Abstract
The aim of the present study was to evaluate the expression of innate immunity receptors belonging to the Toll-like family in the neural plexuses of the different tracts of murine intestine, of the human ileum, and in lower dorsal root ganglia (DRGs) from where extrinsic afferents to these plexuses originate. Results obtained by immunohistochemistry and immunofluorescence on paraffin-embedded tissue and whole-mount preparations show that Toll-like receptors (TLRs) -3 and -7, recognizing viral RNA, and TLR4, recognizing lipopolysaccharide (membrane component of Gram-negative bacteria), are expressed in the myenteric and submucous plexuses of murine intestine and human ileum, and in DRGs primary sensory neurons. They also show that TLR4 immunostaining is stronger in murine distal large bowel. In murine tissue, expression of TLRs was present in both neurons and glial cells. These observations indicate that the enteric neural network might be directly activated by bacterial and viral components and is therefore more in the forefront than previously envisaged in defense responses of the intestinal wall and in the cross-talk with intestinal microbiota. They also highlight the presence of a peripheral neural network that by way of hardwired neurotransmission could potentially convey to the central nervous system specific information on our microbial counterpart and invading or potentially invading pathogens. (J Histochem Cytochem 57:1013–1023, 2009)
Keywords: Toll-like receptors, TLR4, TLR3, TLR7, ENS, mouse, man, DRGs
The enteric nervous system (ENS) is embedded in the wall of the digestive tract and is basically organized into two ganglionated plexuses. The myenteric plexus lies between the outer and the inner smooth muscle layers, and the submucous plexus lies in the submucosa. Plexuses are constituted of intrinsic neurons and glial cells and extrinsic fibers of efferent and afferent nature. Efferent fibers are sympathetic and parasympathetic, and afferent fibers originate from the nodose ganglion of the vagus nerve and from lower thoracic and lumbosacral dorsal root ganglia (DRGs) (Furness 2000; Berthoud et al. 2004). The ENS provides for different functions ranging from motility to secretion control, and several experimental evidences indicate its contribution also to defense mechanisms of the gastrointestinal wall: enteric network reflex activity and neuropeptide release are involved in response to enterotoxins (Castagliuolo et al. 1994; Pothoulakis et al. 1998); enteric neurons can modify their neurochemical and electrophysiological profiles following exposure to immunomodulatory substances or inflammation (Lomax et al. 2006; Vasina et al. 2006); enteric neurons and glial cells produce and respond to cytokines and can affect epithelial proliferation and epithelial barrier permeability (Neunlist et al. 2008). In the general picture portrayed by these studies, ENS recruitment in inflammatory and immune responses always requires an epithelial, enteroendocrine, or immune cell as an intermediary to trigger the neural response (Goehler et al. 2000; Wood 2004). However, quite recently, it has been shown that neurons of the myenteric plexus of the murine jejunum and human ileum express Toll-like receptor 4 (TLR4), an innate immunity receptor belonging to the Toll-like family recognizing lipopolysaccharide, a membrane component typical of Gram-negative bacteria (Rumio et al. 2006). Additionally, TLR4 mRNA transcription has been detected in the nodose ganglion of the vagus nerve (Hosoi et al. 2005).
TLRs are pathogen recognition receptors that allow the innate immune system to rapidly recognize conserved microorganism–associated molecular patterns (MAMPs) of pathogenic, potentially pathogenic, or non-pathogenic and, moreover, useful commensal microorganisms. They can be subdivided into several subfamilies that recognize related MAMPs belonging to bacteria, fungi, parasites, viruses, and hosts (Akira et al. 2006). TLR activation by pathogens triggers rapid and localized responses mediated by phagocytes through different signaling pathways, leading to the production of proinflammatory cytokines, chemokines, and type 1 interferon (Akira et al. 2006). On the other hand, interaction of TLRs with intestinal microbiota appears to be rather multifaceted. Under normal conditions, it contributes to maintenance of the intestinal barrier and gut homeostasis, but when deregulated, it may lead to and/or precipitate inflammatory pathologies (Cario 2005; Harris et al. 2006).
The aim of the present study was to evaluate the expression of two other receptors of the Toll-like family, TLR3 and TLR7, recognizing viral double-stranded and single-stranded RNA respectively, in the ENS of the murine small and large intestine and of the human ileum. Moreover, because microbial density and predominance of Gram-negative bacteria increase from the proximal to the distal intestine (Hooper and Gordon 2001; Macpherson and Uhr 2004a,b), we also wanted to investigate whether a corresponding change in TLR4 immunostaining of enteric neurons could be observed. Additionally, TLR3, -4, and -7 expression has been evaluated in lower thoracic and lumbosacral murine DRGs.
Materials and Methods
Murine Tissue
C57BL/6N female mice, from 8 to 32 weeks old, were purchased from Charles River (Calco, Italy) and housed under specific pathogen-free conditions, maintained at constant temperature and humidity, with food and water given ad libitum. Animals were sacrificed by cervical dislocation. Handling and suppression were performed according to the regulations of the Università degli Studi di Milano. Following sacrifice, the intestinal tube was excised, laid out in a Petri dish, and dissected into its different portions under a steromicroscope. DRGs from lower thoracic and lumbosacral segments were collected from the appropriate intervertebral foramen under a surgical microscope.
Immediately after excision, samples were immersed in one of the following fixatives: 10% formalin in phosphate-buffered saline (PBS, 0.1 M, pH 7.4) for 4 hr or overnight at 4C, 4% paraformaldehyde in PBS for 4 hr or overnight at 4C, or Zamboni fixative (2% formalin, 0.2% picric acid in PBS) overnight at 4C.
