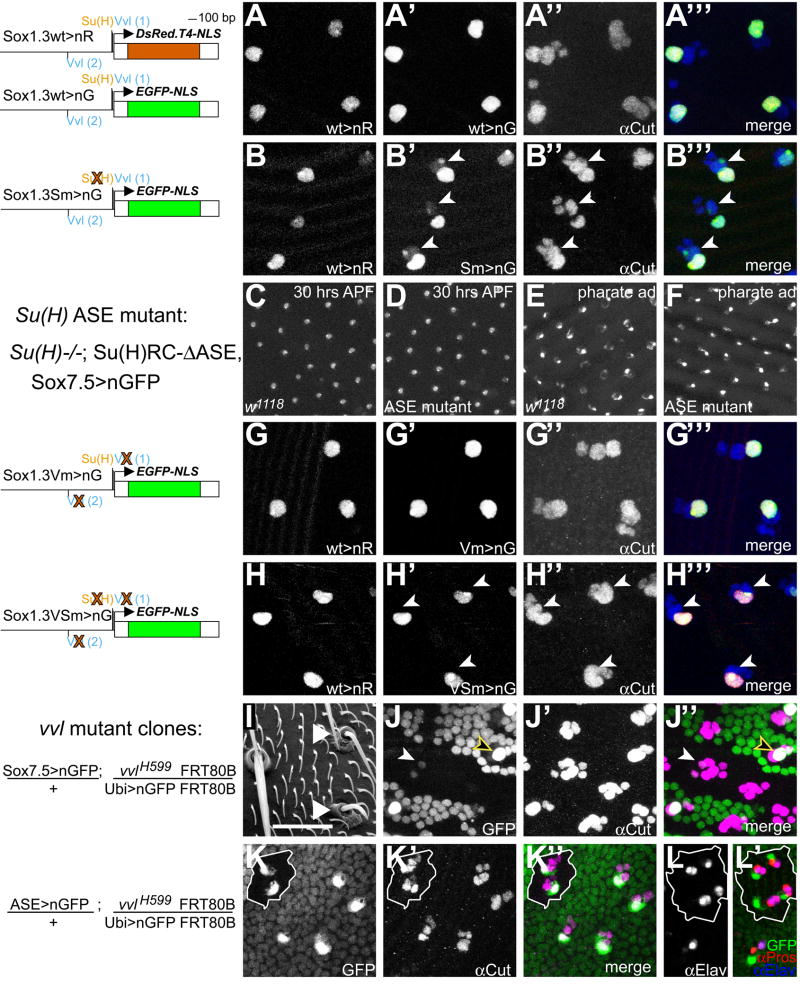
Fig. 4.
Loss of Su(H) and Vvl binding in cis and in trans affects Sox15 socket enhancer activity. (A-A‴) 30-hour-APF notum from a fly bearing one copy each of Sox1.3wt>nRFP (wild-type enhancer) and Sox1.3wt>nGFP (wild-type enhancer), showing the nRFP signal (A, red in A‴), the nGFP signal (A′, green in A‴), and Cut immunoreactivity (A″, blue in A‴). (B-B‴) 30-hour-APF notum from a fly bearing one copy each of Sox1.3wt>nRFP and Sox1.3Sm>nGFP [Su(H) site mutant enhancer], with the different channels displayed as in A-A‴. Arrowheads in B′-B‴ indicate positions of GFP-positive shaft cell nuclei. (C–F) nGFP expression directed by the Sox7.5 enhancer fragment in either a wild-type (w1118; C,E) or Su(H) ASE mutant background [Su(H)AR9/Su(H)SF8; Su(H)RC-ASE; D,F; genotype shown at left], at either 30 hours APF (C,D) or pharate adult (E,F). (G-G‴) 30-hour-APF notum from a fly bearing one copy each of Sox1.3wt>nRFP and Sox1.3Vm>nGFP (Vvl site mutant enhancer), with the different channels displayed as in A-A‴. (H-H‴) 30-hour-APF notum from a fly bearing one copy each of Sox1.3wt>nRFP and Sox1.3VSm>nGFP [Vvl site-Su(H) site double mutant enhancer], with the different channels displayed as in A-A‴. Arrowheads in H′-H‴ indicate positions of GFP-negative shaft cell nuclei (compare with B-B‴). (I) Scanning electron micrograph of notum region of a fly bearing vvl−/− clones. Arrowheads indicate mutant bristles. (J-J″) 36-hour-APF notum from a fly bearing one copy of Sox7.5>nGFP and vvl−/− clones (genotype shown at left; mutant tissue marked by the absence of low-level ubiquitous nGFP), showing the nGFP signal from the reporter gene (J, green in J″) and Cut immunoreactivity (J′, magenta in J″). Filled arrowhead in J and J″ indicates Sox7.5>nGFP expression within the vvl−/− mutant territory; yellow-edged open arrowhead in the same panels indicates expression of the reporter in adjacent vvl+/− tissue for comparison. (K–L′) 30-hour-APF nota from flies bearing one copy of ASE>nGFP and vvl−/− clones (genotype shown at left; mutant tissue marked as in J and J″ and outlined in white). Compare level of ASE>nGFP expression (K, green in K″ and L′) within the vvl−/− mutant territory with that in adjacent vvl+/− tissue; sensory organ cells marked by Cut immunoreactivity (K′, magenta in K″). (L-L′) vvl−/− tissue at 30 hours APF shows two cells positive for the neuron marker Elav in most positions (L, blue in L′), and three cells positive for Pros immunoreactivity (red in L′), of which two are the Elav-positive cells.
1.13.1 Results
1. 14 Sox15 is expressed specifically in the socket cell of external sensory organs
A previous survey of the expression patterns of Sox family genes in Drosophila revealed that transcripts from Sox15 accumulate specifically in the developing peripheral nervous system (PNS) in the embryo, and it was suggested that this expression might be in the socket cells of external sensory organs (Cremazy et al., 2001) (Fig. 1A,B). We thus sought to define the cell-type specificity of Sox15 expression in both the larval and adult PNSs. In the embryo, we detect Sox15 transcript accumulation specifically in the cells that exhibit high levels of Su(H) transcript (Schweisguth and Posakony, 1992), indicating that Sox15 is indeed expressed in socket cells of the larval PNS (Fig. 1C–E). The cell-type specificity of Sox15 expression in the pupal notum at 30 hours APF was investigated by observing its response to experimentally induced cell fate changes (see Supplementary Fig. S1). This analysis shows explicitly that Sox15 expression in the sensory organ lineage is triggered by activation of the N pathway and specification of the socket cell fate. Despite their co-expression in socket cells, we find that the temporal patterns of Su(H) and Sox15 mRNA accumulation in the pupal notum differ markedly, in that high-level expression of Sox15 is considerably delayed relative to that of Su(H) (see Supplementary Fig. S2).
