Abstract
Introduction:
This study examined whether children with cancer are exposed to measurable levels of passive smoke as assessed by parent report and laboratory measures of urine cotinine, an established biomarker of passive smoke exposure (PSE). It also determined whether parents/caretakers of young cancer patients can provide valid reports of their child's PSE during the child's treatment, by examining their association with urine cotinine measures.
Methods:
Participants included 124 parents of a child with cancer who lived with at least one adult smoker in the home and was exposed to tobacco smoke in the home and/or car. Eligible patients were younger than 18 years of age, were receiving active treatment for cancer at a large pediatric oncology institution, were at least 30 days postdiagnosis, and did not smoke. Parents provided information about smoking and their child's PSE by responding to a series of questionnaires. Patients provided urine samples for cotinine analyses.
Results:
Findings showed that parents provided valid short-term accounts of their child's PSE in the context of their child's cancer treatment. Parent reports of PSE showed moderately strong positive relationships with urine cotinine levels which were stronger for reports provided by parents who smoked compared with nonsmoking parents.
Discussion:
Parent reports of PSE were validated by positive and significant associations with urine cotinine. Reports provided in the context of possible verification by biomarker assays can provide sufficiently accurate estimates of PSE to serve as outcome measures for clinical research and clinical care in a pediatric cancer setting.
Introduction
Chronic passive smoke exposure (PSE) is a major public health concern that has been causally linked to premature death and disease in children and adults who do not smoke (U.S. Department of Health and Human Services [USDHHS], 2006). According to the 1999–2000 National Health and Nutrition Examination Survey, 24.9% of children aged 3–11 years and 19.9% of adolescents and young adults aged 12–19 years lived in households with at least one smoker (USDHHS, 2006). Prevalence of PSE in the home is highest among low income and minority populations (Centers for Disease Control and Prevention, 2008). Smoking parents are the most important sources of exposure among young children. Like healthy children, medically compromised children are exposed to toxic passive smoke, despite their increased vulnerability to the adverse health effects of exposure. Tyc et al. (2004) reported, for example, that approximately 45% of children with newly diagnosed cancer from 303 households lived with at least one smoking parent and that many parents smoked in the presence of their child.
PSE increases children's risk of pneumonia, bronchitis, respiratory illness, wheezing, middle ear effusions, and otitis media (Etzel, 1994; National Research Council, 1986; USDHHS, 1986; U.S. Environmental Protection Agency, 1992), and risk of complication increases with higher levels of exposure (DiFranza & Lew, 1996). A number of biological mechanisms that interfere with lung function and growth (Cook & Strachan, 1999) and immune responsiveness (USDHHS, 2006) have been postulated to explain how PSE causes injury and disease. Children with cancer may be especially vulnerable to these insults secondary to disease and treatment-related toxicities that may affect their pulmonary, respiratory, and cardiovascular functioning (Benoist et al., 1982; Lipshultz et al., 1991; O'Driscoll et al., 1990). In addition to greater risk for later cardiovascular or pulmonary disease (USDHHS, 1995), continued PSE may exacerbate pediatric cancer patients’ risk for developing second malignancies (Meisler, 1993; Neglia et al., 1991; Robison & Mertens, 1993).
Interventions designed to protect children from PSE are dependent on reliable measures of PSE. It is critical to know the social context in which PSE takes place and the time course or episodicity of both acute and chronic exposure. Such details are obtained only by observation in public settings or by report of persons routinely in such settings, such as parents in their private home. A number of behavioral, environmental, and biochemical measures have been used to quantify the magnitude of children's PSE in their homes and cars (Matt, Bernert, & Hovell, 2008). Parent reports are the most common method of assessing smoking behavior and exposure rates in the social and physical contexts of the young child (Hovell, Zakarian, Wahlgren, Matt, & Emmons, 2000). Parent reports are noninvasive, relatively inexpensive, and can be repeated over time. However, as is true of all measures, reports can be compromised by memory, distractions, or bias, where risk of penalty might lead parents to underestimate (or overestimate) level of PSE. Thus, it is important to assess the reliability and accuracy of reported measures to set the stage for clinical research and service programs aimed at reducing child PSE (Matt et al., 2000).