Human Tissue
Small fragments encompassing the full thickness of the intestinal wall were obtained from the ileum of obese patients who underwent Scopinaro biliopancreatic diversion in the Department of Surgery, Multimedica Hospital, Sesto San Giovanni, Milan. All patients gave informed written consent. Ethical approval was obtained from the internal local board.
Human biopsies were fixed in 10% formalin in PBS overnight at 4C.
Antibodies
The following rabbit polyclonal antibodies against murine TLRs were used: TLR4 (ab13556 Novus Biologicals, Inc.; Littleton, CO); TLR3 (ab 210-367-R200, Alexis Biochemicals; Lausen, Switzerland); TLR7 (ab IMG-581A, Imgenex; San Diego, CA). Monoclonal antibody MS-280-PO directed against mouse glial fibrillary acidic protein (GFAP) was purchased from Lab Vision (Suffolk, UK).
Swine anti-rabbit immunoglobulins and rabbit peroxidase anti-peroxidase (PAP) were purchased from DAKO (Glostrup, Denmark). For the detection of mouse anti-mouse antibodies in murine tissue, the M.O.M. Kit (Vector Laboratories, Inc.; Burlingame, CA) was used.
Immunohistochemistry on Paraffin-embedded Tissue
Specimens were processed for paraffin embedding. Tissue sections (4-μm to 6-μm) were obtained from paraffin blocks and collected on silane-coated slides. For antigen retrieval, deparaffinized sections were either autoclaved for 6 min at 120C in sodium citrate buffer (0.01 M, pH 6) or treated in a microwave oven. Quenching of endogenous peroxidase activity was performed in 0.3% H2O2 in PBS. Nonspecific sites were blocked incubating slides for 30 min either with a solution consisting of 0.05 M Tris-HCl, 0.15 M NaCl, 0.1% gelatin, 0.5% ovalbumin, 0.05% Tween-20, and 0.2% fish gelatin, or with 10% goat serum. Afterwards, sections were incubated with one of the following primary antibodies: rabbit anti-mouse TLR4 diluted 1:50 in PBS overnight at 4C; rabbit anti-mouse TLR3 diluted 1:50 and rabbit anti-mouse TLR7 diluted 1:100 in PBS for 1 hr at 37C. After rinsing in PBS, sections were incubated in goat anti-rabbit diluted 1:100 in PBS for 1 hr at room temperature and then in rabbit PAP diluted 1:100 in PBS for 1 hr at room temperature. For the development of the reaction, a liquid DAB substrate chromogen system was used (DAB, DAKO). Slides were washed with PBS, dehydrated through an ascending series of ethanols, counterstained with hematoxylin, and mounted with entellan (Merck; Darmstadt, Germany). Controls were performed by replacing the primary antibody with non-immune rabbit serum or dilution buffer, by sequential omission of the secondary antibody and PAP complex, or by incubation with DAB reagent alone to exclude the possibility of non-suppressed endogenous peroxidase activity. Spleen was used as positive control.
Double Immunofluorescence on Paraffin-embedded Tissue
For double immunofluorescence experiments, deparaffinized mouse intestine sections were rinsed in Tris-buffered saline with 1% bovine serum albumin (TBS-BSA) and either autoclaved for 6 min at 120C in sodium citrate buffer (0.01 M, pH 6) or subjected to microwave treatment in sodium citrate buffer (0.01 M, pH 6) for antigen retrieval. To remove tissue autofluorescence, sections were processed with sodium borohydride in TBS (0.1%, three times, 10 min each at 4C). After washing with TBS-BSA, nonspecific binding sites were blocked with 1:10 goat serum in TBS-BSA. Incubation with primary antibodies was performed as follows: rabbit anti-mouse TLR4 diluted 1:50 in TBS-BSA overnight at 4C; rabbit anti-mouse TLR3 and rabbit anti-mouse TLR7 diluted 1:50 in TBS-BSA for 1 hr at 37C. After rinsing in TBS-BSA, sections were incubated with FITC-conjugated goat anti-rabbit antibody diluted 1:200 in TBS-BSA for 1 hr at room temperature. For GFAP detection, sections were subsequently washed in TBS-BSA, and nonspecific sites were blocked with mouse IgG blocking reagent (M.O.M. Kit) for 1 hr, followed by incubation with mouse anti-mouse GFAP for 1 hr at 37C (diluted 1:100 in M.O.M. diluent), with biotinylated anti-mouse IgG for 10 min, and with tetra-rhodamine isothiocyanate–conjugated streptavidin (Jackson Laboratories; West Grove, PA) diluted 1:200 in TBS-BSA for 30 min at room temperature. After several washes in TBS-BSA, sections were incubated with DAPI (Sigma-Aldrich; St. Louis, MO) diluted 1:300,000 in TBS for 5 min. Sections were then mounted with Mowiol 4-88 (Calbiochem; La Jolla, CA). Controls were treated as described above, omitting the primary antibodies.
Immunohistochemistry on Whole-mount Preparations
Segments of murine small intestine, 1 cm long, were fixed by immersion in 4% paraformaldehyde in PBS for 2 hr at 4C, opened along the mesenteric border, and washed in PBS. The longitudinal muscle layer with attached myenteric ganglia was peeled off under the dissecting microscope using fine forceps. This preparation and the remaining inner layers of the intestine containing the submucous plexus were washed in PBS and processed free-floating for immunolabeling with TLR3, TLR4, and TLR7.
