Abstract
The mechanism by which bacterial pathogens activate caspase-1 via Nlrp3 remains poorly understood. Here we show that the ability of Staphylococcus aureus, a leading cause of infection in humans, to activate caspase-1 and induce IL-1β secretion resides in culture supernatants of growing bacteria. Caspase-1 activation induced by S. aureus required α-, β- and γ- hemolysins and the host Nlrp3 inflammasome. Mechanistically, α- and β-hemolysins alone did not trigger caspase-1 activation but they did so in the presence of bacterial lipoproteins released by S. aureus. Notably, caspase-1 activation induced by S. aureus supernatant was independent of the P2X7 receptor and the essential TLR adaptors Myd88 and Trif, but was inhibited by extracellular K+. These results indicate that S. aureus hemolysins circumvent the requirement of ATP and the P2X7 receptor to induce caspase-1 activation via Nlrp3. Furthermore, these studies revealed that hemolysins promote in the presence of lipoproteins the activation of the Nlrp3 inflammasome.
Keywords: S. aureus, hemolysins, toxins, inflammasome, NLR, lipoproteins
Introduction
Staphylococcus aureus is a Gram-positive bacterium responsible for a wide variety of superficial and life-threatening systemic infections (1). It produces an array of virulence factors that allow the bacteria to colonize epithelial surfaces, invade host tissues and evade the immune response (2). The bacterium also produces a number of disease-relevant exotoxins, such as toxic shock syndrome toxin-1, enterotoxins and membrane-damaging toxins (cytolysins), which include hemolysins (α, β, γ and δ) and Panton-Valentine Leucocidin (3, 4). α-hemolysin is secreted as a 33 KDa monomer and oligomerizes forming heptameric transmembrane pores (5). β-hemolysin is a neutral sphingomyelinase (6). Both α- and β-hemolysins have the ability to damage the membrane of human monocytes and promote the secretion of IL-1β, IL-6 and TNF-α (7-10). Furthermore, α-hemolysin can induce K+ efflux in host cells (11, 12). However, the mechanism by which S. aureus hemolysins promote host immune responses remains poorly understood.
Host recognition of bacterial pathogens including S. aureus is mediated in part by pattern recognition receptors, including membrane-bound TLRs and intracellular nucleotide-binding oligomerization domain receptors (NLRs) (13). TLRs recognize conserved bacterial components such as LPS, flagellin, lipoproteins, lipoteichoic acid and unmethylated CpG DNA (14). There is evidence that TLR2 is critical for S. aureus recognition and host defense (15). More recently, nucleotide-binding oligomerization domain containing 2 (Nod2), a NLR protein which senses muramyl dipeptide produced during the synthesis and/or degradation of peptidoglycan, has been implicated in the host response to S. aureus (16). While TLR2 and Nod2 induce immune responses via the activation of the transcription factor NF-κB and MAP kinases (17), another group of NLRs that include Nlrp3 and Nlrc4 are critical for the activation of caspase-1 and IL-1β secretion in response to bacterial and endogenous stimuli (18). Nlrp3 and Nlrc4 link specific microbial stimuli to caspase-1 activation through the formation of a multi-protein platform called the inflammasome that includes the adaptor apoptosis associated speck-like protein (Asc) (18). Upon microbial stimulation, the IL-1β precursor is induced in macrophages via NF-κB activation (19). Subsequently, pro-IL-1β is processed into mature IL-1β by the enzymatically active heterodimer composed of a 10- and a 20-kDa chain of caspase-1 (20). Nlrp3 is essential for caspase-1 activation in response to a variety of microbial molecules in combination with ATP and particulate matter including urate crystals, silica particles and aluminum salts (21-23). Stimulation of the purinergic P2X7 receptor (P2X7R) with ATP induces K+ efflux via the rapid opening of a non-selective cation channel and the gradual opening of a large pore possibly mediated by pannexin-1, both of which are important for Nlrp3-mediated caspase-1 activation in response to ATP and bacterial products (21, 24). In addition to ATP, activation of the Nlrp3 inflammasome is induced in LPS-stimulated macrophages by pore-forming toxin maitotoxin and the ionophore nigericin, which induce changes in cellular K+ concentration (25). However, the mechanism by which bacterial pathogens activate the Nlrp3 inflammasome is poorly understood.
Recent studies showed that the adaptor protein Asc and IL-1β can play a critical role in neutrophil recruitment elicited by S. aureus skin infection and elimination of the bacterium in vivo (26, 27). Furthermore, infection of macrophages with S. aureus induced low level, but significant, caspase-1 activation via Nlrp3 in vitro (25). However, the latter finding remains controversial as some authors did not observe caspase-1 activation and IL-1β secretion in response to S. aureus infection in the absence of exogenous ATP (28). Furthermore, the S. aureus molecules that induce caspase-1 activation and IL-1β secretion remain unknown. The objective of this study was to clarify the mechanism by which S. aureus induces IL-1β and triggers the activation of caspase-1.
Materials and Methods
Mice and macrophages
Nlrp3-/-, Asc-/-, caspase-1-/- mice have been described (29). P2X7R-/- mice were obtained from Dr. George Dubyak, Case Western Reserve University. MyD88-/- and Myd88/Trif-/- mice were obtained from Dr. Akira, Osaka University. All mice were backcrossed onto the C57BL/6 background at least eight times except for the Nlrp3-/- mice which were in mixed 129/C57BL/6 background. Wild-type (WT) C57BL/6 and 129/C57BL/6 mice were maintained in our Animal Facility. All animal studies were approved by the University of Michigan Committee on Use and Care of Animals.
