Abstract
An essential tool for investigating the role of a gene during development is the ability to perform gene knockdown, overexpression, and misexpression studies. In zebrafish (Danio rerio), microinjection of RNA, DNA, proteins, antisense oligonucleotides and other small molecules into the developing embryo provides researchers a quick and robust assay for exploring gene function in vivo. In this video-article, we will demonstrate how to prepare and microinject in vitro synthesized EGFP mRNA and a translational-blocking morpholino oligo against pkd2, a gene associated with autosomal dominant polycystic kidney disease (ADPKD), into 1-cell stage zebrafish embryos. We will then analyze the success of the mRNA and morpholino microinjections by verifying GFP expression and phenotype analysis. Broad applications of this technique include generating transgenic animals and germ-line chimeras, cell-fate mapping and gene screening. Herein we describe a protocol for overexpression of EGFP and knockdown of pkd2 by mRNA and morpholino oligonucleotide injection.
Keywords: Developmental Biology, Issue 27, Zebrafish, microinjection, morpholino antisense oligonucleotide, gene overexpression, gene knockdown
Protocol
Part 1: Preparation of micropipettes, and microinjection chamber plates
Fabricate micropipettes by heating and pulling borosilicate glass capillary tubes (World Precision Instruments, Inc., 1B100-4) in a micropipette puller device (Sutter Instruments Inc., Flaming/Brown P-97). Store in a Petri dish on top of small amount of clay or adhesive tape.
Pour 1.5% agarose (American Bioanlytical, Inc., AB00972-00500) in 1x E3 medium plates molded with wedge-shaped troughs that will serve as an easy method for holding the embryos during the injection (as described in 'The Zebrafish Book") 1. Let agar chamber plates solidify before use and store at 4ºC.
Part 2: Preparation of RNA
One powerful approach for gene function studies is to microinject in vitro transcribed capped RNA in zebrafish embryos. Capped RNA behaves similarly to eukaryotic mRNAs found in vivo due to the presence of the CAP analog. Zebrafish researchers routinely utilize this method to overexpress or misexpress their gene of interest. In this demonstration, we will microinject an EGFP-tagged transcript and use live whole-embryo GFP-expression as a visible readout for a successful injection.
Perform an in vitro CAP RNA transcription reaction on your transcript of interest. In our lab, we routinely insert a cDNA of interest into a pCS2+ vector and perform an in vitro synthesis reaction of large amounts of capped RNA using the mMessage mMachine SP6 kit (Ambion, Inc., AM1340).
Purify the RNA sample by running it through the RNeasy Mini kit (Qiagen, Inc., 74104) or by phenol:chloroform extraction and isopropanol precipitation.
Carefully determine the concentration of the RNA preparation and store at -80ºC until ready for use.
On the day of the injection, thaw the RNA sample, vortex lightly and spin down briefly.
Prepare a working solution with sterile water and a final concentration of 0.025 - 0.050% phenol red (Sigma-Aldrich Co., P0290). Phenol red serves as a visible marker for the injection of the solution into the embryo. In this article, we will prepare a working solution of 100 ng/uL EGFP mRNA with 0.050% phenol red.
Keep working sample on ice and return RNA stock to -80°C.
Proceed to Part 4 when ready to inject this sample.
Part 3: Preparation of morpholino
Morpholino antisense oligonucleotides are widely used to modify gene expression by blocking translation of a targeted protein or by modifying pre-mRNA splicing 2,3. Morpholinos in the zebrafish serve as a powerful reverse genetics tool by knocking down gene function. In this article, we will microinject a morpholino oligo targeted to the translational initiation site of pkd2 (5′-AGGACGAACGCGACTGGAGCTCATC-3′). Based on previously established work, we expect the injected embryos to phenotypically mimic pkd2 mutant fish4.
We routinely order 300 nmol of a morpholino oligo specifically designed for a gene of interest from Gene Tools, LLC (Philomath, OR).
Add 100 uL sterile water to make a 3 mM stock solution. Aliquot the solution and store at -20°C until ready for use.
On the day of the injection, heat the morpholino solution at 65°C for 5 minutes. Snap cool on ice immediately and spin briefly. This step denatures any secondary structures in the oligo and ensures that the solution is completely solubilized.