We complemented this analysis of the pattern of Sox15 transcript accumulation with a parallel examination of Sox15 protein expression. Immunofluorescence analysis utilizing a polyclonal antiserum raised against recombinant GST-Sox15 protein revealed expression specifically in the nuclei of socket cells in both the larval and adult PNSs, as indicated by co-localization with Su(H) immunoreactivity (Fig. 1F–K). Our antibody also revealed the unique pattern of Sox15 protein accumulation in the imaginal discs of late third-instar larvae (Fig. 1M). In wing and haltere discs, Sox15 localizes to the presumptive hinge region, while in leg discs the protein is found in discrete patches on opposite sides of the proximal leg primordium. These patterns of Sox15 protein expression in imaginal discs closely mimic the corresponding patterns of Sox15 transcript accumulation (see Fig. 5A–C) (Cremazy et al., 2001). We note that Sox15 protein accumulation in the wing hinge primordium abuts the more distal zone of Wingless (Wg) protein expression (Fig. 1L–N), which may be suggestive of an important regulatory relationship.
Fig. 5.
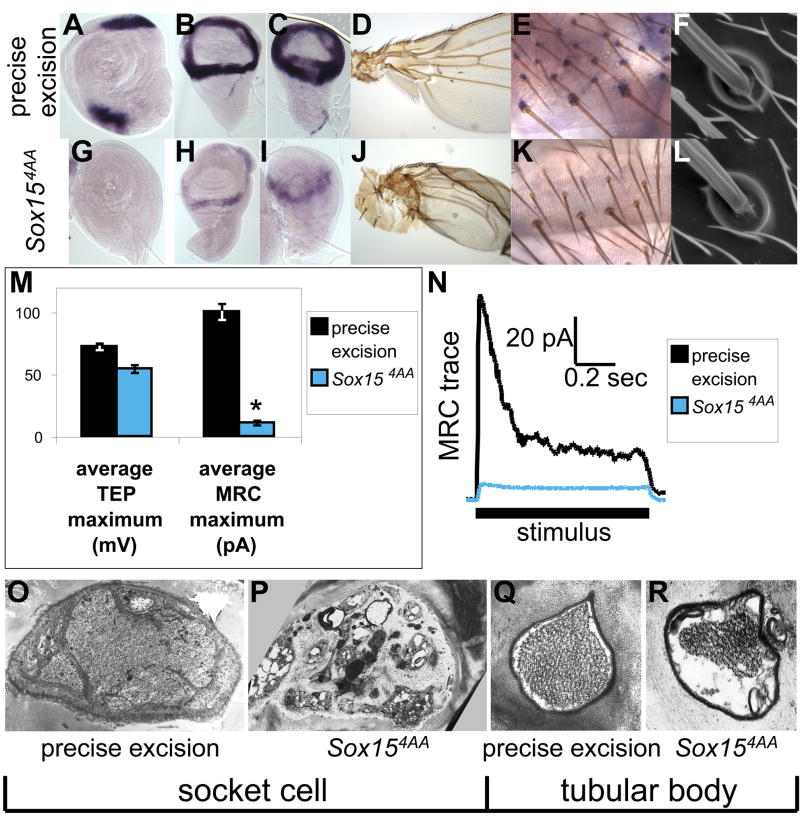
Sox15 loss-of-function phenotypes. (A–F,O,Q) Tissue from wild-type (homozygous precise excision) flies. (G–L,P,R) Tissue from Sox154AA homozygous flies. (A–C,G–I) Pattern of Sox15 mRNA accumulation in imaginal discs of late third-instar larvae; see also (Cremazy et al., 2001). (A,G) Leg discs. (B,H) Wing discs. (C,I) Haltere discs. (D,J) Proximal wing and body wall. (E,K) Sox15 mRNA accumulation in socket cells of mechanosensory bristles on the pharate adult abdomen. (F,L) Scanning electron micrographs of external cuticular structures of representative adult mechanosensory bristles. (M) Average transepithelial potential (TEP) and mechanoreceptor current (MRC) recorded from precise excision (black) and Sox154AA (blue) adult flies. Asterisk indicates statistically significant difference from the precise excision value (p<0.001). (N) Representative MRC traces recorded from the same genotypes. (O–R) Transmission electron micrograph sections through the adult socket cell (O,P) and through the tubular body of the neuronal dendrite (Q,R).
1. 15 Identification and analysis of the Sox15 socket enhancer
To begin to define how Sox15 expression in the PNS is regulated at the transcriptional level, we sought to identify the Sox15 socket cell enhancer module. We took two approaches to this goal: generation of deletion mutations within the Sox15 locus, and analysis of reporter gene expression driven by non-coding genomic DNA fragments from the gene.
The first effort was aided by the availability of the KG09145 line from the BDGP gene disruption collection (Roseman et al., 1995; Bellen et al., 2004), which contains a P-element insertion within the 7.5-kb first intron of Sox15 (Fig. 2A). Five new mutant alleles of Sox15 were created through imprecise excision of this element, resulting in a series of deletions internal to the Sox15 locus (Fig. 2A). Only two of these alleles, Sox154AA and Sox153NN, display altered accumulation of Sox15 mRNA. Sox154AA is a deletion that removes part of the intron proximal to the first exon, as well as the first exon itself (−21 to +3078), while the Sox153NN deletion endpoints (+1812 to +3645) are contained entirely within the intron (which extends from +1124 to +8360). Sox154AA homozygotes have lost expression of the gene in leg imaginal discs as well as in the socket cell, and expression in wing and haltere discs, while not eliminated, is dramatically reduced (see Fig. 5G–I,K). This phenotype, which affects multiple tissues, is likely due in part to the deletion of the transcription Initiator sequence of Sox15 (Juven-Gershon et al., 2008), but it could also be the result of deleting one or more cis-regulatory modules (see below). Sox153NN homozygous embryos have lost most socket cell expression, while adult socket expression appears normal (see Supplementary Fig. S3), suggesting that the embryonic and adult tormogen regulatory elements are at least to some degree distinct.