Bacteria
S. aureus wild-type (wt) strain 8325-4 and its isogenic hemolysin mutants have been previously described (4). S. aureus wt strain SA113 and its isogenic mutant deficient in lipoprotein diacylglyceryl transferase (Δlgt) have also been previously described (30). Clinical isolates of S. aureus were obtained from the laboratory of Clinical Microbiology at the University of Michigan University Hospital with the approval of the Institutional Review Board of the University of Michigan Medical School. Tryptic Soy Broth was used for the growth of the bacteria.
Purified toxins
Purified α-hemolysin was purchased from Sigma (H9395) and β-hemolysin from Toxin Technology (BHT1). For caspase-1 activation experiments with purified toxins, macrophages were stimulated for 3 h either with a 1/10 dilution of S. aureus supernatant collected after 20 h of subculture, washed 3 times with PBS and subsequently incubated with 10 μg/ml of α-hemolysin or β-hemolysin in IMDM.
In vitro S. aureus stimulation
Bacteria in midlogarithmic phase were obtained after subculturing bacteria at a 1:100 dilution of an overnight culture for 3 h. Bone marrow-derived macrophages were infected with S. aureus for 3 h or indicated time at the bacterial-macrophage ratio indicated in Figures and either pulsed for 30 min with 5 mM ATP or left untreated. S. aureus supernatants were prepared by subculturing 1:100 dilutions of overnight S. aureus cultures for different times (0-20 h). The cultures were centrifuged at 4000 g for 10 min and the supernatants were collected and sterilized by filtration. All in vitro stimulation of macrophages with S. aureus supernatants were performed for 3 hours at 1/10 dilution of supernatants collected after 20 h of subculture unless otherwise specified.
Cytotoxicity assay
The percentage of macrophage death was determined by measurement of the release of LDH after incubation with the CytoTox 96 nonradioactive cytotoxicity assay (Promega). The absorbance at 490 nm was measured, and the percentage of cell death was calculated as follows: [(experimental release - spontaneous release) / (maximum release-spontaneous release)]×100, where ‘spontaneous release’ is that found in untreated macrophages and ‘maximum release’ is the value obtained after lysis of macrophages with a solution of 0.1% Triton-X100.
Cutaneous S. aureus infection model
The subcutaneous abscess model has been previously described (26). Briefly, the mice were inoculated subcutaneously with 106 CFUs of midlogarithmic growth phase S. aureus strain 8325-4 in 50 μl of PBS. Lesional skin biopsies were collected 24 h later and homogenized in the presence of proteinase inhibitors (Roche).
Immunoblotting and cytokine measurements
Membranes were probed with antibodies against caspase-1 (a gift of Peter Vandenabeele (Ghent University, Belgium), mature IL-1β (p17, Asp116, Cell Signaling), pro-IL-1β (pIL-1β) (R&D Systems) (27) and α-hemolysin antiserum (Sigma). As a positive control for caspase-1 activation, WT macrophages were incubated with 1 μg/ml of LPS for 3 h and stimulated for 30 min with 5 mM ATP. Levels of IL-1β, mature IL-18 and MIP-2α were determined by ELISA (MBL International).
Statistical analysis
Statistical significance between groups was determined by two tailed Student’s t test. Animals studies were analyzed by one-way ANOVA. Differences were considered significant when p < 0.05.
Results
Culture supernatant of S. aureus is a potent activator of caspase-1
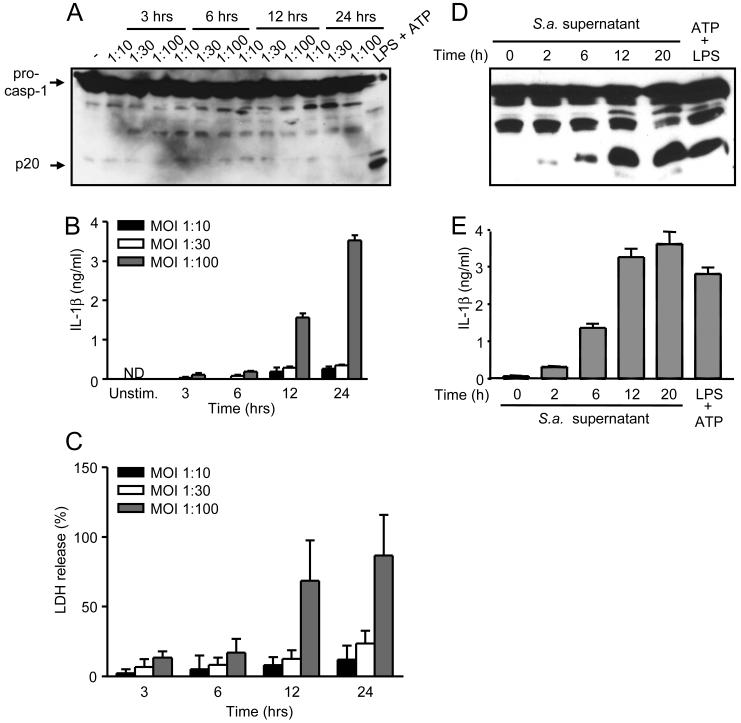
The ability of S. aureus to active caspase-1 in macrophages remains controversial (25, 28). Consistent with our previous results (28), infection of macrophages with S. aureus did not induce detectable activation of caspase-1 at different bacterial-macrophage ratio for up to 24 hrs of culture (Fig. 1A). However, incubation of macrophages with the highest MOI of S. aureus tested and for extended periods of time (12-24 hours) led to significant release of IL-1β (Fig. 1B) which was associated with elevated cytotoxicity (Fig. 1C). To further assess the ability of S. aureus to activate caspase-1, we incubated macrophages with S. aureus supernatants collected after bacterial growth in culture for different times. Notably, stimulation of macrophages for 3 hrs with culture supernatants from S. aureus collected after 2 h of growth elicited significant caspase-1 activation, which increased by 6 h and peaked by 12 h of bacterial culture (Fig. 1D). Consistently, a parallel increase in IL-1β secretion was elicited by culture supernatants of growing S. aureus (Fig. 1E). Caspase-1 activation and IL-1β secretion triggered by culture supernatant from S. aureus were comparable to that observed with LPS, but unlike that induced with the TLR4 agonist, that triggered by the bacterium supernatant did not require exogenous ATP stimulation (Fig. 1D and E). These results indicate that S. aureus releases factor(s) during bacterial growth that activate caspase-1 in the absence of exogenous ATP.