Whole-mount preparations were permeabilized in TBS containing 0.3% Triton X-100 (TBS-T) for 30 min, and incubated in 0.3% H2O2 in TBS for 30 min to inactivate endogenous peroxidase and then in 0.1% Na2B4O7 in TBS for 10 min to remove excess aldehydes. After rinsing in TBS-T, nonspecific sites were blocked, incubating samples for 30 min in a solution consisting of 0.05 M Tris-HCl, 0.15 M NaCl, 0.1% gelatin, 0.5% ovalbumin, 0.05% Tween-20, and 0.2% fish gelatin. Whole-mounts were then incubated for 36 hr at 4C with one of the following primary antibodies: rabbit anti-mouse TLR4 diluted 1:50 in TBS-T, rabbit anti-mouse TLR3 diluted 1:50 in TBS-T, or rabbit anti-mouse TLR7 diluted 1:100 in TBS-T. After several rinsings in TBS-T, samples were incubated with the secondary antibody, goat anti-rabbit diluted 1:100 in TBS-T for 2 hr at room temperature, and then with rabbit PAP diluted 1:100 in TBS-T for 2 hr at room temperature. The reaction was developed using the liquid DAB substrate chromogen system. Preparations were collected on silane-coated slides, air dried, and mounted with entellan.
Immunohistochemical reactions on whole-mount preparations and paraffin sections were observed with a Nikon Eclipse 80i (Nikon; Tokyo, Japan) equipped with bright-field high-quality objectives [20×, Nikon, Plan Fluor, numerical aperture (NA) 0.50; 40×, Nikon, Plan Fluor, NA 1, oil immersion; 60×, Nikon, Plan Apo, NA 1.40, oil immersion]. Images were acquired by digital camera (Nikon Digital Sight 5MC) and image acquisition software (ACT-2U).
Evaluation of immunofluorescence samples was performed with a Nikon C1 laser scanning confocal unit (Nikon D-Eclipse C1) attached to an inverted fluorescence microscope (Nikon Eclipse Ti-U) with a 60× objective (Nikon, Plan Apo, NA 1.40, oil immersion). Excitation was obtained with an air-cooled argon laser (488 nm) and a helium/neon laser (561 nm). Appropriate filter sets were used to collect fluorophore emissions. Conditions were set to avoid photo bleaching. Images were acquired digitally and processed using the operation software EZ-C1 for Nikon C1 confocal microscope. For each field of observation, 40 consecutive optical sections were obtained (steps of 0.20 μm). Three-dimensional images and galleries of the optical sections were analyzed to evaluate the expression of TLR3, -4, and -7 in GFAP-positive cells.
Quantitative Evaluation of TLR3, TLR4, and TLR7 Immunohistochemistry in Murine Myenteric Plexus
To evaluate differences in the level of immunostaining between the proximal small bowel and distal large bowel, staining intensity was computed as optical density (OD) and measured in 21 samples for the large intestine and 18 samples for the small intestine.
Digitally fixed images of slices at 40× magnification were analyzed using an image analyzer (Image Pro Plus 4.5.019, Media Cybernetics Inc.; Silver Spring, MD). For each ganglion, the unbiased OD was calculated by evaluating its OD and subtracting from this value the OD of an equivalent area surrounding the ganglion.
Data are presented as mean ± SEM. Statistical significance was evaluated by Student's t-test for unpaired samples and set at 1% (p=0.01).
Semiquantitative Evaluation of TLR4 Immunohistochemistry in Murine Myenteric Plexus
For semiquantitative evaluation of TLR4 immunostaining of murine myenteric plexus, sections from proximal small bowel and distal large bowel were scored independently by two observers for labeling intensity as follows: −, undetectable immunostaining; +, moderate immunostaining; ++, strong immunostaining.
Western Blotting
Proteins were extracted from DRGs by lysis in 0.001 M Tris-HCl, pH 7.6, 0.1 M NaCl, 0.001 M EDTA, pH 8, 1% Triton X-100, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 10 mg/ml pepstatin, and 100 mg/ml phenylmethylsulfonylfluoride for 1 hr at 4C, followed by centrifugation for 15 min at 5000 rpm at 4C. The supranatant was then harvested at −80C. Proteins were estimated quantitatively using the BCA Protein Assay Kit (Pierce; Rockford, IL), measuring at 562 nm with a spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences; Little Chalfont, UK). Protein samples (10 μg) were fractionated on an 8% acrylamide slab gel containing 0.1% SDS (Sigma-Aldrich) and transferred onto a nitrocellulose filter (Amersham Biosciences). As positive control, spleen extract was treated as described above. After incubation for 1 hr in TBS with 1% Tween-20 (Sigma-Aldrich) and 5% milk powder to block nonspecific binding sites, the filter was incubated with primary antibodies directed against rabbit anti-mouse TLR4 (1:200 in TBS, 0.1% Tween-20, and 1% milk powder) for 2 hr, and against rabbit anti-mouse TLR3 and rabbit anti-mouse TLR7 (both 1:500 in 1% TBS) for 1 hr at room temperature. After three washes of 20 min each in TBS, 1% Tween-20, and 1% milk powder, the filter was incubated with a goat anti-rabbit peroxidase-conjugated secondary antibody (1:1000 in TBS, 0.1% Tween-20, and 5% milk powder) for 1 hr at room temperature and then washed three times in 1% TBS for 10 min. Bands were visualized using enhanced chemiluminescence Western blotting detection reagents and autoradiography film (Amersham Biosciences).