We next tested the capacity of a number of genomic DNA fragments from the Sox15 locus to direct GFP reporter gene expression in the socket cell. A fragment comprising the entire 7.5-kb intron (Sox7.5; Fig. 2A) drives reporter expression in late third-instar wing and haltere imaginal discs (Fig. 2B), in a pattern recapitulating that of transcript accumulation from the endogenous gene (see Fig. 5B,C). This fragment also directs reporter activity in single cells in both the embryonic and adult PNSs; these are confirmed to be socket cells by the co-expression of the socket-specific Su(H) reporter ASE>nRFP (Fig. 2C,D). A shorter fragment containing the intronic 1.3 kb proximal to exon 1 (Sox1.3wt; Fig. 2A) is likewise sufficient to recapitulate this full socket cell expression pattern, while even smaller subfragments (Regions A–C, Fig. 2A) are capable of directing expression in only subsets of embryonic and adult socket cells (Fig. 2E). The 5.5 kb of the first intron distal to exon 1 (Region F, Fig. 2A) directs reporter gene expression only in wing and haltere discs (data not shown). Sox158AX, the imprecise excision allele bearing the largest deletion within the intron, has its distal endpoint 1.2 kb from exon 2 (Fig. 2A), and flies homozygous for this allele show no obvious defect in endogenous Sox15 mRNA accumulation (see Fig. S3; data not shown). Thus, the combined evidence from deletion mutations of Sox15 and from enhancer fragment activity localizes the wing/haltere and socket cell regulatory sequences to opposite ends of the gene’s large first intron, with the former presumed to be in the 1.2 kb distal and the latter in the 1.3 kb proximal to exon 1 (Fig. 2A).
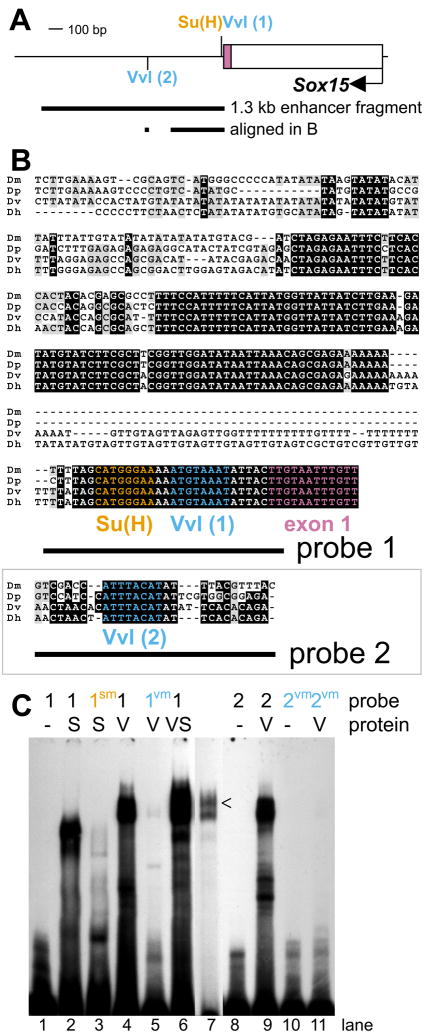
Since the 1.3-kb proximal enhancer region is the smallest fragment tested capable of recapitulating the full socket cell expression dynamics of Sox15 mRNA, we examined this region (Fig. 3A) for conserved sequence elements that might function as binding sites for important regulatory factors. This analysis revealed two occurrences of the sequence ATGTAAAT (Fig. 3A,B), which is a single-base-pair mismatch to the strong binding site ATGCAAAT for the POU-domain transcription factor Vvl (Certel et al., 1996; Ma et al., 2000). In the adult mechanosensory bristle lineage, Vvl is detectable in all cells from the SOP to the post-mitotic cell types, at least initially (Inbal et al., 2003). By 42 hours APF, however, expression persists only in the socket cell, and mosaic analysis has revealed differentiation defects in both the socket and shaft cuticular structures in vvl mutant sensory organs (see Fig. 4I) (Inbal et al., 2003). We find that a purified GST-Vvl fusion protein binds specifically in vitro to each of the Vvl motifs in the Sox15 socket enhancer (Fig. 3C). Six bp distal to Vvl(1), the more proximal of these sites, is the conserved sequence CATGGGAA (Fig. 3A,B), previously shown to be bound strongly by Su(H) in vitro (Nellesen et al., 1999). Indeed we find that this sequence in the Sox15 enhancer is bound specifically by purified His-tagged Su(H) (Fig. 3C). With both Vvl and Su(H) as strong candidates for key regulatory factors, we next investigated their possible roles in the operation of the Sox15 socket cell module.
Fig. 3.
Analysis of conserved sequence motifs in the Sox15 socket enhancer reveals Su(H) and Vvl as potential regulatory inputs. (A) Schematic of the 1.3-kb socket enhancer fragment in relation to the first exon of Sox15, indicating the positions of two Vvl and one Su(H) binding motifs. The sequences aligned in B are also indicated. (B) Alignment of the region of high sequence conservation among D. melanogaster (Dm), D. pseudoobscura (Dp), D. virilis (Dv), and D. hydei (Dh) with the Su(H) site (orange) and Vvl site 1 (blue) highlighted. Also shown is an alignment of the region surrounding Vvl site 2 (blue) from the same species. Sequences included in oligonucleotide probes used in gel shift assays (see C) are indicated. (C) Electrophoretic mobility shift assays showing the ability of both His-tagged Su(H) (S) and a GST-Vvl fusion protein (V) to bind radiolabelled oligonucleotides from the D. melanogaster sequence (S: lanes 2,6,7; V: lanes 4,6,7,9), while failing to bind mutant oligonucleotides (S: lane 3; V: lanes 5,11). Lane 7 is a shorter exposure of lane 6, to indicate the slower mobility band (caret) seen when both His-Su(H) and GST-Vvl are allowed to bind probe 1.
1. 16 Sox15 is a direct target of the N pathway in the socket cell
Interfering with Su(H) regulation of N target genes often results in two concurrent defects in gene expression (Barolo and Posakony, 2002). One effect is loss of gene activation in the N signal-receiving cell due to loss of the Su(H)/NICD/Mam activation complex at the enhancer (Bailey and Posakony, 1995; Lecourtois and Schweisguth, 1995). The second phenotype is de-repression of N target genes in the N signal-sending cell because of the inability of the Su(H)/H/Gro/CtBP repression complex to be recruited to the enhancer (Barolo et al., 2000; Morel and Schweisguth, 2000; Castro et al., 2005; Koelzer and Klein, 2006). Su(H) binding in vitro and in vivo can be abolished by mutating the YRTGDGAA motif to YRTGDCAA (Fig. 3C, lane 3) (Bailey and Posakony, 1995). When applied to the 1.3-kb socket enhancer fragment, this single-base-pair mutation results in weak ectopic activation of reporter gene expression in a neighboring large Cut-expressing nucleus, that of the shaft cell, by 30 hours APF (Fig. 4A,B). Such de-repression in the N signal-sending shaft cell indicates a role for default repression of Sox15 in this cell by Su(H) (Barolo and Posakony, 2002). While we can detect ectopic activation of the Sox15 socket enhancer in the shaft cell when its Su(H) site is mutated, we fail to detect any effect on activation of the reporter gene throughout the life of the socket cell (Fig. 4B and data not shown). Moreover, when the socket cell lacks the function of the Su(H) ASE, there is no effect on either wild-type 7.5-kb reporter gene expression (Fig. 4C–F) or accumulation of endogenous Sox15 transcript in this cell (data not shown). Collectively, these data indicate that the principal direct effect of normal N signaling on Sox15 socket enhancer activity is the relief of default repression in the socket cell. This allows activators other than Su(H), one or more of which would be present in both the socket and shaft cells, to initiate transcription of Sox15 only in the appropriate cell.