Figure 1.
Factors released during S. aureus growth activate caspase-1. A-C, S. aureus (S.a.) does not activate caspase-1 in macrophages in vitro. Macrophages were infected with a S. aureus strain 8325-4 macrophage/bacteria ratio of 1:10, 1:30 and 1:100 for 3, 6, 12 and 24 hrs. Caspase-1 activation was measured by immunoblotting with anti-caspase-1 antibody (A), IL-1β in culture supernatant by ELISA (B) and macrophage viability by LDH assay (C). D and E, S. aureus supernatant activates caspase-1. S. aureus culture supernatants were prepared by subculturing S. aureus for the indicated amount of time. Macrophages were incubated for 3 hours with a 1/10 dilution of each S. aureus supernatant and IL-1β and caspase-1 processing was determined. Stimulation with LPS plus ATP is shown as a control. ND, not detectable. Values represent mean ± SEM (n = 3). Results are representative of at least three separate experiments.
α-, β- and γ-hemolysins are required for the induction of caspase-1 activation by S. aureus supernatant
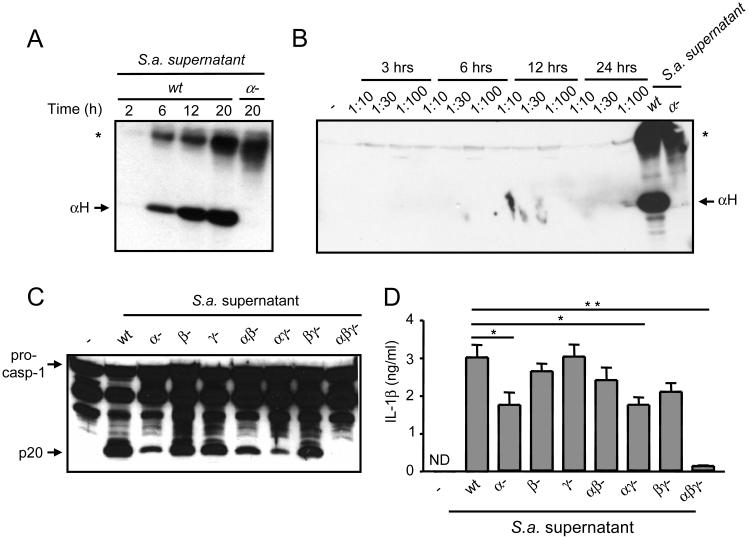
S. aureus secretes several toxins including hemolysins that function as pores and/or membrane-damaging factors (4, 7, 8). To begin to assess a role for hemolysis in caspase-1 activation, we determined the production of α-hemolysin by S. aureus during bacterial growth culture. Immunoblotting analysis revealed that α-hemolysin was first detectable in the culture supernatants by 6 h and increased by 12 h of bacterial culture (Figure 2A). To assess whether α-hemolysin was induced during infection, we determined the production of α-hemolysin during culture of S. aureus with macrophages under conditions similar to those shown in Fig. 1A in which caspase-1 was not activated. Under these conditions, we did not observe detectable production of α-hemolysin (Fig. 2B). To assesses the role of hemolysins in caspase-1 activation, culture supernatants from wt S. aureus or its isogenic mutants lacking α-, β- or γ-hemolysins and all possible combinations were tested for their ability to induce caspase-1 activation in macrophages. The culture supernatants from single hemolysin mutant strains, particularly α-hemolysin, induced significant, although reduced, levels of caspase-1 activation when compared to wt S. aureus supernatant (Fig. 2C). Notably, combined deficiency of α-, β- and γ-hemolysins abolished the ability of the S. aureus culture supernatant to activate caspase-1 (Fig. 2C) and to induce IL-1β secretion (Fig. 2D). Thus, α-, β- and γ-hemolysins released by S. aureus play an essential, although redundant, role in the activation of caspase-1 and the secretion of IL-1β in macrophages.
Figure 2.