Prepare a working solution by diluting the morpholino solution in sterile water and a final concentration of 0.025 - 0.050% phenol red. In this demonstration, we will be preparing a working solution of 0.50 mM pkd2 morpholino with 0.050% phenol red.
Keep working solution at room temperature.
Proceed to Part 4 when ready to inject this sample.----
Part 4: Filling the micropipette with your working solution
Cut the distal tip of a micropipette with forceps or a surgical razorblade to create an opening that is just visible under a dissecting microscope (Nikon Instruments, Inc., SM2645) at 50X magnification.
Place 2 uL of your working solution as a drop onto a coverslip.
Attach the back of a micropipette to a 5 mL syringe fitted with tubing on a hypodermic needle and slightly submerge the distal tip of the micropipette into your drop of working solution. Take care not to break the tip of the micropipette.
Siphon the working solution through the distal end of the micropipette by pulling the plunger of the syringe.
Part 5: Calibrating the micropipette injection volume
Place a drop of mineral oil (American Bioanalytical, Inc., AB00921-00025) on a micrometer slide (Fisher, 12-561-SM1).
Turn on power and air supply to the pressure-pulsed micro injector apparatus (World Precision Instruments Inc., PV830).
Attach the micropipette to the micropipette holder of the micro injector apparatus. The micro injector is pressure regulated and discharges are activated by pressing the foot pedal.
Under the dissection microscope, test the injection volume by using the micro injector to place a drop of your working solution onto the micrometer oil. Measure the diameter of the drop as it floats as a sphere on top of the oil.
Adjust the duration and pressure of injection to carefully calibrate your volume of injection. This step aids in the reproducibility of the microinjection experiment. In this demonstration, all microinjections will be done with a drop diameter of 0.15 mm (approximately 1.76 nL in volume).
Once your injection volume has been calibrated, use the “Hold” knob on the micro injector to prevent backfilling and leaking of the micropipette.
Part 6: Preparing fertilized zebrafish embryos for microinjection
Zebrafish will randomly mate in the first few hours of each morning. Collect embryos at the 1-cell stage and place them in 1x E3 medium.
Using a 3 mL transfer pipet (Becton Dickinson Labware, 357524), arrange the embryos along the wedge-shaped troughs in the microinjection chamber plates.
Remove the medium so that the embryos are shallowly submerged and not flooded. This step aids in settling the embryos to the bottom of the troughs as well as in the penetration of the chorion by the micropipette.
Part 7: Microinjection through the chorion
Manipulate the embryos with the micropipette so that the cytoplasm of the 1-cell stage embryo is visible under the dissecting microscope. Take care not to break the tip of the micropipette.
Penetrate the chorion and then the yolk with the micropipette in order to inject into the embryo. In this demonstration, we will be first microinjecting into the yolk directly below the cell, and allow cytoplasmic flow and diffusion to bring the working solution into the cell. This flow is visible due to the phenol red added to the working solution.
We will also demonstrate direct injection into the cell cytoplasm. Direct microinjection into the cell is more robust but time-consuming as proper embryo orientation and microinjection technique is required. As the cell membrane is more rigorous than the yolk membrane, it is often quicker to enter the micropipette through the yolk to reach the cell cytoplasm. Orienting the animal pole towards the bottom of the trough and working with steady hands may aid in this method of injection.
After microinjection, place the embryos into a Petri dish with E3 medium and incubate them at 28.5°C for normal development.
Over the next several hours and days of embryo development, observe the embryos for your phenotypes of interest.
Part 8: Representative results
EGFP mRNA overexpression: To verify the success of the microinjection, we will monitor the expression of GFP in the developing embryos beginning at shield stage (~6 hpf) by in vivo whole-embryo fluorescence microscopy (Leica Microsystems GmBh, MZFLIII). Expression of the construct is most strong during the early events of embryonic development and will often diminish during development as the injected capped RNA is gradually degraded and depends on the stability of the expressed protein.
pkd2 translational-blocking morpholino: Based on previously published results, we expect the pkd2 morphants to mimic the dorsal body axis curvature as found in pkd2 mutant zebrafish and present kidney cysts at approximately 2 - 3 dpf4.