1. 17 Vvl activates Sox15 enhancer activity in both the socket and shaft cells
The presence of Vvl binding sites in the 1.3-kb Sox15 socket cell enhancer, combined with the Vvl expression pattern and the phenotype of vvl mutant bristles (Inbal et al., 2003) (see Fig. 4I), suggested that Vvl might function as one of the activators of the enhancer. Mutating the two identified Vvl sites (see Fig. 3A,B) in a manner that abolishes binding in vitro (see Fig. 3C), however, does not result in loss of reporter gene expression in the socket cell (Fig. 4G). When these sites are mutated in combination with the Su(H) site mutation, though, we find that ectopic reporter expression in the shaft cell is eliminated (Fig. 4H), suggesting a role for Vvl as an activator in the shaft cell at a minimum. The lack of an effect on reporter activity in the socket cell could indicate that Vvl is not required for activation in this cell, that Vvl could be working through additional sequence motifs in the socket enhancer region, or that Vvl functions, at least in part, indirectly in the activation of the socket module. To help distinguish among these possibilities, we examined the expression of a wild-type reporter gene in vvl mutant clones. First, however, we further investigated the effects of the loss of vvl function on the sensory organ lineage (Inbal et al., 2003).
vvl mutant bristles on the adult notum have smaller, deformed shafts and disorganized socket structures (Fig. 4I). At 42 hours APF, supernumerary cells expressing Cut protein are detected at these positions, suggesting that one or more cells in the lineage may undergo inappropriate divisions (Inbal et al., 2003). At 30 hours APF, we find that most vvl mutant bristle positions display five Cut-positive nuclei (Fig. 4K,L). To determine which cells might be dividing inappropriately, we stained wild-type and mutant tissue with anti-Prospero (Pros), which identifies the sheath cell, and anti-Elav, which labels the neuron. Wild-type positions contain one Pros-positive, Elav-negative cell (sheath) and one Elav-positive, Pros-negative cell (neuron). vvl mutant positions, however, often have three cells expressing Pros, and in most of these positions two of the Pros-positive cells also express Elav (Fig. 4L). By contrast, loss of vvl function appears not to affect expression of ASE>nGFP, as the GFP level and pattern are indistinguishable from that in wild-type territories (Fig. 4K,L), and reporter activity is detected in a single large Cut-positive nucleus at 30 hours APF (Fig. 4K). The normal expression of ASE>nGFP in vvl mutant territory indicates that vvl function is not required for the high levels of Su(H) expression in the socket cell, and suggests that loss of vvl activity does not affect specification of the socket fate, as ASE>nGFP is one of the earliest and most specific markers of this event.
The smaller size of the socket and shaft structures in adult external sensory organs within vvl mutant clones suggests that the external cells divide inappropriately; we confirm this by detecting two nuclei expressing ASE>nGFP in vvl clones around the time of eclosion (data not shown). By 36 hours APF, some vvl mutant microchaete positions contain up to eight Cut-positive nuclei, implying the occurrence of additional divisions of one or more normally post-mitotic cells in the bristle lineage. The observation that in these positions none of the nuclei are as large as either the wild-type socket cell or shaft cell nuclei may indicate that indeed the external cells are dividing at this time, and/or that they have failed to undergo the normal rounds of endoreplication.
It is only at 36 hours APF, 19 hours after it comes on in the wild-type positions outside of vvl clone boundaries, that we can first detect any expression of the Sox7.5>nGFP reporter in vvl mutant territory. Figure 4J shows a mutant position at which weak activity of the Sox7.5>nGFP reporter is observed in two small nuclei; compare reporter gene expression in wild-type (open arrowhead) and mutant (filled arrowhead) cells. The lack of an expression delay in the case of the ASE>nGFP reporter suggests that Vvl is required for proper regulation of Sox15 expression. However, since the Sox15 reporter gene still becomes expressed in the socket cell, albeit with a long delay, Vvl is likely not the only factor contributing to the activation of Sox15 in this cell.
1. 18 Sox15 activity in the socket cell is required for normal mechano sensory organ function
Because of the specificity and timing of its expression in the socket cell (see Fig. 1 and Supplementary Figs. S1 and S2), Sox15 is well positioned as a potential regulator of differentiative gene expression subsequent to N signaling-dependent cell fate specification. The imprecise excision allele Sox154AA proved to be a crucial reagent for analyzing the function of Sox15 in the socket cell. Most of the endogenous expression pattern is affected in Sox154AA homozygous flies; no transcript is detected in late third-instar leg imaginal discs and adult socket cells, and only weak expression is detected in wing and haltere discs (Fig. 5; compare A,B,C,E with G,H,I,K, respectively). The reduction of expression in these latter tissues results in a strong mutant phenotype in the adult wing and haltere, mainly affecting the formation of the hinge region (Fig. 5D,J). Such a phenotype is observed in a mis-expression mutant of another Sox gene, Dichaete, which Russell has proposed is possibly due to a dominant-negative effect on another endogenous Sox gene (Russell, 2000). Our observations would indicate that Sox15 is that gene. The absence of leg disc expression in Sox154AA homozygotes does not cause an obvious physical deformity, but results in reduced movement at the coxa-trochanter joint, preventing extension of the femur and interfering with the ability to walk (data not shown). The Sox154AA deletion allele, in addition to removing the Initiator sequence at the gene’s transcription start site, also removes the first exon and the proximal first intron sequence that contains the socket cell regulatory information, as indicated above. For these reasons Sox154AA homozygotes fail to accumulate either Sox 15 transcript or Sox15 protein in the socket cells of developing notum bristles at 30 hours APF (data not shown); likewise, they lack detectable Sox15 transcript in the socket cells of abdominal bristles in pharate adults (Fig. 5E,K). Despite this lack of expression, the socket fate is properly specified, and by scanning electron microscopy we could detect no defect in the cuticular structures elaborated by the external cells (Fig. 5F,L). This result suggests that Sox15 may be required instead for proper functional differentiation of the socket and possibly other cells in the bristle organ. Indeed, we have reported earlier that Su(H) ASE mutants, while displaying apparently normal specification of the socket cell fate, exhibit dramatic reductions of both the trans-epithelial potential (TEP) and the mechanoreceptor current (MRC) of adult mechanosensory organs (Barolo et al., 2000; Walker et al., 2000). We find that Sox154AA flies display only a mild TEP phenotype but show a strong MRC defect (Fig. 5M,N). Interestingly, this particular type of TEP/MRC phenotype is most often observed with mutations affecting neuronal, as opposed to socket cell, function (Avidor-Reiss et al., 2004).