Results
TLR3, TLR4, and TLR7 Immunolocalization in the Neural Plexuses of Murine Intestine and Human Ileum
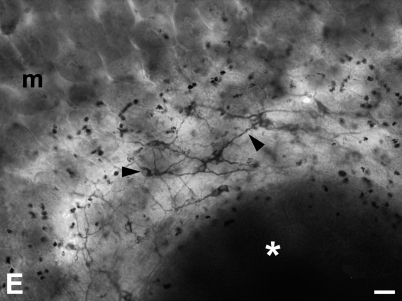
Immunolocalizations performed on paraffin-embedded tissue (Figures 1 and 2) and whole-mount preparations (Figure 3), revealed that TLR3, -4, and -7 are expressed in the myenteric and submucous plexuses of murine small and large intestine. In whole-mount preparations, it was clearly visible that ganglia and nerve fibers connecting them were immunolabeled, as well as nerve fibers traveling in the muscle layers and in the submucosa (Figure 3). In the latter, association of immunopositive neurons with Peyer's patches was also observed (Figure 3E). Many TLR3-immunopositive fibers were endowed with varicosities (Figure 3A). TLR3 and TLR7 expression could also be detected in the myenteric plexus of the human ileum (Figure 4).
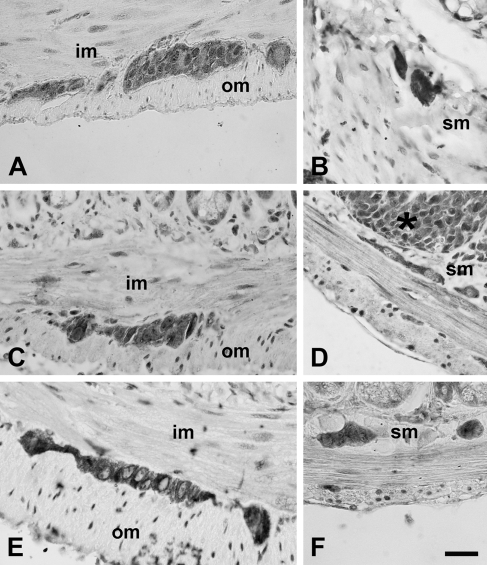
Figure 1.
Immunohistochemical labeling for Toll-like receptor 3 (TLR3) (A,B), TLR4 (C,D), and TLR7 (E,F) in the myenteric plexus (left column) and in the submucous plexus (right column) of murine intestine. im, inner muscle layer; om, outer muscle layer; sm, submucosa. Asterisk, Peyer's patch. Bar = 30 μm.
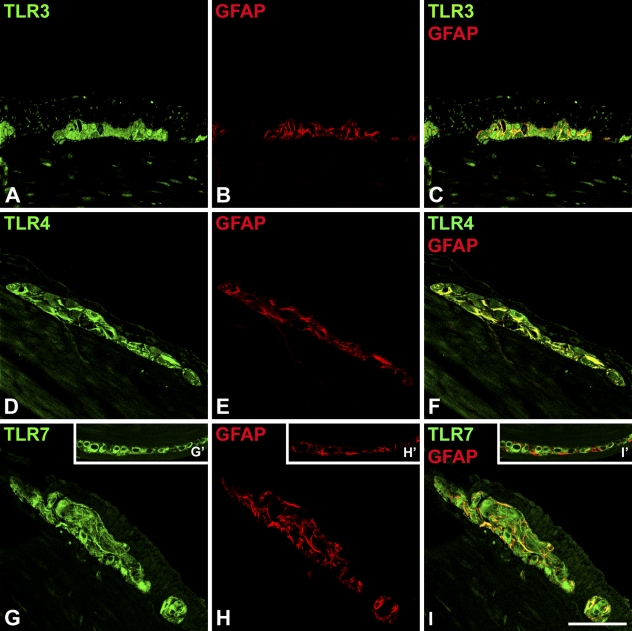
Figure 2.
Confocal scanning microscopy analysis of TLR3 (A), TLR4 (D), TLR7 (G), and glial fibrillary acidic protein (GFAP) (B,E,H) in murine myenteric plexus. For each antigen, a single optical confocal section is shown. Merged images (C,F,I) evidence colocalization of TLRs and GFAP (yellow). In the inserts (G′,H′,I′), a plexus where glial cells are negative for TLR7 is shown. Bar = 30 μm.
Figure 3.
Whole-mount immunohistochemistry of murine small bowel. (A–C) Whole-mount preparations of murine myenteric plexus and outer muscle layer showing ganglia and strands of nerve fibers positive for TLR3 (A), TLR4 (B), and TLR7 (C). (A) Arrows indicate nerve fibers with varicosities. In the right column, two examples of whole-mount preparations of the submucous plexus and inner smooth muscle layer are shown. (D) Immunostaining for TLR3. (E) TLR7-immunopositive submucous plexus (arrowheads) surrounding a Peyer's patch (asterisk). m, mucosa where flattened villi are visiible. Bars: C,D = 30 μm; E = 120 μm.
Figure 4.
Immunolabeling for TLR3 (A) and TLR7 (B) in the myenteric plexus of the human intestine. Bar = 30 μm.