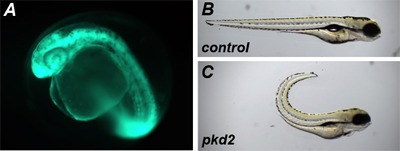 Figure 1: Representative results from microinjection of EGFP mRNA and pkd2 AUG morpholino. (a) TAB embryos were microinjected at the 1-cell stage with 0.17 ng of EGFP mRNA and visualized at 1 dpf for in vivo GFP expression. (b,c) TAB embryos were microinjected at the 1-cell stage with ~1.7 nl of a pkd2 translational blocking morpholino at 0.50 mM and scored for dorsal body curvature at 4 dpf.
Figure 1: Representative results from microinjection of EGFP mRNA and pkd2 AUG morpholino. (a) TAB embryos were microinjected at the 1-cell stage with 0.17 ng of EGFP mRNA and visualized at 1 dpf for in vivo GFP expression. (b,c) TAB embryos were microinjected at the 1-cell stage with ~1.7 nl of a pkd2 translational blocking morpholino at 0.50 mM and scored for dorsal body curvature at 4 dpf.
Discussion
Microinjection into zebrafish embryos is a well-established and robust technique for exploring the role of a particular gene in development. Applications include overexpression, misexpression, and knockdown assays of your gene of interest as well as epistatic analysis between multiple genes. Microinjection in zebrafish has been widely used for generating transgenic animals, and mapping cell fate in early blastula embryos5,6,7,8,9. In addition, the application of this technique serves as a key step in generating germ-line chimeras by cell transplantation methods10.
One alternative method for exploring a gene’s ‘gain-of-function’ phenotype is to microinject constitutively active forms of that gene. In addition, a gene’s pseudo ‘loss-of-function’ phenotype may be explored by microinjection of dominant negative forms of that gene. Microinjection into zebrafish embryos may also include DNA and small molecules11,12.
A critical step in this microinjection technique is the quality of the micropipette needle. We make our micropipette from capillary tubes shaped by a pipette puller device. It is essential that the pipette puller is properly calibrated to yield optimal needle shape and size. Micropipettes that are too long and narrow often lack rigidity, break easily and struggle to penetrate the chorion and yolk. Short micropipettes are often more prone to damaging the embryo during microinjection.
Acknowledgments
This work was supported by the NIH and PKD foundation to ZS. All animal experiments were conducted according to Yale Animal Resources Center (YARC) and Institutional Animal Care and Use Committee (IACUC) guidelines.
References
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) 4th. University of Oregon; 2000. [Google Scholar]
- Nasevicius A, Ekker S. Effective targeted gene 'knockdown' in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Draper B, Morcos P, Kimmel C. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- Sun Z, et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- Stuart G, McMurray J, Westerfield M. Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development. 1988;103:403–412. doi: 10.1242/dev.103.2.403. [DOI] [PubMed] [Google Scholar]
- Stuart G, Vielkind J, McMurray J, Westerfield M. Stable lines of transgenic zebrafish exhibit reproducible patterns of transgene expression. Development. 1990;109:577–584. doi: 10.1242/dev.109.3.577. [DOI] [PubMed] [Google Scholar]
- Culp P, Nüsslein-Volhard C, Hopkins N. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc Natl Acad Sci U S A. 1991;88:7953–7957. doi: 10.1073/pnas.88.18.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehlow D, Heinrich G, Gilbert W. The fates of the blastomeres of the 16-cell zebrafish embryo. Development. 1994;120:1791–1798. doi: 10.1242/dev.120.7.1791. [DOI] [PubMed] [Google Scholar]
- Helde K, Wilson E, Cretekos C, Grunwald D. Contribution of early cells to the fate map of the zebrafish gastrula. Science. 1994;265:517–520. doi: 10.1126/science.8036493. [DOI] [PubMed] [Google Scholar]
- Lin S, Long W, Chen J, Hopkins N. Production of germ-line chimeras in zebrafish by cell transplants from genetically pigmented to albino embryos. Proc Natl Acad Sci U S A. 1992;89:4519–4523. doi: 10.1073/pnas.89.10.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q, et al. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- Murphey R, Zon L. Small molecule screening in the zebrafish. Methods. 2006;39:255–261. doi: 10.1016/j.ymeth.2005.09.019. [DOI] [PubMed] [Google Scholar]