We further investigated the Sox154AA electrophysiological defect by looking for abnormalities in socket cell and/or neuronal internal structure using transmission electron microscopy. Consistent with the physiological phenotype, we find a reduction in the quantity of microtubules in the tubular body of the ciliary dendrite of the neuron (Fig. 5Q,R), which could account for the MRC defect. In addition to this neuronal abnormality, the socket cell exhibits signs of cell death. The cell loses its characteristic apical membrane folds, and the cytoplasm contains multiple large vesicles lacking electron density (Fig. 5O,P). Indeed, the results of studies presented in the Supplementary Material (see Fig. S4) suggest that the Sox15 mutant socket cell undergoes necrosis (not apoptosis).
1. 19 Sox15 and Su(H) function together to repress sv expression and the shaft differentiation program in the socket cell
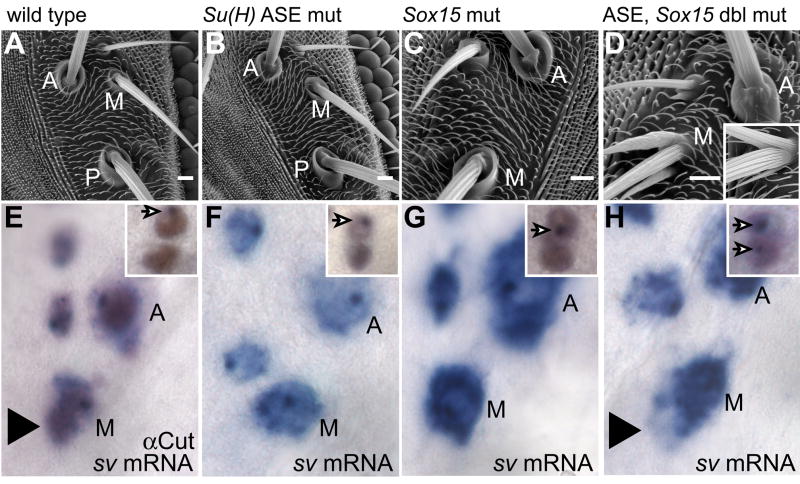
Data presented above and previously (Barolo et al., 2000) support the conclusion that both Su(H) ASE function and Sox15 play critical roles in the differentiation, but not the fate specification, of the socket cell. Loss of either of these activities causes severe defects in the sensory organ’s electrophysiological function. Given the similarities in mutant phenotype, we sought to examine the effect of loss of both Sox15 and Su(H) ASE activity on the socket cell (Fig. 6). We find that removing the function of both of these factors results in a more dramatic socket differentiation defect than loss of either alone. The majority of double-mutant bristle positions display a convex, rather than a concave, socket-shaft cuticular interface (Fig. 6A–D). Some of these bulbous socket structures extend one or more small cuticular projections, while others extend one or two distinctly shaft-like structures (Fig. 6D). This latter phenotype is the most severe, and is primarily restricted to the medial orbital macrochaetes. It is reminiscent of the effect of mis-expressing in the socket cell the Pax transcription factor Shaven (Sv, formerly D-Pax2), a high-level regulator of shaft cell differentiation (see inset in Fig. 6D) (Kavaler et al., 1999). Sv is expressed in the bristle lineage starting late in SOP development and persisting through the specification of each of the post-mitotic cell types. Once specified, however, the socket cell and neuron fail to maintain Sv expression, and by 32 hours APF it is undetectable in the socket (Kavaler et al., 1999). We thus sought to examine whether the Su(H) ASE-Sox15 double mutant phenotype we observe could result in part from the maintenance of sv transcription in the socket cell. Using RNA in situ hybridization probes generated from sv coding sequence, we often detect a cloud of transcript accumulation around the socket cell nuclei of orbital macrochaetes only in Su(H) ASE-Sox15 double mutants at 36 hours APF (Fig. 6E–H). We confirmed late socket cell transcription of sv in the microchaete field using RNA in situ hybridization probes generated from intron sequence to detect nascent transcript (insets in Fig. 6E–H). These data indicate that Su(H) and Sox15 act together early in socket cell differentiation to inhibit the maintenance of sv expression and hence to prevent inappropriate execution of the shaft differentiation program in this cell.
Fig. 6.
Socket cell phenotype of the Su(H) ASE-Sox15 double mutant. (A–D) Scanning electron micrographs of adult cuticle and (E–H) in situ hybridization detection of sv mRNA at 36 hours APF using a cDNA probe, highlighting orbital bristles of the following genotypes: (A,E) Wild type (w1118). (B,F) Su(H)AR9/Su(H)SF8; Su(H)RC-ASE. (C,G) Sox154AA. (D,H) Su(H)AR9 Sox154AA/Su(H)SF8 Sox154AA; Su(H)RC-ASE. Inset in D shows bristle organ of a fly in which sv is misexpressed specifically in the socket cell (genotype ASE-GAL4/+; UAS-sv/+). A: anterior orbital; M: middle orbital; P: posterior orbital. Large arrowhead in E and H points to the position of the socket cell as indicated by Cut immunoreactivity shown in E. Insets in E–H are thoracic microchaete socket-shaft pairs stained with anti-Cut (brown) and a sv intron in situ hybridization probe to detect nascent transcript (purple nuclear dots, arrows).
1.19.1 Discussion
1.20 Distinct transcriptional regulation of Sox15 and the Su(H) ASE in the socket cell
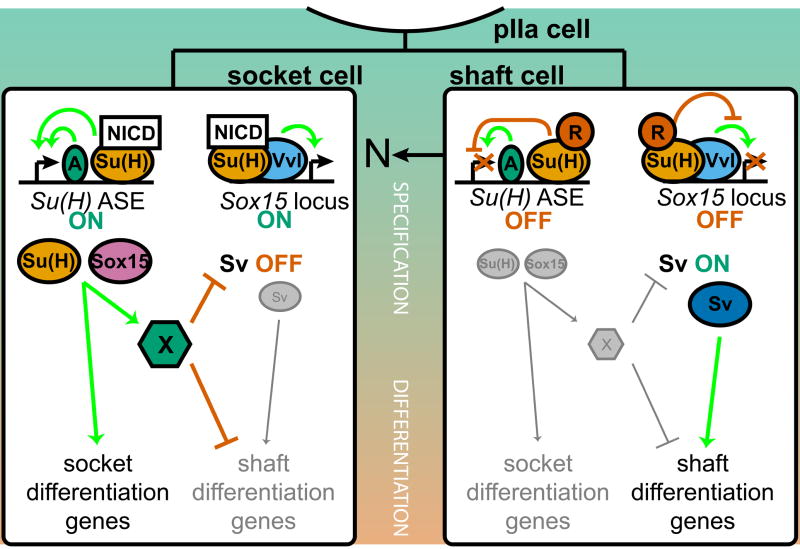
After Su(H) (Barolo et al., 2000), Sox15 is the second transcription factor gene known to be activated specifically in the postmitotic socket cell of the Drosophila external sensory organ lineage. Three observations reported here indicate that although both genes come to be expressed at high levels in this cell, the underlying regulatory logic may be quite different (Fig. 7).