α-, β- and γ-hemolysins secreted during S. aureus growth activate caspase-1. A,, S. aureus secretes α-hemolysin during in vitro growth. S. aureus (S a.) was subcultured for the indicated amount of time and supernatants were immunobloted with an anti-α-hemolysin antiserum. An isogenic strain deficient in α-hemolysin was used as a specificity control. The arrow indicates the α-hemolysin 33 kDa monomer. B, Production of α-hemolysin during infection of macrophages with S. aureus under conditions similar to those used for Fig. 1A. Cell extracts were immunoblotted with anti-α-hemolysin antiserum. Culture supernatant from growing S. aureus (wt) was used as a positive control. C and D, α-, β- and γ-hemolysins mediate caspase-1 activation induced by S. aureus supernatant. Macrophages were stimulated for 3 h with supernatant from wt S. aureus or its isogenic hemolysin mutants. Cell extracts were immunobloted with anti-caspase-1 antibody and IL-1β measured in the cell supernatants. Values represent mean ± SEM (n = 3). ND, not detectable. *, indicates a no-specific band. Results are representative of at least three separate experiments. C, * p < 0.05, ** p < 0.01 (Student’s t test).
S. aureus supernatant activates caspase-1 via the Nlrp3 inflammasome independently of P2X7R and TLR signaling
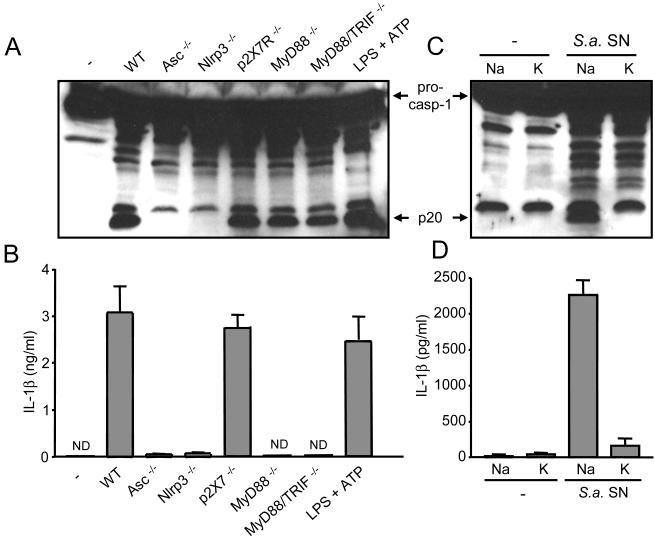
To study the host recognition system involved in hemolysin-mediated caspase-1 activation, we analyzed macrophages deficient in several inflammasome components as well as critical TLR adaptors and the P2X7R. Caspase-1 activation and IL-1β secretion induced by S. aureus supernatant was abolished in macrophages deficient in Nlrp3 or Asc (Fig. 3A and 3B). In contrast, Nlrc4, a NLR family member required for caspase-1 activation in response to Salmonella, was dispensable for caspase-1 activation and IL-1β secretion induced by S. aureus supernatant (data not shown). Nlrp3-mediated caspase-1 activation induced by bacterial molecules including LPS requires ATP-mediated stimulation of the P2X7R (25). However, processing of pro-caspase-1 and IL-1β secretion induced by S. aureus supernatant was unimpaired in macrophages deficient in the P2X7R (Fig. 3A and 3B). Furthermore, caspase-1 activation proceeded normally in macrophages deficient in MyD88 or both MyD88 and Trif, the adaptors that are essential for TLR signaling (Fig. 3A). In contrast, IL-1β secretion induced by S. aureus supernatant was abrogated in macrophages deficient in MyD88 or MyD88 and Trif (Fig. 3B). Thus, TLR signaling is required for IL-1β secretion but not caspase-1 activation induced by S. aureus supernatant.
Figure 3.
S. aureus activates the Nlrp3 inflammasome independently of TLR signaling. A and B, macrophages from WT, Asc-/-, Nlrp3-/-, p2X7R-/-, MyD88-/-, MyD88/Trif-/- were stimulated for 3 h with supernatant from wt S. aureus. Stimulation with LPS and ATP is shown as a control. C and D, Caspase-1 activation and IL-1β secretion by S. aureus supernatant requires K+ efflux. Macrophages were pretreated with 130 mM of NaCl or KCl for 30 min and subsequently left unstimulated or wt S. aureus supernatant (S. a. SN) was added to a final dilution of 1/10. Three hours later supernatants were collected and analyzed for IL-1β and cell extracts were immunobloted with anti-caspase-1 antibody. ND, not detectable. Values represent mean ± SEM (n = 3). Results are representative of at least three separate experiments.
Activation of the Nlrp3 inflammasome in response to ATP or particulate matter in combination with TLR agonists is inhibited by high concentration of extracellular K+ (31, 32). Furthermore, the K+ efflux elicited by α- and β- hemolysin is necessary for IL-1β secretion (8, 33). Consistently, incubation of macrophages in medium rich in K+, but not Na+, impaired caspase-1 activation and IL-1β secretion induced by S. aureus supernatant (Fig. 3C and 3D). These results suggest that the K+ efflux triggered by hemolysins released during S. aureus growth regulates Nlrp3 inflammasome activation independently of the P2X7R.