Past studies have demonstrated that smoking mothers can provide reliable and valid reports of sources and patterns of their child's PSE (Emerson et al., 1995; Emmons et al., 1992; Emmons, Hammond, & Abrams, 1994; Matt et al., 1999, 2000), with 20%–40% of the variance in biomarkers of exposure accounted for by quantitative parent reports of exposure. For example, Matt et al. (2000) reported correlations of .62 and .75 between mothers’ reported smoking rates and infant urine cotinine and air nicotine levels, respectively. However, no studies to date have examined quantitative parent reports of PSE for children undergoing cancer treatment.
Managing the demands of their child's cancer treatment may result in emotional stress that can reduce parental attention to and memory of ongoing PSE and/or increase parents’ smoking rate and level of child PSE. The social undesirability of exposing a sick child to PSE may also increase a parent's guilt about smoking in the presence of the child, particularly if the medical setting is one that discourages smoking and advises parents to stop smoking around their child. These conditions are likely to compromise the reliability of reports provided by parents of children with cancer as well as promote systematic underreporting on some occasions (Matt et al., 2000).
This study was designed to document the magnitude of PSE using both parent reports of exposure and laboratory assays of PSE for children undergoing treatment for cancer. It extends earlier research on PSE measurement by investigating whether parents/guardians can provide valid reports of the child's PSE during cancer treatment. In addition to validity tests, this study was designed to determine whether parent smoking status, demographic, or treatment-related variables alter the validity of parents’ reports of children's PSE. Results of the study will inform the generalizability of reported measures of PSE to medically vulnerable children and their families.
Methods
Participants
One-hundred and twenty-four parents or guardians of a child with cancer who lived with at least one adult smoker in the home and was exposed to tobacco smoke in the home and/or car participated in this study. Parents/guardians were eligible for participation regardless of their smoking status. Patients were eligible for this study if they were younger than 18 years of age, were receiving active treatment for cancer, were at least 30 days postdiagnosis, and were nonsmokers. Recruitment took place in the outpatient clinic of a large pediatric oncology hospital. Eligible families were invited to participate in a randomized intervention trial to reduce PSE among pediatric cancer patients. The data presented represent the baseline data for parents and children who agreed to participate in this trial.
A small group of nonsmoking patients (n = 29) who lived in nonsmoking households (cotinine control group) was also recruited in order to assess the validity of our urine cotinine measures. The cotinine control group met similar eligibility criteria as the study patients, with the exception that they lived in nonsmoking households. Cotinine control group patients were selected to be comparable to a random subsample of the study participants according to age (±1 year), gender, and race (White/non-White).
Procedure
Eligible parents were asked to provide information about smoking and their child's PSE by completing structured interviews, as described below. Patients provided urine samples for cotinine analyses.
Measures of PSE
The primary dependent variable in this study was the child's exposure to PSE from all persons who smoked in the child's environment. Parents provided information about their child's PSE in response to a 15-item PSE Questionnaire that included questions about the number of smokers in the home, parent/family smoking patterns and exposure, as well as home smoking rules. Specifically, parents were asked, “How many cigarettes did you smoke in your home and to how many was your child exposed?” They were also asked this question for each smoker living in or visiting the home and were similarly asked to report smoking and exposure occurring in the car. Exposure was defined as the number of cigarettes smoked in the same room or car as the child.
Parents were required to record a specific number of cigarettes smoked and exposed along a continuum ranging from yesterday through 7 days ago. However, the number of cigarettes smoked and the number of cigarettes to which the child was exposed over the previous 72 hr (3 days) was of primary interest for this study, as this timeframe was used to validate against measures of cotinine whose half-life falls within this window (Collier, Goldstein, Shrewsbury, Zhang, & Williams, 1990). Smoking parents participating in the study were asked to report on the number of cigarettes smoked and exposed from himself/herself and all other smokers living in the home and from those who visited. Nonsmoking parents were asked to report on the number of cigarettes smoked and the number of cigarettes to which the child was exposed from the smoking spouse/partner and all other smokers living in the home or visiting. Responses were used to calculate the parents’ and all sources’ average daily smoking and the child's average daily exposure to the cigarettes smoked in the home or car.