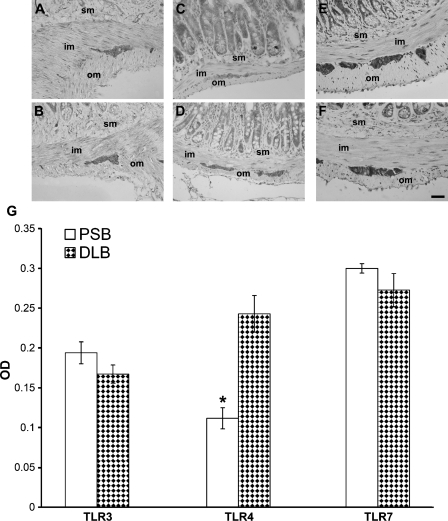
In murine samples, the intensity of TLR4 immunostaining was stronger in the distal colon (Figure 5D) than in the proximal small bowel (Figure 5C), where weaker labeling intensity and lack of staining in some ganglia were visible. Semiquantitative (Table 1) and quantitative (Figure 5G) evaluations performed on sections from the proximal small bowel and from the distal large bowel confirmed this finding. Conversely, intensity of TLR3 (Figures 5A, 5B, and 5G) and TLR7 (Figures 5E–5G) immunostaining did not vary along the intestinal axis.
Figure 5.
Immunolabeling for TLR3 (A,B), TLR4 (C,D), and TLR7 (E,F) in murine proximal small bowel (upper row) and distal large bowel (lower row). TLR4 immunostaining appears to be stronger in the distal large bowel (D). im, inner muscle layer; om, outer muscle layer; sm, submucosa. Bar = 30 μm. (G) Quantitative analysis of TLR3, TLR4, and TLR7 immunostaining in proximal small bowel (PSB) and distal large bowel (DLB), determined by optical density (OD). Values are presented as mean ± SEM. Statistically significant difference was reached only for TLR4. *p<0.01.
Table 1.
Semiquantitative analysis of TLR4 immunolabeling of murine myenteric plexus in proximal small bowel and distal large bowela
| Number of ganglionated plexuses evaluated | − | + | ++ | |
|---|---|---|---|---|
| PSB | 18 | 11.1% | 22.2% | 66.7% |
| DLB | 21 | 0 | 9.5% | 90.5% |
++, strong immunostaining; +, moderate immunostaining; −, undetectable immunostaining.
TLR4, Toll-like receptor 4; PSB, proximal small bowel; DLB, distal large bowel.
Double immunofluorescence of TLRs with the glial cell marker GFAP in murine tissue showed that GFAP-positive profiles were also positive for TLR3 and TLR4, whereas colocalization with TLR7 was not always present (Figure 2).
Immunolabeling could also be observed in Peyer's patches, in cells of probable macrophagic and lynfocitic nature in the submucosa, and in the epithelial lining. Labeling for TLRs was mostly cytoplasmic. However, in paraffin-embedded tissue, but apparently not in whole mounts, nuclear staining was present in some neurons (most notably for TLR3) and in smooth muscle cells. Specificity of the immunolabeling was verified using positive and negative controls as described in Materials and Methods. The different types of fixation did not change the quality of the immunoreactivity.
Expression of TLR4, TLR3, and TLR7 in Murine DRGs
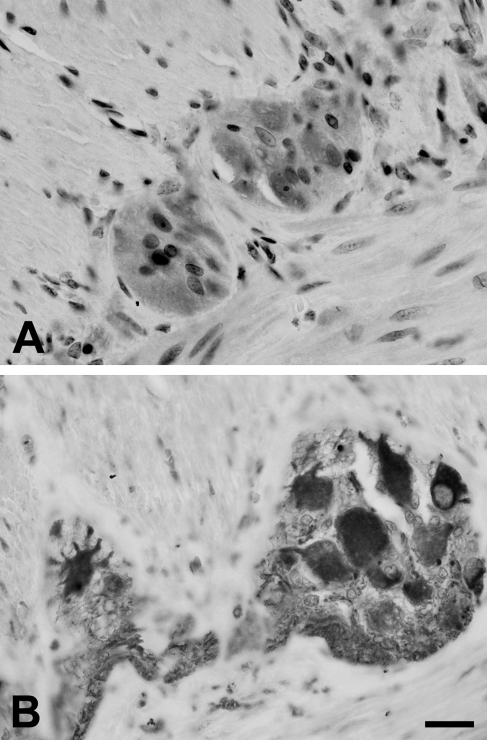
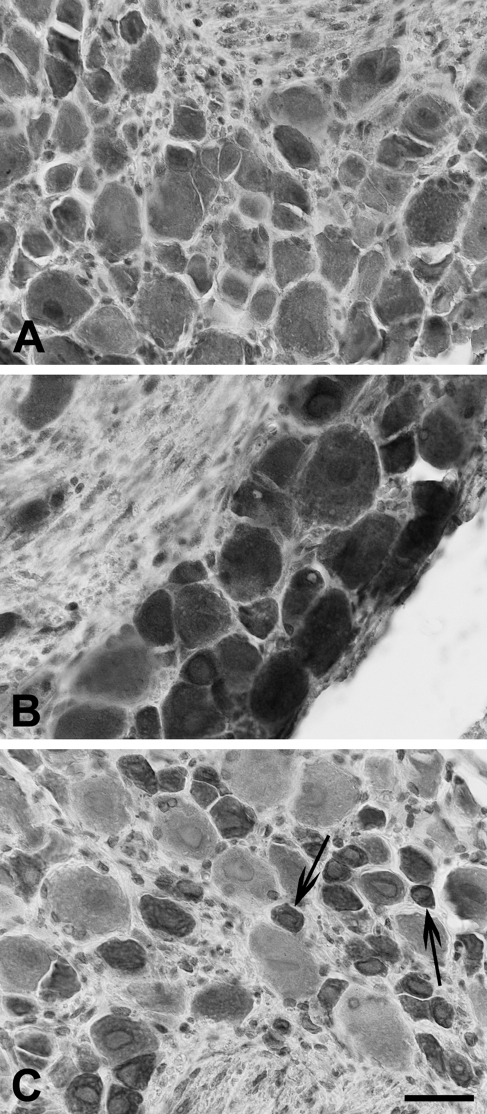
Immunohistochemistry (Figure 6) and Western blot analysis (Figure 7) showed that murine lower thoracic and lumbosacral DRGs express TLR3, TLR4, and TLR7.