Hemolysins and the Nlrp3 inflammasome are required for the activation of caspase-1 by S. aureus in vivo
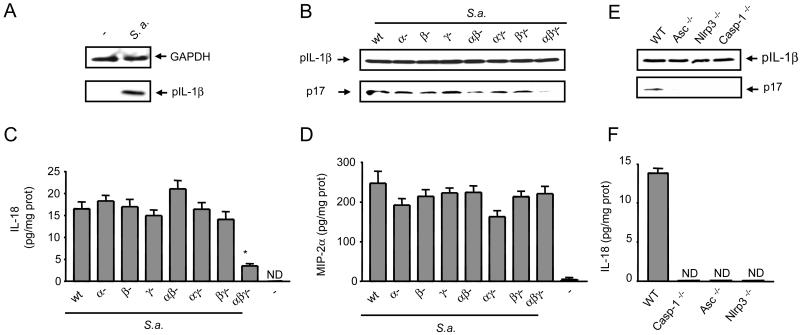
To study the role of hemolysins in caspase-1 activation in vivo we used a previously described model of S. aureus subcutaneous infection (26, 27). WT mice were injected subcutaneously with 1 × 106 CFUs of wt S. aureus or its isogenic hemolysin mutants and tissue extracts of the lesions were prepared 24 h post-infection. Immunoblotting of the skin tissue revealed that infection with S. aureus induced the production of proIL-1β (Fig. 4A). Importantly, S. aureus lacking α-, β- and γ-hemolysins was impaired in the production of the processed IL-1β (p17) and IL-18 in the tissue, whereas that induced by single or double mutants were slightly reduced or unimpaired (Fig. 4B and 4C). In contrast, MIP-2α induction by the triple hemolysin S. aureus mutant was unimpaired (Fig. 4D), providing specificity for the response. Counting the number of viable bacteria in the lesions revealed similar bacterial burdens in mice infected with wild-type and the isogenic S. aureus mutants (Fig. S1).
Figure 4.
α-, β- and γ-hemolysins activate the Nlrp3 inflammasome in vivo. WT, Caspase-1-/-, Asc-/- and Nlrp3-/- mice (n=6) were injected subcutaneously with 106 CFUs of wt S. aureus (S.a.) or its isogenic hemolysin mutants. Tissue biopsies were collected 24 h after injection and analyzed by immunoblotting for the expression of proIL-1β (pIL-1β) and the cleavage of proIL-1β into its mature form (p17) (A,C,E) and for the production of IL-18 and MIP-2a (B,D,F). ND, not detectable. Values represent mean ± SEM. Results are representative of at least three separate experiments. (*) p > 0.05 (one-way ANOVA).
We next determined the role of the Nlrp3 inflammasome in the processing of pro-IL-1β and secretion of IL-18 in vivo. Immunoblotting of skin tissue extracts prepared after infection with S. aureus showed that production of the mature IL-1β (p17) was reduced in tissue from mice deficient in Nlrp3, Asc and caspase-1 (Fig. 4E). Furthermore, the production of IL-18 was abrogated in mice deficient in Nlrp3, Asc and caspase-1 (Fig. 4F). Taken together, these results indicate that S. aureus α-, β- and γ-hemolysins play a critical role in the production of mature IL-1β and IL-18 through the Nlrp3 inflammasome in vivo.
Clinical samples of S. aureus induce caspase-1 activation via Nlrp3 independently of TLR signaling
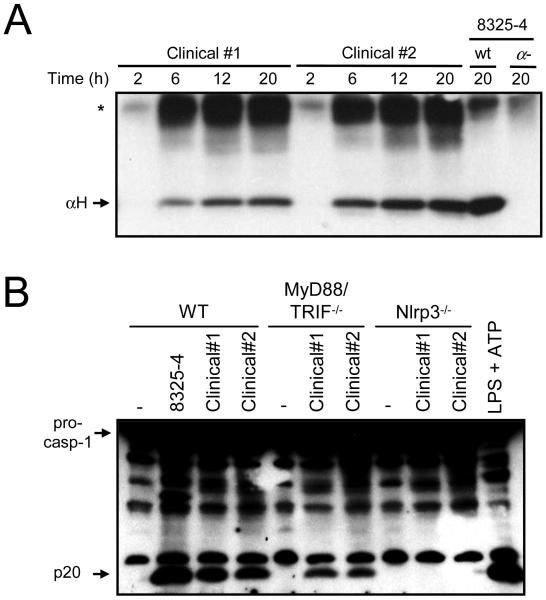
To validate the results obtained with the S. aureus laboratory strain 8325-4, we next determined whether bacterial strains isolated from patients suffering from S. aureus infection induce caspase-1 activation via Nlrp3. In line with our previous results, α-hemolysin was released to the culture media during in vitro growth of clinical S. aureus clinical isolates (Fig. 5A). Importantly, caspase-1 activation induced by the culture supernatant of the clinical S. aureus strains required Nlrp3 but was independent of MyD88 and Trif (Fig. 5B). These results indicate that the culture supernatant from S. aureus strains isolated from infected patients can induce caspase-1 activation via Nlrp3 independently of TLR signaling.
Figure 5.
S. aureus clinical isolates activate the Nlrp3 inflammasome independently of TLR signalling. A, S. aureus clinical isolates were subcultured for the indicated amounts of time, supernatants were prepared and immunoblotted with anti-serum against α-hemolysin. S. aureus strain 8325-4 and its isogenic α-hemolysin deficient mutant (α-) were used as specificity controls. *, indicates a non-specific band. B, WT, MyD88/Trif-/- and Nlrp3-/- macrophages were stimulated for 3 h with supernatants of S. aureus clinical isolates and strain 8325-4. Stimulation with LPS and ATP is shown as a control. Cell extracts were immunoblotted with anti-caspase-1 antibody. Results are representative of at least 3 different experiments.