Urine collection
For toilet-trained children, urine samples were obtained in the clinic using standard urine collection methods. Children urinated into standard urine collection cups, and urine was transferred to plastic test tubes for freezing and analyses. For non–toilet-trained children (n = 15), samples were collected using a sterile pediatric urine collection bag (Pediabag; n = 14) or via cotton rolls placed in the diaper (n = 1). Obtained urine was expressed into a collection cup.
Obtained samples were split and frozen in a standard freezer with tubes labeled with a randomly assigned identification number for laboratory use. Twenty-one (16.9%) subjects were unable to provide sufficient urine to split (<10 ml of urine in a single void). Batched samples were packed in dry ice and shipped to the Mass Spectrometry laboratories at San Diego State University, San Diego, CA, for analyses of cotinine levels. All samples were assayed by a high-performance liquid chromatography and tandem mass spectrometry method (Bernert et al., 1997).
Statistical analyses
Cotinine values below detection were set to 0.1 ng/ml, the reported minimal detection level; only children in the control group from nonsmoking households had levels below detection at baseline (12/57 control samples or 8/29 children). In parametric analyses, cotinine values and reported average daily exposure values (+0.1) were log10 transformed given their skewed distributions so that more robust results would be obtained as done in prior studies (Matt et al., 1999, 2000). Means and interquartile ranges (IQRs or 25th and 75th percentiles) of the logged data are shown back-transformed to their original metric (i.e., resulting in geometric means) unless otherwise noted.
Cotinine split-half reliability was assessed by evaluating whether approximately 95% of the differences of paired split samples fell within ±2 SDs of the mean difference, which should be near 0 (Bland & Altman, 1986). Intraclass correlations and SEs of measurement (or intersubject deviation) were calculated using analysis of variance methods (Bland & Altman, 1996). For remaining cotinine analyses, the average of replicated assessments was used. Cotinine values for the control group (no smokers in the home) were compared with the study group (smokers in the home) using the nonparametric rank sum test. Spearman's rank correlation was used to investigate the relationship between cotinine values and reported measures of smoke exposure for the target parent and others, as well as the relationship between reported smoking and exposure. Regression methods were used to assess the predictive ability of cotinine on reported exposure to determine if this relationship (intercept or slope) differed according to participant characteristics. These analyses were implemented in SAS 9.1 (Cary, NC). p Values less than .05 were considered significant, and no adjustments were made for multiple testing.
Results
Demographic and medical variables
Table 1 presents the demographic characteristics of the 124 patients who provided urine samples. The demographic characteristics of a smaller related cotinine control sample (children who did not reside in homes with smokers; n = 29) selected to be comparable to the children in our study sample based on age, gender, and race are also provided. The median patient age of the study sample was 7.2 years (range, 0.4–17.7 years), and the median time from diagnosis was 0.3 years (range, 0.1–4.8 years). For the cotinine control group, the median patient age was 9.4 years (range, 2.2–17.2 years) with a median time from diagnosis of 0.6 years (range, 0.1–2.4 years).
Table 1.