Fig. 7.
Summary model of the collaborative roles of Su(H) and Sox15 as N pathway targets required for socket cell differentiation. In the N signal-resistant shaft cell (right), Su(H) in its default repression mode keeps both the Su(H) ASE and the Sox15 socket enhancer off (in the latter case, despite the presence of Vvl). The result is that the Pax family factor Sv accumulates to high levels in the shaft cell and directs its differentiation program. Successful activation of the N pathway in the socket cell (left) relieves Su(H)-mediated repression of the two enhancers in this cell. The initially low basal level of Su(H), now complexed with NICD and Mam, contributes critically to the rapid (auto)activation of the Su(H) ASE, but acts permissively on the Sox15 enhancer, allowing Vvl and other factors to activate Sox15 gradually. Su(H) and Sox15 then function collaboratively (not hierarchically) to activate target genes required primarily for the later (physiological) phase of socket cell differentiation. They also collaborate to shut off continued transcription of sv, probably indirectly through the action of a hypothetical repressor X. This inactivates the shaft differentiation program in the socket cell.
The first is the distinct dynamics of ASE-stimulated Su(H) transcription versus Sox15 expression. Su(H) is immediately activated at high levels following the specification of the socket cell, due at least in part to the establishment of an autoregulatory loop working through the ASE. Sox15 expression, however, exhibits a significant delay between socket cell specification and the time peak levels of transcript accumulation are achieved.
The second observation concerns the role played by Vvl in the activation of the Sox15 socket enhancer and the Su(H) ASE. Conserved within the ASE lies a motif, CATAAAT, that might act as a weak Vvl binding site (not shown) (Certel et al., 1996), suggesting the possibility that Vvl could play a part in the high-level activation of Su(H) in the socket cell. However, this appears not to be the case, since ASE-GFP is activated within the same temporal window, and just as strongly, in vvl mutant clones as in neighboring wild-type tissue. By contrast, while Sox7.5>GFP is also activated in vvl mutant sensory organs, there is a substantial delay in this expression, which is often not detectable until the socket cell has begun to divide aberrantly. At this time, neighboring wild-type sensory organs are already strongly expressing Sox7.5>GFP. Vvl thus appears to be one factor present in the socket cell that is necessary for the full activation of Sox15, but not of Su(H).
Finally, there is the observed role of N-activated Su(H) in contributing to the transcriptional activation of the Sox15 socket enhancer versus the Su(H) ASE. A major difference between the two genes is made apparent by the contrasting effects on reporter gene expression of mutating the high-affinity Su(H) site(s) in their respective socket cell enhancers. In the case of the Su(H) ASE, mutation of the Su(H) sites causes a strong reduction in socket cell activity at early times, along with ectopic activity in the shaft cell; by the adult stage, the mutant enhancer is inactive (Barolo et al., 2000). Thus, N-activated Su(H) contributes critically to the transcriptional activation of the Su(H) ASE. The Su(H)-site-mutant Sox15 enhancer, on the other hand, shows no apparent diminution of its socket cell activity early (when it also drives ectopic expression in the shaft cell), and remains fully active in the pharate adult. In the case of Sox15, then, activation of Su(H) by the N signaling event appears to serve only the purpose of relieving Su(H)-mediated default repression; activation of the enhancer is evidently accomplished entirely through the action of other factors such as Vvl. This distinction in the role of N signaling in enhancer activation has been referred to as “Notch instructive” [Su(H) ASE] versus “Notch permissive” (Sox15 socket enhancer) (Bray and Furriols, 2001).
1.21 Functions of Sox15 and Su(H) in socket cell and sensory organ differentiation
Our investigation of the loss-of-function phenotype of Sox15 has revealed that, like Su(H), it has an important role in controlling the socket cell differentiation program (Fig. 7). Comparison of the phenotypic effects of losing Sox15 function, Su(H) function, or both, suggests an incomplete overlap in the target gene batteries regulated by the two factors. Loss of either Sox15 or Su(H) ASE activity causes a serious defect in mechanosensory organ function. The lack of Su(H) ASE activity confers the more severe phenotype, including significant reductions of both TEP and MRC. The TEP defect signifies an inability of the socket cell to establish the receptor lymph cavity itself, the proper ionic composition of the receptor lymph, or a combination of the two. The genes required for these events have yet to be identified, but it is likely that Su(H) plays a role in regulating their expression in the socket cell. Sox15, on the other hand, does not appear to share this role, based on the apparent lack of a major TEP defect in Sox15 mutants. Instead, Sox15 appears to regulate targets that contribute to socket cell viability. Without these target factors, the cell eventually becomes necrotic. In addition, the principal physiological phenotype of Sox15 mutants is the MRC defect, which is also conferred by loss of Su(H) ASE function. Loss of MRC is indicative of a failure in neuronal function, yet both Sox 15 and the Su(H) ASE are active specifically in the socket cell. This apparent paradox indicates an important role for the socket cell as a support cell for the mechanosensory neuron. To date three proteins — Sox15 (this paper), Su(H) (Barolo et al., 2000), and the cytochrome P450 Cyp303a1 (Willingham and Keil, 2004) — expressed in and required specifically for socket cell differentiation appear to contribute to neuronal function in mechanosensation. Given that the socket cell envelops the other cells of the sensory organ as it develops, the socket may be intimately involved in their normal differentiation and in the establishment of structural and functional connectivity between them. Defects in these processes could readily manifest themselves in an MRC phenotype. Thus, the abnormal microtubule bundling in the sensory dendrite in Sox15 mutants may very well be the result of a defect in the socket cell’s ability to contribute as it should to the neuron’s normal development. It is unclear at this point if the dendrite defect is due to a failure to activate Sox15-dependent target genes directly involved in the socket cell’s support function, or if it is an indirect consequence of the degeneration of the socket cell.