α- and β-hemolysins and another factor(s) released by S. aureus activate the Nlrp3 inflammasome
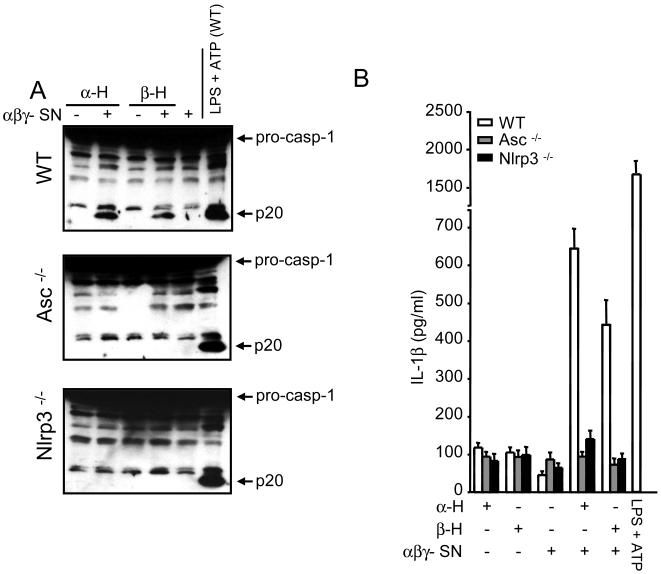
To further assess the ability of hemolysins to activate the Nlrp3 inflammasome, we tested whether purified α- and β-hemolysins could restore the ability to activate caspase-1 of the supernatant from S. aureus deficient in α-, β- and γ-hemolysins. Notably, incubation of macrophages with purified hemolysins did not activate caspase-1 (Fig. 6A). However, addition of purified α- or β-hemolysin to supernatant from α-, β- and γ-deficient S. aureus restored its ability to induce caspase-1 activation and IL-1β secretion in macrophages (Fig. 6A and 6B). Consistent with our previous results, this activity was abrogated in macrophages deficient in Nlrp3 or Asc (Fig. 6A and 6B), suggesting that that α- and β-hemolysins cooperate with other factor(s) released by the bacteria to activate the Nlrp3 inflammasome.
Figure 6.
α- and β-hemolysins and another factor(s) released by S. aureus activate the Nlrp3 inflammasome. A and B, Purified α- or β-hemolysin restores the ability of α-, β- and γ- hemolysin-deficient S. aureus supernatant (αβγ- SN) to activate caspase-1 (A) and to induce IL-1β secretion (B). Macrophages from WT, Asc-/- and Nlrp3-/- mice were stimulated with purified α- or β-hemolysin alone (-) or in the presence of αβγ- SN (+). Lysate prepared from WT macrophages stimulated with LPS and ATP (LPS+ATP WT) is shown as a reference control in all blots. Results are representative of at least three different experiments.
Lipoproteins released during S. aureus growth induce expression of pro-IL-1β and promote caspase-1 activation
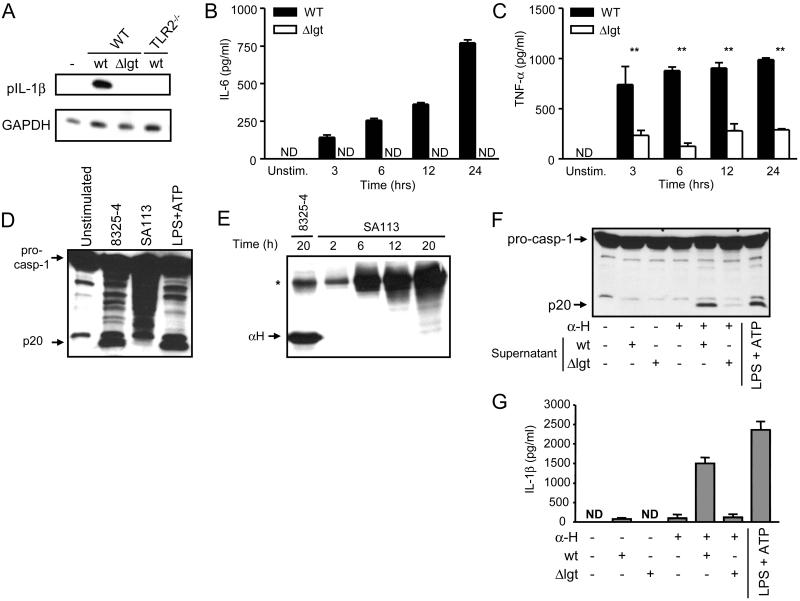
The bacterial cell wall of S. aureus contains several immunostimulatory molecules which are released during bacterial growth (30, 34). To identify the factor(s) present in the S. aureus supernatant that is required with hemolysins to activate caspase-1, we next investigated the role of the lipoproteins released by S. aureus in the secretion of IL-1β. The enzyme lipoprotein diacyglyceryl transferase, encoded in the lgt locus, catalizes the first step of lipoprotein biosynthesis (30). To determine if lipoproteins are required for IL-1β secretion, we incubated WT and TLR2-/- macrophages with culture supernatants from the wt S. aureus strain SA113 or its isogenic lgt deletion mutant (Δlgt) and assessed the induction of proIL-1β. Immunoblotting analysis revealed that the mutant Δlgt strain was impaired in its ability to induce pro-IL-1β when compared to the wt SA113 strain (Fig. 7A). Consistently, TLR2 deficient macrophages failed to induce pro-IL-1β in response to wt supernatant (Fig. 7A). In line with previous studies (30, 34), we found that IL-6 secretion was abrogated and TNF-α secretion greatly reduced in macrophages stimulated with lipoprotein deficient bacterial supernatants (Fig. 7B and 7C).