Child demographic characteristics for study sample and cotinine control sample
| Study sample (n = 124) |
Control sample (n = 29) |
|||
| Parent or child variable | n | % | n | % |
| Child variables | ||||
| Child age (years) | ||||
| 0–5 | 52 | 41.9 | 10 | 34.5 |
| 6–12 | 37 | 29.9 | 10 | 34.5 |
| 13–17 | 35 | 28.2 | 9 | 31.0 |
| Child gender | ||||
| Male | 63 | 50.8 | 15 | 51.7 |
| Female | 61 | 49.2 | 15 | 48.3 |
| Child race | ||||
| White | 97 | 78.2 | 24 | 82.8 |
| Non-White | 27 | 21.8 | 5 | 17.2 |
| Diagnosis | ||||
| CNS | 10 | 8.1 | 2 | 6.9 |
| Leukemia/lymphoma | 82 | 66.1 | 22 | 75.9 |
| Solid tumor | 32 | 25.8 | 5 | 17.2 |
| Time since diagnosis | ||||
| <6 months | 87 | 70.2 | 13 | 44.8 |
| ≥6 months | 37 | 29.8 | 16 | 55.2 |
Note. CNS = central nervous system.
The median age for the parents/guardians in our study sample was 33.4 years (19.6–61.2 years). Parents/guardians participating in the study included 75.8% mothers/stepmothers, 17.7% fathers/stepfathers, and 6.5% guardians identified as a grandmother or aunt. The majority of the sample (82.3%) was White with 17.7% of the sample identified as non-White. Approximately 52% of the sample was of low socioeconomic status (SES), as determined by a score of 1 or 2 on the Hollingshead (1975) Index. Roughly 58% of parents were married and 42% of parents were single. Almost 70% (n = 90) of the parents/guardians participating in the trial were self-reported smokers.
Cotinine reliability
In order to examine the split-half reliability of the cotinine measurements obtained in this study, intraclass correlations were computed for split-half cotinine samples obtained from 103 patient participants. The intraclass correlation was 0.970, indicating that 3% of the total variance was due to within-subject variability. Pearson's correlation for data on the log scale was also computed at .970. The SE of measurement (or root mean square error) was 0.110 on the log scale. The mean difference (SD) on the log scale was 0.002 (0.156), which was not significantly different from 0 (t102 = 0.099, p = .92), as expected given the random assignment of labels (first or second split). Importantly, 95/103 (92.2%) of the differences fell within 0 ± 2 SD, indicating acceptable reliability (Bland & Altman, 1996).
Cotinine validity
Cotinine levels for patients who came from smoking households were also significantly higher than those obtained from patients living in nonsmoking homes who comprised our cotinine control group (mean [IQR] = 3.9 [1.4–11.5] vs. 0.5 ng/ml [0.1–0.9], respectively; p < .001), thereby supporting the construct validity of the laboratory cotinine measures used in the study.
Predictive validity of parent-reported PSE
Table 2 presents the average daily number of cigarettes smoked and the average daily number of cigarettes to which the child was exposed over the previous 72 hr, as reported separately by smoking and nonsmoking parents. Average parent-reported exposure levels from all sources in the child's environment over a 3-day period were similar when reported by both smoking and nonsmoking parents (1.5 and 1.3 cigarettes/day, respectively). Children were reportedly exposed to nearly half of the average number of cigarettes smoked by all sources.
Table 2.
Average daily smoking and exposure over 3 days as reported by smoking and nonsmoking parents
| Variable | 3 Days |
|||
| Reporter (source) | N | M | 25th percentile | 75th percentile |
| Average daily cigarettes smoked | ||||
| Smoking parent (self) | 90 | 1.6 | 0.7 | 6.7 |
| Smoking parent (all sources)a | 88 | 4.1 | 1.2 | 16.5 |
| Nonsmoking parent (smoking parent)b | 33 | 1.6 | 0 | 14.3 |
| Nonsmoking parent (all sources) | 34 | 3.0 | 0.3 | 15.0 |
| All parents (smoking parents)b | 123 | 2.2 | 0.7 | 10.3 |
| All parents (all sources) | 122 | 5.8 | 1.0 | 16.0 |
| Average daily cigarettes exposed | ||||
| Smoking parent (self) | 90 | 0.6 | 0 | 2.3 |
| Smoking parent (all sources) | 90 | 1.5 | 0.3 | 7.7 |
| Nonsmoking parent (smoking parent)b | 33 | 0.6 | 0 | 3.3 |
| Nonsmoking parent (all sources) | 34 | 1.3 | 0 | 8.3 |
| All parents (smoking parents)b | 123 | 0.8 | 0 | 3.3 |
| All parents (all sources) | 124 | 1.5 | 0 | 7.8 |
Note. Average daily smoking and exposure are calculated by adding smoking and exposure values across 3 days and dividing by 3. These values are log-transformed prior to calculating descriptive statistics, and means and interquartile ranges are back-transformed to the original scale.