1.22 Inhibition of the sister cell differentiation program is one consequence of N - mediated cell fate specification in the bristle lineage
Previous studies have established that both daughters of the pIIa secondary precursor division are bipotent cells that can adopt either the shaft or socket cell fate (Bang and Posakony, 1992). Asymmetric N signaling specifies that the posterior daughter expresses only the signal-dependent socket fate and the anterior daughter only the signal-independent shaft fate. Correspondingly, our investigation of socket cell fate specification has largely focused on its positive aspects; i.e., those ways in which the N signaling event promotes the socket cell from the “default” (signal-independent) shaft fate to the alternative fate, triggering its execution of the distinctive socket differentiation program. We have shown here that socket cell-specific activation of Sox15 expression is an important component of this program. But the present study has also revealed the other side of the coin, by showing that the N signaling event also results in the activation of a mechanism for suppressing in the socket cell the capacity to execute the shaft differentiation program (Fig. 7). We have shown that this suppression mechanism involves the combined action of Sox15 and Su(H) in inhibiting transcription of the sv gene, which encodes a Pax transcription factor that is a high-level activator of the shaft differentiation program (Kavaler et al., 1999). Without this inhibition, the socket cell generates both socket and shaft cuticular structures. It is clear, then, that much of the network circuitry necessary for the execution of the shaft differentiation program remains intact in the socket cell even after its fate has been specified. Our results show that robust N-mediated cell fate specification in the mechanosensory bristle lineage involves not only promoting the signal-dependent fate, but also actively inhibiting the alternative program.
It is likely that at least Su(H)’s role in inhibiting sv expression in the socket cell is indirect, and occurs via an as yet unidentified repressor (Fig. 7). An attractive candidate for this factor X would be one or more basic helix-loop-helix (bHLH) repressors encoded in the Enhancer of split Complex [E(spl)-C]. Multiple E(spl)-C bHLH repressor genes are activated directly by Su(H) in response to N signaling in a variety of developmental contexts (Bailey and Posakony, 1995; Lecourtois and Schweisguth, 1995). Consistent with this possibility, we have observed (data not shown) that socket cell-specific overexpression of E(spl)m7-VP16, a form of the E(spl)m7 bHLH repressor that has been converted to a strong activator, phenocopies the ectopic-shaft effect of sv overexpression in the same cell (see Fig. 6D, inset).
1.23 Separable regulation of early and late phases of the socket cell differentiation program
The results of this and earlier studies (Barolo et al., 2000) afford us a glimpse of the regulatory architecture of the socket differentiation program, which is set in motion by the N signaling event that specifies the socket cell fate. It seems useful to distinguish two broad phases of this program, which no doubt overlap each other in time and are also very likely to share at least some components of the regulatory network. These two phases might be referred to as the earlier “morphogenetic” and the later “physiological” subdivisions of the socket program. The distinction is prompted by our observations of the phenotypes conferred by loss of the two socket cell-specific transcription factor activities identified so far, Su(H) and Sox15. In both cases, we find that many characteristic aspects of the socket’s cellular differentiation proceed completely normally, most notably the construction of the complex socket cuticular structure that surrounds the shaft structure (morphogenesis). By contrast, loss of Su(H) or Sox15 function in the socket cell results in major deficits in the electrophysiological capacity of the sensory organ (physiological differentiation). As described above, the specifics of these deficits differ for Su(H) versus Sox15 mutants, and include distinctive cell-autonomous defects in the socket cell and defects in other cells likely due to the failure of some aspects of the socket cell’s support function. But the phenotypic commonalities (emphasizing the physiological and not the morphogenetic) are striking nonetheless. It is perhaps reasonable to speculate that transcription factors like Su(H) and Sox15 that are activated for the first time in the sensory organ lineage specifically in the socket cell will tend to function primarily in the later physiological phase of the differentiative program. By contrast, we may expect that the earlier morphogenetic phase is controlled primarily by factors first expressed earlier in the lineage, at least in the pIIa precursor cell and perhaps in the SOP. Vvl exemplifies this notion: It is first expressed in the SOP, and loss of its activity causes visible defects in the socket cuticular structure, as well as aberrations in the mitotic status of the normally postmitotic socket cell. Investigation of the roles of additional transcriptional regulators in directing the socket differentiation program will test the viability of this broad conceptual framework.
Overall, our comparison of the roles of Sox15, Su(H), and Vvl in controlling aspects of the socket differentiation program indicates that they function largely in parallel, and collaboratively, rather than in a hierarchical fashion. This may suggest that the socket program will prove to be characterized by an ensemble of such parallel regulatory inputs that collectively direct the complex differentiation of the cell. It is perhaps useful to note that this picture contrasts already with what is known about the control of the shaft differentiation program, which is dominated by the function of Sv as a high-level regulator (Kavaler et al., 1999). Whether this reflects some important difference in how the differentiative programs of N-responsive versus N-non-responsive cell types are controlled will become clearer as we learn more about the gene regulatory network that underlies mechanosensory organ development.
Supplementary Material
Acknowledgments
We dedicate this paper to the memory of Danny L. Brower, undergraduate advisor, role model, and friend to S.W.M. We are indebted to Jamy Peng for making the GST-Sox15 construct, to Tammie Stone for prepping His-Su(H) protein, and to Sui Zhang for critical steps in Sox15 antibody production and characterization. We would like to thank Scott Barolo, Nick Reeves, and Mark Rebeiz for useful discussions throughout this project, members of the Posakony lab for critical comments on the manuscript, and two anonymous reviewers for their helpful suggestions. S.W.M. received support from NIH pre-doctoral training grant GM07240. This work was supported by Grant 07-04-01127 from the Russian Foundation for Basic Research to A.P. and by NIH grants GM062279 and GM046993 to J.W.P.