Figure 7.
Bacterial lipoproteins released by S. aureus induce the expression of proIL-1β, IL-6 and TNF-α and promote caspase-1 activation. A, Induction of proIL-1β by S. aureus supernatant is mediated by bacterial lipoproteins and TLR2 signaling. WT and TLR2-/- macrophages were stimulated for 3 h with S. aureus supernatant from wt strain SA113 (wt) or its isogenic mutant deficient in lipoprotein diacylglyceryl transferase (Δlgt). B and C, Bacterial lipoproteins released by S. aureus trigger the secretion of IL-6 and TNF-α. ND, not detectable. D and E, WT S. aureus strain SA113 supernatant does not activate caspase-1 and does not release α-hemolysin (αH) during in vitro growth. Stimulation with LPS and ATP is shown as a control. F and G, Caspase-1 activation by SA113 supernatant complemented with purified α-hemolysin is mediated by bacterial lipoproteins. Macrophages were incubated for 3 h with wt or Δlgt S. aureus supernatant and subsequently stimulated with 10 μg/ml of α-hemolysin for 30 min. Stimulation with LPS and ATP is shown as a control. ND, not detectable. *, indicates a non-specific band. Values represent mean ± SEM. Results are representative of at least three separate experiments. ** p < 0.0001.
We next tested whether bacterial lipoproteins present in the S. aureus supernatant contribute to caspase-1 activation. Unlike the 8325-4 strain, the culture supernatant from the wt SA113 S. aureus did not induce detectable caspase-1 activation (Fig. 7D) and this was associated with undetectable levels of α-hemolysin (Fig. 7E). Therefore, we tested whether purified α-hemolysin could restore the ability of strain SA113 supernatant to activate caspase-1. We found that the addition of α-hemolysin to macrophages previously stimulated with culture supernatant from the wt strain SA113 induced caspase-1 activation and IL-1β secretion, but each factor alone did not (Fig. 7F and 7G). Importantly, culture supernatant from Δlgt mutant strain exhibited impaired caspase-1 activation and IL-1β secretion in the presence of purified α-hemolysin, when compared to that induced by supernatant from the wt parental strain (Fig. 7F and 7G). These results indicate that lipoproteins released by S. aureus are important in inducing caspase-1 activation and IL-1β secretion.
Discussion
Previous studies have shown that IL-1β signaling and the adaptor protein Asc play a critical role in the clearance of S. aureus infection in the skin (26, 27). However, the mechanism by which the bacterium induces such host responses remained poorly understood. Here we provide evidence both in vitro and in vivo for a critical but redundant function of S. aureus α-, β- and γ-hemolysins in eliciting caspase-1 activation via the Nlrp3 inflammasome. This redundancy may explain, at least in part, why a previous study analyzing single hemolysin S. aureus mutants (25) did not reveal any significant role for these exotoxins in caspase-1 activation. Unlike the present work, these authors observed weak but significant caspase-1 activation after S. aureus infection in LPS-stimulated macrophages (25). These differences in results could be due to the use of different culture conditions or the priming step with LPS prior to the stimulation with the bacteria. We also show that the main caspase-1 activating activity resides in the supernatants of growing S. aureus which is consistent with the fact that hemolysins are exotoxins secreted by the bacteria (2). Experiments with purified α- and β-hemolysins showed that these toxins are necessary but not sufficient to activate the Nlrp3 inflammasome, as they required the presence of other bacterial products to activate caspase-1. Specifically, we provide evidence that lipoproteins released by the bacterium are important for the induction of proIL-1β and the activation of the Nlrp3 inflammasome. Although S. aureus supernatant did not require P2X7R signaling to activate the Nlrp3 inflammasome, caspase-1 activation was blocked by incubation of macrophages in medium containing high concentration of K+. These results suggest that S. aureus hemolysins released by S. aureus elicit K+ efflux independently of P2X7R which is important for the activation of caspase-1. In line with these results, purified α-hemolysin can induce K+ efflux (11) and this activity is thought to be necessary for IL-1β maturation (8, 35).
TLR2 plays an important role in the innate immune response to S. aureus and mediates the production of proinflammatory cytokines in macrophages and monocytes (36, 37). In this study, we examined the role of TLRs and bacterial lipoproteins in caspase-1 activation and IL-1β secretion induced by S. aureus in macrophages. Using macrophages that are deficient in the adaptor proteins MyD88 and Trif, and that therefore cannot signal via TLRs, we found that caspase-1 activation induced by S. aureus supernatant is independent of TLR signaling. The results suggest that products released by S. aureus induce TLR-independent caspase-1 activation in combination with pore forming toxins. In contrast, lipoproteins in the bacterial supernatant are important for pro-IL-1β induction through TLR2 signaling. Collectively, these results reveal distinct but critical roles for lipoproteins and hemolysins as well as the host TLRs and the Nlrp3 inflammasome in IL-1β secretion in response to S. aureus in macrophages.