Figure 6.
Immunolabeling for TLR3 (A), TLR4 (B), and TLR7 (C) in murine dorsal root ganglia (DRGs). In C, arrows indicate small-sized neurons strongly reactive for TLR7. Bar = 30 μm.
Figure 7.
Western blot analysis of protein extract from mouse lower thoracic and lumbosacral DRGs, probed with the antibodies anti-TLR4 (A), anti-TLR3 (B), and anti-TLR7 (C). Spleen protein extract was used as positive control.
Cytoplasmic staining of neuronal cell bodies was often associated with nuclear or perinuclear labeling. Nerve fibers were also immunoreactive. TLR4 immunolabeling was very strong in neurons of all sizes (Figure 6B), whereas for TLR3, the intensity of the immunostaining was more heterogeneously distributed (Figure 6A). In the case of TLR7, small-sized neurons were strongly reactive, compared with those of larger size, whose immunolabeling was much weaker or absent (Figure 6C).
Discussion
In the present study, we show for the first time that TLR3 and TLR7, recognizing viral RNA, are expressed in neurons and glial cells of the myenteric and submucous plexuses of murine small and large bowel and in plexuses of the human ileum. We also show that TLR4, besides being expressed in the neurons of the myenteric plexus of the murine jejunum (Rumio et al. 2006), is also expressed in the remaining intestine and in the submucous plexus, in both neurons and glial cells, and that the intensity of its immunolabeling is stronger in the distal large bowel. Furthermore, we report the expression of the three receptors in lower thoracic and lumbosacral murine DRGs.
Expression of TLRs by the enteric neural network highlights the presence of a TLR-based neural surveillance system hardwired in the intestinal wall, and strongly suggests that viral and bacterial agents could directly activate intestinal neural responses without the interposition of immune or epithelial cells. The close proximity to a physiological and potentially pathological microbial environment might partly explain the difference in TLR expression between the ENS and the central nervous system (CNS). In the latter, relevant TLR expression can be observed in neurons only during pathological conditions (Jackson et al. 2006; Lafon et al. 2006), whereas it is constitutively present in glial cells and microglia (Bsibsi et al. 2002; Kielian 2006).
Closeness to a heavily loaded microbial environment (Hooper and Gordon 2001; Wood 2004) and adaptation to microorganisms normally present in the intestinal lumen might also explain the finding of stronger TLR4 immunolabeling in the distal colon, as also suggested by Ortega-Cava et al. (2003), who observed the same gradient in TLR4 mRNA and protein expression in the gut mucosa.
To date, cellular localization of TLR3 and TLR7 in the intestinal wall has not been much described and, to our knowledge, only Furrie et al. (2005) reported TLR3 expression in mature epithelial cells and lamina propria cells. Also, although TLR involvement in bacterial infections of the gastrointestinal tract has been addressed in several studies (Cario 2005; Harris et al. 2006), much less is known about the contribution of TLRs to the intestinal responses to viruses (Harris et al. 2006). The gastrointestinal tract provides the main route of access to the host for several viruses (Oberste et al. 2000), including the human immunodeficiency virus (Smith et al. 2003) and poliovirus (Mueller et al. 2005), and for unconventional viruses or agents such as those involved in prion diseases (McBride et al. 2001). These viruses can cause gastroenteritis, which is characterized by electrolyte and fluid secretion that seems to be triggered by local neural reflexes whose mechanism of activation is at present unknown (Morris and Estes 2001; Kordasti et al. 2004). Some enteroviruses also show different degrees of neurotropism and neurovirulence that can lead to overt cytotoxic effects, latent infection, or viral spreading to the CNS (Irie et al. 1989; Morrison et al. 1991; Chen et al. 2003; Holland-Cunz et al. 2006; Selgrad et al. 2009). Although we did not perform any functional evaluation of TLR3 and TLR7 expression in enteric neurons and glial cells in this study, it is likely that they may be part of the signaling pathways that neurons have developed to respond to viral invasions (Patterson et al. 2002; Orvedahl and Levine 2008). In the CNS, a defensive role for TLR3 in neurons and glial cells has been demonstrated (Carpentier et al. 2005; Lafon et al. 2006; Daffis et al. 2008), whereas for TLR7, very few data are available (Lewis et al. 2008).
In our study, we observed widespread cytoplasmic labeling of neurons and fiber tracts connecting ganglia. Very little is known about the intracellular trafficking of TLRs in neurons (Lafon et al. 2006; Ménager et al. 2009). However, data are available from other cell types. Viral TLRs mostly localize to the endoplasmic reticulum and to endosomes where contact with viral nucleic acids takes place (Kawai and Akira 2007). In neurons, endocytic activity is also present in the somatodendritic domain, and following endocytosis, endosome-containing viral particles have been shown in the different compartments of the cell, including dendrites and axon (Lewis and Lentz 1998). TLR4 is classified as a membrane receptor. Nonetheless, there are several reports that indicate a cytoplasmic localization as well, including the Golgi apparatus and microtubules (Uronen-Hansson et al. 2004; Hassan et al. 2006).
