Abstract
Shiga toxin has the potential to induce expression of inflammation-associated genes, although the underlying mechanisms are not well understood. We examined the effects of subtilase cytotoxin (SubAB), an AB5 toxin produced by some Shiga toxigenic Escherichia coli, on the activation of NF-κB. SubAB is known to be a protease which selectively degrades GRP78/Bip. Treatment of NRK-52E cells with SubAB caused rapid cleavage of GRP78. Following the degradation of GRP78, transient activation of NF-κB was observed with a peak at 6–12 h; the activation subsided within 24 h despite the continuous absence of intact GRP78. The activation of NF-κB was preceded by transient phosphorylation of Akt. Treatment of the cells with a selective inhibitor of Akt1/2 or an inhibitor of PI3K attenuated SubAB-induced NF-κB activation, suggesting that activation of Akt is an event upstream of NF-κB. Degradation of GRP78 caused the unfolded protein response (UPR), and inducers of the UPR mimicked the stimulatory effects of SubAB on Akt and NF-κB. SubAB triggered the three major branches of the UPR including the IRE1-XBP1, PERK, and ATF6 pathways. Dominant-negative inhibition of IRE1α, XBP1, or PERK did not attenuate activation of NF-κB by SubAB. In contrast, genetic and pharmacological inhibition of ATF6 significantly suppressed SubAB-triggered Akt phosphorylation and NF-κB activation. These results suggested that loss of GRP78 by SubAB leads to transient phosphorylation of Akt and consequent activation of NF-κB through the ATF6 branch of the UPR.
Shiga toxin-producing Escherichia coli causes renal tubular injury and hemolytic uremic syndrome (HUS),4 the most common causes of acute renal failure in children (1, 2). Resident cell activation and subsequent recruitment of leukocytes underlie the development of tissue injury including microangiopathy (3, 4). Previous reports suggested that Shiga toxin induces leukocyte adhesion to endothelial cells by up-regulation of adhesion proteins including E-selectin, intracellular adhesion molecule-1, and vascular cell adhesion molecule-1 (5), all of which are regulated by NF-κB (6). Other reports also showed that, through activation of NF-κB, Shiga toxin increases endothelial expression of chemokines (IL-8 and MCP-1) and tubular expression of TNF-α (7, 8). Currently, however, it is unknown how Shiga toxin causes activation of NF-κB, the crucial event involved in the development of HUS and renal injury.
AB5 toxins are key virulence factors of various bacteria including Shiga toxigenic E. coli (9). Subtilase cytotoxin (SubAB) was discovered in a highly virulent Shiga toxigenic E. coli, which is responsible for an outbreak of HUS (10, 11). Recently, we showed that i.p. injection of SubAB in mice caused microangiopathic hemolytic anemia, thrombocytopenia, and renal injury typical of Shiga toxin-induced HUS. Infiltration of neutrophils was also observed in the livers, kidneys, and spleens (12). These findings indicate that SubAB may contribute to the development of renal injury and HUS in which activation of NF-κB plays a crucial role. We previously reported that SubAB selectively degrades 78 kDa glucose-regulated protein (GRP78), a master chaperone in the endoplasmic reticulum (ER), by cleavage between two leucine residues at positions 416 and 417 (13). We hypothesized that degradation of GRP78 by SubAB may be responsible for activation of NF-κB and NF-κB-dependent gene expression. In the present report, we first show that, following acute deprivation of GRP78 by SubAB, transient activation of NF-κB occurs and that the activation is caused by phosphorylation of Akt. We also demonstrate that loss of GRP78 causes the unfolded protein response (UPR), which mediates the stimulatory effects of SubAB on Akt and NF-κB. Furthermore, among three major branches of the UPR triggered by deprivation of GRP78, we provide evidence that the activating transcription factor 6 (ATF6) pathway is involved in the induction of the Akt-NF-κB signaling triggered by SubAB.
Materials and Methods
Reagents
SubAB and its inactive mutant SubAA272B were purified by Ni-NTA chromatography from recombinant E. coli, as described before (10, 12). Endotoxin-free SubAB (SubABET–) was purified by the same procedure, using a lpxM– E. coli BL21(DE3) host strain, which has penta- rather than hexa-acylated LPS. Tunicamycin, thapsigargin, LPS (E. coli 0111, B4), wortmannin, and 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) were purchased from Sigma-Aldrich. Human rIL-1β and rTNF-α were obtained from R&D Systems, and salubrinal and Akti-1/2 were from Calbiochem.