Several bacteria activate caspase-1 through the formation of membrane pores. Salmonella, for instance, requires the presence of a functional type III secretion system to activate the Nlrc4 inflammasome (29). Similarly, L. monocytogenes requires the expression of the pore-forming protein listerolysin O to activate caspase-1 (38). In the current work, we show that α-, β- and γ-hemolysins produced by S. aureus are critical for activating the Nlrp3 inflammasome both in vitro and in vivo. A common feature of these observations is the formation of pores which alter the permeability of host membranes and may allow the cytosolic delivery of microbial molecules. Consistent with this hypothesis, we have previously shown that ATP triggers through pannexin-1 the intracellular delivery of MDP, which induces caspase-1 activation via Nlrp3 (39). Alternatively, lipoproteins and other microbial molecules released by S. aureus may provide a priming signal, at least in part, independently of TLRs for inflammasome activation and hemolysins may mimic ATP and P2X7R signaling to activate caspase-1. Because a direct association between microbial molecules and Nlrp3 proteins has not yet been identified, a likely model is that the activation of the inflammasome by microbial ligands is indirectly induced via an endogenous mediator. Consistently, there is evidence that reactive oxygen species, calcium-independent phospholipase A2, and cathepsin B may contribute to the activation of the Nlrp3 inflammasome at least in response to some stimuli (23, 32, 40, 41). Further work is needed to understand the mechanism whereby bacterial pathogens and host factors interact to induce inflammasome activation.
Supplementary Material
Acknowledgments
We thank Millenium Pharmaceuticals, Shizuo Akira and George Dubyak for generously supplying mutant mice, Timothy Foster and Friedrich Götz for kindly providing bacterial strains, Joel Whitfield for technical support, Peter Vandenabeele for providing anti caspase-1 antibody and Sherry Koonse for excellent animal husbandry.
Abbreviations used in this paper
- NLR
nucleotide-binding oligomerization domain-like receptors
- P2X7R
P2X7 receptor
- WT
wild-type
Footnotes
This work was supported by grants AI063331 and AI064748 (to G. N.). R. M.-P. was supported in part by a Fulbright-Ramón Areces Fellowship and L. F. was supported by a Fellowship grant from the Arthritis Foundation.
Disclosures
The authors declare that no competing interests exist.
Bibliography
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Iwatsuki K, Yamasaki O, Morizane S, Oono T. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203–214. doi: 10.1016/j.jdermsci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson IM, Hartford O, Foster T, Tarkowski A. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect Immun. 1999;67:1045–1049. doi: 10.1128/iai.67.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 6.Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, Bohach GA, Schlievert PM, Ohlendorf DH, Earhart CA. Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol. 2007;189:8719–8726. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walev I, Weller U, Strauch S, Foster T, Bhakdi S. Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus. Infect Immun. 1996;64:2974–2979. doi: 10.1128/iai.64.8.2974-2979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onogawa T. Staphylococcal alpha-toxin synergistically enhances inflammation caused by bacterial components. FEMS Immunol Med Microbiol. 2002;33:15–21. doi: 10.1111/j.1574-695X.2002.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 10.Yarovinsky TO, Monick MM, Husmann M, Hunninghake GW. Interferons increase cell resistance to Staphylococcal alpha-toxin. Infect Immun. 2008;76:571–577. doi: 10.1128/IAI.01088-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walev I, Martin E, Jonas D, Mohamadzadeh M, Muller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Leulier F, Lemaitre B. Toll-like receptors--taking an evolutionary approach. Nat Rev Genet. 2008;9:165–178. doi: 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 16.Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR, Fowler VG., Jr. The Critical Role of NOD2 in Regulating the Immune Response to Staphylococcus aureus. Infect Immun. 2009 doi: 10.1128/IAI.00940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 18.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 20.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 21.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 23.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 26.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O’Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 28.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 29.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 30.Stoll H, Dengjel J, Nerz C, Gotz F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun. 2005;73:2411–2423. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 32.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warny M, Kelly CP. Monocytic cell necrosis is mediated by potassium depletion and caspase-like proteases. Am J Physiol. 1999;276:C717–724. doi: 10.1152/ajpcell.1999.276.3.C717. [DOI] [PubMed] [Google Scholar]
- 34.van Langevelde P, van Dissel JT, Ravensbergen E, Appelmelk BJ, Schrijver IA, Groeneveld PH. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gratz N, Siller M, Schaljo B, Pirzada ZA, Gattermeier I, Vojtek I, Kirschning CJ, Wagner H, Akira S, Charpentier E, Kovarik P. Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9. J Biol Chem. 2008;283:19879–19887. doi: 10.1074/jbc.M802848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loof TG, Goldmann O, Medina E. Immune recognition of Streptococcus pyogenes by dendritic cells. Infect Immun. 2008;76:2785–2792. doi: 10.1128/IAI.01680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nunez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 39.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via Cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 40.Walev I, Klein J, Husmann M, Valeva A, Strauch S, Wirtz H, Weichel O, Bhakdi S. Potassium regulates IL-1 beta processing via calcium-independent phospholipase A2. J Immunol. 2000;164:5120–5124. doi: 10.4049/jimmunol.164.10.5120. [DOI] [PubMed] [Google Scholar]
- 41.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.