Two families did not have results for cigarettes smoked, as reporting parent was unaware of other parent's smoking.
One family had a patient's brother as the identified smoker, so was excluded from parent results.
The relationship between reported level of smoking and reported level of PSE was also examined. Spearman's correlation for all sources of exposure and smoking was .81 as reported by all parents regardless of smoking status (p < .001). Correlations were also .81 when examined separately for smoking and nonsmoking parents (p values < .001). The reported levels of smoking and PSE only from parental sources resulted in correlations of .75 when reports were provided by all parents irrespective of smoking status and .75 and .82 when reports were provided by smoking and nonsmoking parents, respectively (p values < .001).
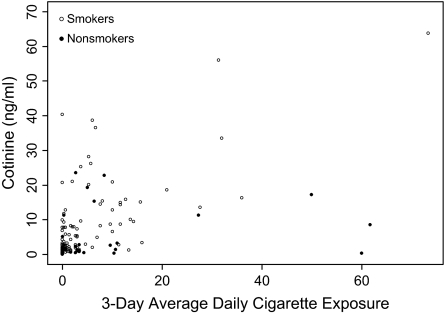
Figure 1 shows a scatter plot of urine cotinine and reported average cigarette exposure over 3 days from all sources according to the reporting parent's/guardian's smoking status. Results indicate a cluster of potential “false-positive,” cases (low urine cotinine and high parent-reported exposure) as well as some potential “false-negative” cases (high urine cotinine and low parent-reported exposure). Closer examination revealed a tendency for nonsmoking mothers to report higher levels of reported exposure than expected based on levels of cotinine.
Figure 1.
Cotinine versus 3-day average cigarette exposure from all sources according to the reporting parent's/guardian's smoking status.
In order to examine whether parents could provide valid reports of the child's PSE, Spearman's correlations between average daily parent-reported exposure with children's urine cotinine levels over a 3-day period were calculated according to smoking status of the reporting parent. These results are provided in Table 3. Reports from smoking parents yielded correlations of .35 for exposure from self and .54 for exposure from all sources. The proportion of shared variance between cotinine and 3-day parent-reported exposure increased by an absolute difference of 15% (Pearson's r2 = .13–.28) when other sources of exposure (other parent, relatives, friends) are considered over and above exposure by the reporting smoking parent. The mean urine cotinine level for patients whose reporting parent was a smoker was 5.1 ng/ml (IQR = 1.9–13.6 ng/ml).
Table 3.
Spearman's correlations between average daily parent-reported exposure and child urine cotinine levels over 3 days
| Variable | N | Spearman's correlation |
| Smoking parent (self) | 90 | .35** |
| Smoking parent (all sources) | 90 | .54*** |
| Nonsmoking parent (smoking parent) | 33 | .16 |
| Nonsmoking parent (all sources) | 34 | .38* |
| All parents (smoking parent) | 123 | .34** |
| All parents (all sources) | 124 | .48** |
Note. In one family, the patient's brother was the identified smoker and was excluded from parent results.
*p < .05; **p < .001; ***p < .0001.
Lower correlations were found between child cotinine levels and 3-day exposure reported by nonsmoking parents. Spearman's correlations were .16 for exposure from the smoking spouse alone and .38 for exposure from all sources. Also, the proportion of shared variance between cotinine and 3-day parent-reported exposure increased by an absolute difference of 13% (Pearson's r2 = .04–.17) when other sources of exposure are considered over and above the smoking spouse/partner when that report was provided by the nonsmoking parent. The mean cotinine level for patients whose reporting parent was a nonsmoker was 2.0 ng/ml (IQR = 0.7–5.2 ng/ml).