Under normal conditions, the ENS does not appear to be directly exposed to the luminal content, inasmuch as neural projections into the intestinal lumen have not been demonstrated. The first possible site of interaction between enteric neurons and microbial components seems therefore to be limited to the subepithelial compartment. However, several pathological events of infective and non-infective nature cause disruption of the epithelial layer integrity (Svensson et al. 2004; Laukoetter et al. 2008), and bacteria and viruses can also translocate across the integer epithelial barrier by way of specialized cells able to convey them to underlying immune cells located in the mucosa and Peyer's patches (Bomsel and Alfsen 2003; Niedergang and Kweon 2005). At these sites, close apposition between immune cells and nerve fibers has been demonstrated (Ma et al. 2007; Vulchanova et al. 2007). In addition to pathological conditions, bacterial translocation is also normally present and contributes to gut-associated lymphoid tissue development, induction of tolerance to commensal microbiota, and constant stimulation of immune cells (Macpherson and Uhr 2004a,b). Physiological “close encounters” between enteric neurons and commensal microbiota are therefore not unlikely and might be at the base of a steady stimulation of neuroimmunomodulatory mechanisms participating in gut homeostasis. Modulatory effects of prebiotics and probiotics on nerve cells have already been demonstrated for enteric neurons (Kamm et al. 2004), DRGs (Kamiya et al. 2006), and rat brain neurons or pheochromocytoma-differentiated cells (Sobol et al. 2005).
We also investigated TLR3, TLR4, and TLR7 expression in lower thoracic and lumbosacral DRGs. This part of the study was undertaken to determine whether spinal ganglia harboring neurons projecting to the intestine expressed TLRs and for the more general purpose of ascertaining whether TLR expression could be considered a somehow diffuse feature of the adult peripheral nervous system. Although we did not specifically identify primary sensory neurons projecting to the intestine, it is reasonable to assume that they express the three receptors on the basis of the following observations: different degrees of TLR3 and TLR4 immunoreactivity could be observed in all DRGs neurons examined; and strong TLR7 immunostaining was present in all small- to medium-sized neurons—the size range of visceral sensory neurons.
The presence of a direct mechanism of recognition of MAMPs in primary sensory neurons could be very meaningful when considering both the afferent function and efferent function of these cells (Holzer and Maggi 1998). By directly recognizing a characteristic microbial component and not only a generic inflammatory event, DRGs sensory neurons could be the first element in a labeled neural pathway, conveying to the CNS early and specific information on the nature and localization of microorganisms detected in the intestinal wall. Involvement of sensory pathways in conveying non-circulating microorganism-related signals has already been shown for the vagus nerve (Goehler et al. 2005,2007), and because its sensory ganglion expresses TLR4 (Hosoi et al. 2005), a direct activation by pathogens has been hypothesized (Goehler et al. 2007). It has also been shown that electrophysiological responses of DRGs sensory neurons projecting to the intestine can be modulated by oral inoculation of probiotics (Kamiya et al. 2006).
As to their efferent function, our findings suggest that TLRs might be part of the signaling pathways through which sensory neurons participate in rapid protective actions of the intestinal wall. These actions include activation of local axon reflexes such as the anti-inflammatory reflex and peripheral release from sensory endings of different mediators and neuropeptides in response to local threats (Holzer and Maggi 1998; Holzer 2006,2007).
Acknowledgments
We thank Dr. Michele Sciarabba for help with quantitative analysis of TLR4 immunostaining, Marianna Gaman for help in tissue processing, and Dr. Elena Donetti for kindly revising the manuscript.
References
- Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D (2004) Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16(suppl 1):28–33 [DOI] [PubMed] [Google Scholar]
- Bomsel M, Alfsen A (2003) Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol 4:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013–1021 [DOI] [PubMed] [Google Scholar]
- Cario E (2005) Bacterial interaction with cells of the intestinal mucosa: Toll-like receptors and Nod2. Gut 54:1182–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49:360–374 [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, LaMont JT, Letourneau R, Kelly C, O'Keane JC, Jaffer A, Theoharides TC, et al. (1994) Neuronal involvement in the intestinal effects of clostridium difficile toxin A and vibrio cholerae enterotoxin in rat ileum. Gastroenterology 107:657–665 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Gershon AA, Li ZS, Lungu O, Gershon MD (2003) Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J Med Virol 70(suppl 1):S71–78 [DOI] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Gale M Jr, Diamond MS (2008) Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol 82:10349–10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB (2000) Types of neurons in the enteric nervous system. J Auton Nerv Syst 81:87–96 [DOI] [PubMed] [Google Scholar]
- Furrie E, Macfarlane S, Thomson G, Macfarlane GT, Microbiology & Gut Biology Group, Tayside Tissue & Tumour Bank (2005) Toll-like receptors 2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology 115:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR (2000) Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci 85:49–59 [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M (2005) Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun 19:334–344 [DOI] [PubMed] [Google Scholar]
- Goehler LE, Lyte M, Gaykema RP (2007) Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun 21:721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, KuoLee R, Chen W (2006) Role of Toll-like receptors in health and diseases of gastrointestinal tract. World J Gastroenterol 12:2149–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F, Islam S, Tumurkhuu G, Naiki Y, Koide N, Mori I, Yoshida T, et al. (2006) Intracellular expression of toll-like receptor 4 in neuroblastoma cells and their unresponsiveness to lipopolysaccharide. BMC Cancer 8:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland-Cunz S, Goppl M, Rauch U, Bar C, Klotz M, Schafer KH (2006) Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J Pediatr Surg 41:e29–e31 [DOI] [PubMed] [Google Scholar]