1.23.2 References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Bailey AM, Posakony JW. Suppressor of Hairless directly activates transcription of Enhancer of split Complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- Bang AG, Hartenstein V, Posakony JW. Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development. 1991;111:89–104. doi: 10.1242/dev.111.1.89. [DOI] [PubMed] [Google Scholar]
- Bang AG, Posakony JW. The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev. 1992;6:1752–1769. doi: 10.1101/gad.6.9.1752. [DOI] [PubMed] [Google Scholar]
- Barolo S, Carver LA, Posakony JW. GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726–732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36:436–442. doi: 10.2144/04363ST03. [DOI] [PubMed] [Google Scholar]
- Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. doi: 10.1101/gad.976502. [DOI] [PubMed] [Google Scholar]
- Barolo S, Stone T, Bang AG, Posakony JW. Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 2002;16:1964–1976. doi: 10.1101/gad.987402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW. A Notch-independent activity of Suppressor of Hairless is required for normal mechanoreceptor physiology. Cell. 2000;103:957–969. doi: 10.1016/s0092-8674(00)00198-7. [DOI] [PubMed] [Google Scholar]
- Bellen H, Levis R, Liao G, He Y, Carlson J, Tsang G, Evans-Holm M, Hiesinger P, Schulze K, Rubin G, Hoskins R, Spradling A. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Furriols M. Notch pathway: making sense of Suppressor of Hairless. Curr Biol. 2001;11:R217–221. doi: 10.1016/s0960-9822(01)00109-9. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Brizuela BJ, Elfring L, Ballard J, Tamkun JW, Kennison JA. Genetic analysis of the brahma gene of Drosophila melanogaster and polytene chromosome subdivisions 72AB. Genetics. 1994;137:803–813. doi: 10.1093/genetics/137.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NH, Kafatos FC. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988;203:425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Castro B, Barolo S, Bailey AM, Posakony JW. Lateral inhibition in proneural clusters: Cis-regulatory logic and default repression by Suppressor of Hairless. Development. 2005;132:3333–3344. doi: 10.1242/dev.01920. [DOI] [PubMed] [Google Scholar]
- Certel K, Anderson MG, Shrigley RJ, Johnson WA. Distinct variant DNA-binding sites determine cell-specific autoregulated expression of the Drosophila POU domain transcription factor Drifter in midline glia or trachea. Mol Cell Biol. 1996;16:1813–1823. doi: 10.1128/mcb.16.4.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L, Kelley R, Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988;239:1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Tyler DM, Furriols M, Chalkiadaki A, Delidakis C, Bray S. Spatially restricted factors cooperate with Notch in the regulation of Enhancer of split genes. Dev Biol. 2000;221:390–403. doi: 10.1006/dbio.2000.9691. [DOI] [PubMed] [Google Scholar]
- Cremazy F, Berta P, Girard F. Genome-wide analysis of Sox genes in Drosophila melanogaster. Mech Dev. 2001;109:371–375. doi: 10.1016/s0925-4773(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Furriols M, Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Gho M, Bellaïche Y, Schweisguth F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- Goldstein LSB, Fyrberg EA, editors. Methods Cell Biol. Vol. 44. Academic Press; San Diego: 1994. Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. [Google Scholar]
- Golic K, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Posakony JW. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989;107:389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Posakony JW. A dual function of the Notch gene in Drosophila sensillum development. Dev Biol. 1990;142:13–30. doi: 10.1016/0012-1606(90)90147-b. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Inbal A, Levanon D, Salzberg A. Multiple roles for u-turn/ventral veinless in the development of Drosophila PNS. Development. 2003;130:2467–2478. doi: 10.1242/dev.00475. [DOI] [PubMed] [Google Scholar]
- Janknecht R, De Martynoff G, Lou J, Hipskind RA, Nordheim A, Stunnenberg HG. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci USA. 1991;88:8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP. Studies of mechanosensation using the fly. Hum Mol Genet. 2002;11:1215–1218. doi: 10.1093/hmg/11.10.1215. [DOI] [PubMed] [Google Scholar]
- Juven-Gershon T, Hsu JY, Theisen JW, Kadonaga JT. The RNA polymerase II core promoter - the gateway to transcription. Curr Opin Cell Biol. 2008;20:253–259. doi: 10.1016/j.ceb.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaler J, Fu W, Duan H, Noll M, Posakony JW. An essential role for the Drosophila Pax2 homolog in the differentiation of adult sensory organs. Development. 1999;126:2261–2272. doi: 10.1242/dev.126.10.2261. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: Mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Koelzer S, Klein T. Regulation of expression of Vg and establishment of the dorsoventral compartment boundary in the wing imaginal disc by Suppressor of Hairless. Dev Biol. 2006;289:77–90. doi: 10.1016/j.ydbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kosman D, Mizutani CM, Lemons D, Cox WG, Mcginnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lai EC, Bodner R, Posakony JW. The Enhancer of split Complex of Drosophila includes four Notch-regulated members of the Bearded gene family. Development. 2000;127:3441–3455. doi: 10.1242/dev.127.16.3441. [DOI] [PubMed] [Google Scholar]
- Lecourtois M, Schweisguth F. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split Complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- Ma Y, Certel K, Gao Y, Niemitz E, Mosher J, Mukherjee A, Mutsuddi M, Huseinovic N, Crews ST, Johnson WA, Nambu JR. Functional interactions between Drosophila bHLH/PAS, Sox, and POU transcription factors regulate CNS midline expression of the slit gene. J Neurosci. 2000;20:4596–4605. doi: 10.1523/JNEUROSCI.20-12-04596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Jordan NC, Aisemberg G. Affinity purification of GST fusion proteins for immunohistochemical studies of gene expression. Protein Expr Purif. 2002;26:260–265. doi: 10.1016/s1046-5928(02)00524-7. [DOI] [PubMed] [Google Scholar]
- Morel V, Lecourtois M, Massiani O, Maier D, Preiss A, Schweisguth F. Transcriptional repression by Suppressor of Hairless involves the binding of a Hairless-dCtBP complex in Drosophila. Curr Biol. 2001;11:789–792. doi: 10.1016/s0960-9822(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Morel V, Schweisguth F. Repression by Suppressor of Hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- Nellesen DT, Lai EC, Posakony JW. Discrete enhancer elements mediate selective responsiveness of Enhancer of split Complex genes to common transcriptional activators. Dev Biol. 1999;213:33–53. doi: 10.1006/dbio.1999.9324. [DOI] [PubMed] [Google Scholar]
- O’Neill JW, Bier E. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques. 1994;17:870, 874–875. [PubMed] [Google Scholar]
- Posakony JW. Nature versus nurture: asymmetric cell divisions in Drosophila bristle development. Cell. 1994;76:415–418. doi: 10.1016/0092-8674(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Rebeiz M, Posakony JW. GenePalette: A universal software tool for genome sequence visualization and analysis. Dev Biol. 2004;271:431–438. doi: 10.1016/j.ydbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Reeves N, Posakony JW. Genetic programs activated by proneural proteins in the developing Drosophila PNS. Dev Cell. 2005;8:413–425. doi: 10.1016/j.devcel.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Roseman RR, Johnson EA, Rodesch CK, Bjerke M, Nagoshi RN, Geyer PK. A P element containing suppressor of Hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics. 1995;141:1061–1074. doi: 10.1093/genetics/141.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Russell S. The Drosophila dominant wing mutation Dichaete results from ectopic expression of a Sox-domain gene. Mol Gen Genet. 2000;263:690–701. doi: 10.1007/s004380051218. [DOI] [PubMed] [Google Scholar]
- Schweisguth F, Posakony JW. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992;69:1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Willingham AT, Keil T. A tissue specific cytochrome P450 required for the structure and function of Drosophila sensory organs. Mech Dev. 2004;121:1289–1297. doi: 10.1016/j.mod.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.