To examine how well 3-day parent reports of all PSE sources could be predicted from the child's urine cotinine levels, the SE of estimate (SEE) or root mean square was obtained. Nonsmoking parent reports (n = 33) were excluded from the analysis, as they were not strongly correlated with cotinine and we wanted to examine the most precise predictions. The SEE or root mean square was 0.70 on the log scale for predicting exposure from urine cotinine, and the 95% prediction intervals for 3-day exposure at the average value of 1.5 cigarettes/day ranged from 0 to 41.8 cigarettes. That is, if urine cotinine was used to predict the child's exposure at an average 3-day reported exposure of 1.5 cigarettes/day as reported by smoking parents, 95% of these predictions of exposure would fall between 0 and 41.8 cigarettes. This finding suggests that the true level of exposure could be 0 or as high as 25 times that “observed.”
We also examined whether the relationship between 3-day all source exposure as reported by smoking parents (excluding nonsmoking parents) and cotinine levels varied according to patient demographic and medical variables. An ordinary least squares regression model predicting exposure from cotinine was fit that assessed whether intercepts or slopes differed according to patient characteristics. Results indicated no significant differences in intercepts or slopes of the regression lines according to age group, gender, race (White vs. non-White), SES group (low vs. middle vs. high), child diagnosis (leukemia vs. central nervous system/solid tumor), and time since diagnosis (<6 vs. ≥6 months).
Discussion
This is the first study to document the magnitude of PSE among children undergoing treatment for cancer using both quantitative parent report and urine cotinine measures. Our results showed substantial level of exposure as reported by parents, and these reports were validated by cotinine assays. Children in our sample were exposed to approximately half the cigarettes smoked by their parents or other sources. Cotinine data appeared to provide reliable and valid objective measurement of recent exposure, across a range of exposures, in our sample of pediatric cancer patients. Levels of exposure, as measured by the number of cigarettes to which the child was exposed or urine cotinine assays, were comparable to those reported in other studies of childhood samples employing similar outcomes (Hovell et al., 2002; Matt et al., 1999, 2000) and reflect the generalizability of these measures across vulnerable pediatric populations.
Parent reports of recent PSE among children treated for cancer showed moderately strong positive relationships with urine cotinine levels that were of similar magnitude to those previously reported for healthy children and children with asthma (Hovell et al., 2002; Matt et al., 1999, 2000). The potential obstacles for assessing a child's exposure in the medical setting (e.g., demands of caring for a child with cancer, a medical environment that discourages smoking) did not appear to interfere with parents’ ability to provide valid reports of their own smoking and exposure behaviors or those of others in the child's environment. Even under relatively stressful conditions, parent reports can provide reasonable short-term estimates of PSE from which clinical interventions designed to reduce child exposure can be assessed. Patient demographic and medical variables did not affect the validity of parents’ report of exposure. However, validity of the parental reported exposure was impacted by the smoking status of the parent providing the report.
Exposure reports by parents in our sample who smoked were more strongly correlated with urine cotinine levels in their children than exposure reported by nonsmoking parents. Nonsmoking parents tended to overestimate the number of cigarettes to which their child was exposed compared with what was suggested by their child's cotinine levels, a pattern noted in prior studies (Matt et al., 1999). A weaker association was observed between cotinine and PSE from another smoker when reported by nonsmoking parents and compared with the association observed when smoking parents reported on their own exposure. The less accurate reports of exposure by nonsmoking parents are not surprising, as they are not the source of the child's exposure. Nonsmoking parents may estimate exposure based on historical smoking patterns they may have observed, speculate about exposure that is not directly observed, and tend to overestimate the salient exposure episodes when reporting overall exposure. Additionally, some nonsmokers could exaggerate reports of exposure in an attempt to draw attention to the smoker's behavior in relation to the child's exposure and health status.