- Holzer P (2006) Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci 125:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P (2007) Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol 7:563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Maggi CA (1998) Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience 86:389–398 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 292:1115–1118 [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Matsuda T, Nomura Y (2005) Novel pathway for LPS-induced afferent vagus nerve activation: possibile role of nodose ganglion. Auton Neurosci 120:104–107 [DOI] [PubMed] [Google Scholar]
- Irie H, Harada Y, Yoshihashi H, Kimura T, Kojima M, Kataoka M, Saito M, et al. (1989) Spread of herpes simplex virus type-1 (Miyama +GC strain) to the central nervous system after intraperitoneal inoculation: the role of the myenteric plexus of the gut. Arch Virol 105:247–257 [DOI] [PubMed] [Google Scholar]
- Jackson AC, Rossiter JP, Lafon M (2006) Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol 12:229–234 [DOI] [PubMed] [Google Scholar]
- Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, et al. (2006) Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 55:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K, Hoppe S, Breves G, Schröder B, Schemann M (2004) Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil 16:53–60 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S (2007) Antiviral signaling through pattern recognition receptors. J Biochem 141:137–145 [DOI] [PubMed] [Google Scholar]
- Kielian T (2006) Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 83:711–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordasti S, Sjöval H, Lundgren O, Svensson L (2004) Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut 53:952–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon M, Megret F, Lafage M, Prehaud C (2006) The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci 29:185–194 [DOI] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Nusrat A (2008) Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol 14:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Lentz TL (1998) Rabies virus entry into cultured rat hippocampal neurons. J Neurocytol 27:559–573 [DOI] [PubMed] [Google Scholar]
- Lewis SD, Butchi NB, Khaleduzzaman M, Morgan TW, Du M, Pourciau S, Baker DG, et al. (2008) Toll-like receptor 7 is not necessary for retroviral neuropathogenesis but does contribute to virus-induced neuroinflammation. J Neurovirol 17:1–11 [DOI] [PubMed] [Google Scholar]
- Lomax AE, Linden DR, Mawe GM, Sharkey KA (2006) Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci 126–127:250–257 [DOI] [PubMed] [Google Scholar]
- Ma B, von Wasielewski R, Lindenmaier W, Dittmar KE (2007) Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol 36:62–74 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T (2004a) Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci 1029:36–43 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T (2004b) Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662–1665 [DOI] [PubMed] [Google Scholar]
- McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M (2001) Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol 75:9320–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménager P, Roux P, Mégret F, Bourgeois JP, Le Sourd AM, Danckaert A, Lafage M, et al. (2009) Toll-like receptor 3 (TLR3) plays a major role in the formation of rabies virus Negri Bodies. PLoS Pathog 5:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Estes MK (2001) Microbes and microbial toxins: paradigms for microbial-mucosal interactions.VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am J Physiol Gastrointest Liver Physiol 281:G303–310 [DOI] [PubMed] [Google Scholar]
- Morrison LA, Sidman RL, Fields BN (1991) Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci USA 88:3852–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wimmer E, Cello J (2005) Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus Res 111:175–193 [DOI] [PubMed] [Google Scholar]
- Neunlist M, Van Landeghem L, Bourreille A, Savidge T (2008) Neuro-glial crosstalk in inflammatory bowel disease. J Intern Med 263:577–583 [DOI] [PubMed] [Google Scholar]
- Niedergang F, Kweon MN (2005) New trends in antigen uptake in the gut mucosa. Trends Microbiol 13:485–490 [DOI] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Flemister MR, Marchetti G, Kilpatrick DR, Pallansch MA (2000) Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J Clin Microbiol 38:1170–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Cava CF, Ishihara Shunji Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, et al. (2003) Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 170:3977–3985 [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Levine B (2008) Autophagy and viral neurovirulence. Cell Microbiol 10:1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Daley JK, Rall GF (2002) Neuronal survival strategies in the face of RNA viral infection. J Infect Dis 186(suppl 2):215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C, Castigliuolo I, LaMont JT (1998) Nerves and intestinal mast cells modulate responses to enterotoxins. News Physiol Sci 13:58–63 [DOI] [PubMed] [Google Scholar]
- Rumio C, Besusso D, Arnaboldi F, Palazzo M, Selleri S, Gariboldi S, Akira S, et al. (2006) Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. J Cell Physiol 178:47–54 [DOI] [PubMed] [Google Scholar]
- Selgrad M, De Giorgio R, Fini L, Cogliandro RF, Williams S, Stanghellini V, Barbara G, et al. (2009) JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut 58:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Meng G, Salazar-Gonzalez JF, Shaw GM (2003) Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leukoc Biol 74:642–649 [DOI] [PubMed] [Google Scholar]
- Sobol CV, Belostotsskaya GB, Kenworthy MW (2005) Calcium signalling in rat brain neurons and differentiation of PC-12 cells induced by application of a probiotic product. Neurophysiol 3:284–293 [Google Scholar]
- Svensson L, Bergquist J, Wennerås C (2004) Neuromodulation of experimental Shigella infection reduces damage to the gut mucosa. Microbes Infect 6:256–264 [DOI] [PubMed] [Google Scholar]
- Uronen-Hansson H, Allen J, Osman M, Squires G, Klein N, Callard RE (2004) Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology 111:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, et al. (2006) Enteric neuroplasticity evoked by inflammation. Auton Neurosci 126–127:264–272 [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Casey MA, Crabb GW, Kennedy WR, Brown DR (2007) Anatomical evidence for enteric neuroimmune interactions in Peyer's patches. J Neuroimmunol 185:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD (2004) Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 127:635–657 [DOI] [PubMed] [Google Scholar]