While there was general correspondence (up to ∼30% in shared variance) between parental reports of exposure and urine cotinine levels in this sample, the two measures are far from perfect agreement and provide independent information. The strength of these relationships does not allow, for example, for the precise prediction of the number of cigarettes exposed based on cotinine levels. Residual variance may be accounted for by individual physiological differences, metabolic differences, sources and locations of exposure, and limitations in parental reports of exposure. Biological measures do not inform when the exposure occurred, the pattern of exposure, or the magnitude of exposure at each occurrence. Comprehensive exposure assessments in future studies of PSE involving pediatric cancer patients would, therefore, benefit from combinations of biological and reported estimates from parents.
It should be noted that only single, intermittent urine cotinine samples were obtained in this study, providing a relatively crude index of typical and maximal exposure. However, single cotinine measurements may not be sufficient to precisely characterize overall exposure level or exposure over a variable time course (Matt et al., 2007). The large CIs on predicted exposure levels obtained in our study suggest considerable variability in exposure outcomes. Given the variability of children's exposure, estimates of exposure may be artifactually inflated or reduced if the timing of the urine samples reflects episodic high or low level exposure events. For example, children in our sample exposed to high levels of smoke in the car during travel to the hospital may have high urine cotinine levels if the samples are collected upon arrival at the hospital. Alternatively, children being treated at the smoke-free hospital environment for greater periods of time prior to urine sample collection may have less opportunity for exposure. These factors may have affected the less than perfect correspondence between cotinine and parent reports. More frequent and targeted cotinine measurements may be necessary to resolve temporal sampling problems and obtain more representative levels of exposure for this patient population (Matt et al., 2007). Information about exposure profiles may be critical for research questions involving behavior change and disease risk information (Matt et al., 1999).
Our findings suggest that the smoking status of the parent providing the PSE report will need to be considered in future studies. Combining the PSE reports obtained from smoking parents with those of nonsmoking parents could attenuate the relationship between reported exposure and cotinine, such that reports from these two parent cohorts should be examined separately. In order to maximize the validity of parent-reported exposure, reports from smoking sources in the child's environment may also be necessary to enhance the reports from nonsmoking parents. Additionally, nonsmoking parents may require instruction or counseling to become better observers of exposure behaviors when participating in PSE exposure trials.
Although our results are promising, they should be interpreted within the context of a limited sample comprising parents who were recruited to participate in a study designed to reduce PSE to their child being treated for cancer. Additionally, conclusions about nonsmoking parents providing reports of exposure in our study were based on a small number of participants, which limited the power for statistical testing and warrant future investigation. Replicating this study with families of young cancer patients may further increase our understanding of the factors that impact the validity of parent reports. While this study did not examine the specific social and environmental contexts in which the child's exposure took place, such information is critical to understanding the “etiology” of child exposure. Clearly, children are not exposed in the hospital or other clinical settings where smoking is prohibited and exposure is severely controlled. Understanding what might be added to homes, cars, and other microenvironments to move them closer to the functional conditions that now prevail in hospitals and in other such settings may enable much greater reduction in children's PSE.
Funding
Supported in part by grants CA 085406 and CA 21765 from the National Cancer Institute and the American Lebanese Associated Charities.
Declaration of Interests
None declared.
Supplementary Material
References
- Benoist MR, Lemerle J, Jean R, Rufin P, Scheinmann P, Paupe J. Effects of pulmonary function of whole lung irradiation for Wilm's tumour in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clinical Chemistry. 1997;43:2281–2291. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measurement error. British Medical Journal. 1996;312:1654. doi: 10.1136/bmj.312.7047.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Disparities in secondhand smoke exposure—United States, 1988–1994 and 1999–2004. MMWR Morbidity and Mortality Weekly Report. 2008;57:744–747. [PubMed] [Google Scholar]
- Collier AM, Goldstein GM, Shrewsbury RP, Zhang CA, Williams RW. Urine cotinine elimination half-life in young children exposed to side-stream cigarette smoke. Proceedings of the Fifth International Conference on Indoor Air Quality and Climate. 1990;2:195–200. [Google Scholar]
- Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics. 1996;97:560–568. [PubMed] [Google Scholar]
- Emerson JA, Hovell MF, Meltzer SB, Zakarian JM, Hofstetter CR, Wahlgren DR, et al. The accuracy of environmental tobacco smoke exposure measures among asthmatic children. Journal of Clinical Epidemiology. 1995;48:1251–1259. doi: 10.1016/0895-4356(95)00021-u. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Abrams DB, Marshall RJ, Etzel RA, Novotny TE, Marcus BH, et al. Exposure to environmental tobacco smoke in naturalistic settings. American Journal of Public Health. 1992;82:24–28. doi: 10.2105/ajph.82.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Hammond SK, Abrams DB. Smoking at home: The impact of smoking cessation on nonsmokers’ exposure to environmental tobacco smoke. Health Psychology. 1994;13:516–520. doi: 10.1037//0278-6133.13.6.516. [DOI] [PubMed] [Google Scholar]
- Etzel RA. Environmental tobacco smoke. Immunology and Allergy Clinics of North America. 1994;14:621–633. [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975. Unpublished manuscript, New Haven, CT: Yale University. [Google Scholar]
- Hovell MF, Meltzer SB, Wahlgren DR, Matt GE, Hofstetter CR, Jones JA, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110:946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Zakarian JM, Wahlgren DR, Matt GE, Emmons KM. Reported measures of environmental tobacco smoke exposure: Trials and tribulations. Tobacco Control. 2000;9:iii22–iii28. doi: 10.1136/tc.9.suppl_3.iii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. New England Journal of Medicine. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- Matt GE, Bernert JT, Hovell MF. Measuring secondhand smoke exposure in children: An ecological measurement approach. Journal of Pediatric Psychology. 2008;33:156–175. doi: 10.1093/jpepsy/jsm123. [DOI] [PubMed] [Google Scholar]
- Matt GE, Hovell MF, Quintana PJ, Zakarian J, Liles S, Meltzer SB, et al. The variability of urinary cotinine levels in young children: Implications for measuring ETS exposure. Nicotine & Tobacco Research. 2007;9:83–92. doi: 10.1080/14622200601078335. [DOI] [PubMed] [Google Scholar]
- Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring secondhand smoke exposure in babies: The reliability and validity of mother reports in a sample of low-income families. Health Psychology. 2000;19:232–241. doi: 10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- Matt GE, Wahlgren DR, Hovell MF, Zakarian JM, Bernert JT, Meltzer SB, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: Empirical findings and discussion. Tobacco Control. 1999;8:282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler L. Late effects of childhood cancer therapy. Current Problems in Pediatrics. 1993;23:102–131. doi: 10.1016/0045-9380(93)90019-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington, DC: National Academy Press; 1986. [PubMed] [Google Scholar]
- Neglia JP, Meadows AT, Robison LL, Kim TH, Newton WA, Ruymann FB, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. New England Journal of Medicine. 1991;325:1330–1336. doi: 10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- O'Driscoll BR, Hasleton PS, Taylor PM, Poulter LW, Gattameneni HR, Woodcock AA. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. New England Journal of Medicine. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- Robison LL, Mertens A. Second tumors after treatment of childhood malignancies. Hematology/Oncology Clinics of North America. 1993;7:401–415. [PubMed] [Google Scholar]
- Tyc VL, Throckmorton-Belzer L, Klosky JL, Greeson FL, Lensing S, Rai SN, et al. Smoking among parents of pediatric cancer patients and children's exposure to environmental tobacco smoke. Journal of Child Health Care. 2004;8:288–300. doi: 10.1177/1367493504047319. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of involuntary smoking: A report by the U.S. Surgeon General. Rockville, MD: 1986. Author. [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of smoking—chronic obstructive lung disease: A report of the Surgeon General. Rockville, MD: 1995. Author. [Google Scholar]
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the Surgeon General. Atlanta, GA: 2006. Author. [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Respiratory health effects of passive smoking: Lung cancer and other disorders. Washington, DC: 1992. (EPA/600/6–90/006F) Author. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.