Cell culture
The rat renal tubular epithelial cell line NRK-52E was purchased from American Type Culture Collection. The human mesothelial cell line MeT5A (14) was provided by Dr. Curtis C. Harris (National Institutes of Health, Bethesda, MD), and immortalized mouse podocytes were provided by Dr. Karlhans Endlich (University of Heidelberg, Heidelberg, Germany) (15). SM/NFκB-SEAP cells, the reporter rat mesangial cells that express secreted alkaline phosphatase (SEAP) under the control of the NF-κB enhancer elements, were established, as described previously (16). Wild-type mouse embryonic fibroblasts (MEF) and iATF6 MEF that stably express ATF6α siRNA (17) were provided by Dr. Laurie H. Glimcher (Harvard Medical School, Boston, MA). Cells were maintained in DMEM/F-12 (Life Technologies) supplemented with 5% FBS.
Establishment of stable transfectants
NRK-52E cells were stably transfected with pNFκB-SEAP (BD Biosciences) that introduces a SEAP gene under the control of NF-κB, and NRK/NFκB-SEAP reporter cells were established. NRK-52E cells were also stably transfected with pNFκB-Luc (Panomics) or pUPRE-Luc (provided by Dr. Laurie H. Glimcher) (17) that introduces a luciferase gene under the control of the κB site or the UPR element (UPRE), and NRK/NFκB-Luc cells and NRK/UPRE-Luc cells were established. NRK/ATF6-DN cells stably expressing a dominant-negative mutant of ATF6α (ATF6-DN) were established by transfection of NRK-52E cells with pcDNA3.1-ATF6α(171–373)ΔAD (provided by Dr. Kazutoshi Mori, Kyoto University, Kyoto, Japan) (18).
Transient transfection
Using electroporation, NRK-52E cells were transiently transfected with pCMV-3×FLAG-ATF6 (provided by Dr. Ron Prywes, Columbia University, New York, NY) (19), treated with SubAB and subjected to Western blot analysis to evaluate the level of p90ATF6 (20). To examine roles of individual branches of the UPR, cells were transiently cotransfected with pNFκB-Luc together with pCAG-hIRE1αK699A (provided by Dr. Masayuki Miura, University of Tokyo, Tokyo, Japan) (21), pcDNA3.1-dnXBP1 (provided by Dr. Laurie H. Glimcher) (22), pcDNA3-hPERK.K621M (provided by Dr. Ronald C. Wek, Indiana University School of Medicine, Indianapolis, IN) (23), or pcDNA3.1-ATF6α (171– 373)ΔAD encoding a dominant-negative mutant (DN) of inositol-requiring ER-to-nucleus signal kinase 1α (IRE1α), X-box binding protein 1 (XBP1), RNA-dependent protein kinase-like ER kinase (PERK), or ATF6 at 1:4 ratio, stimulated by SubAB and subjected to chemiluminescent assay to evaluate luciferase activity.
Luciferase assay
Activity of luciferase was evaluated by Luciferase Assay System (Promega) according to the manufacturer's protocol (24).
Northern blot analysis
Total RNA was extracted by a single-step method, and Northern blot analysis was performed as described before (25). cDNAs for SEAP (BD Biosciences), MCP-1 (26), GRP78 (provided by Dr. Kazunori Imaizumi, University of Miyazaki, Miyazaki, Japan) (27), and ATF4 (provided by Dr. David Ron, New York University School of Medicine, New York, NY) were used to prepare radiolabeled probes. Expression of GAPDH was used as a loading control.
Western blot analysis
Western blot analysis was performed by the ECL system (Amersham Biosciences), as described previously (25). Primary Abs used were; anti-KDEL Ab (1/1000 dilution; Stressgen), anti-FLAG Ab (1/1000; Sigma-Aldrich), anti-IκBα Ab (1/200 dilution; Santa Cruz Biotechnology), and anti-IκBβ Ab (1/200 dilution; Santa Cruz Biotechnology). Levels of total Akt protein and phosphorylated Akt were evaluated by using PhosphoPlus Akt (Ser473) Ab Kit (Cell Signaling Technology). As a loading control, identical filters were reprobed for β-actin using anti-β-actin Ab (1/6000 dilution; Sigma-Aldrich).
RT-PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen) and subjected to reverse transcription using Omniscript Reverse Transcriptase (Qiagen). Reaction mixtures without reverse transcriptase were used as negative controls. Splicing of XBP1 mRNA was examined using the following primers purchased from Sigma-Aldrich Japan: 5′-CCATGGGAAGATGTTCTGGG-3′ and 5′-ACACGCTTGGGGATGAATGC-3′.
Statistical analysis
In reporter assays, experiments were performed in quadruplicate, and data were expressed as means ± SE. Statistical analysis was performed using the nonparametric Mann-Whitney U test to compare data in different groups. A p value <0.05 was considered to indicate a statistically significant difference.
Results
Transient activation of NF-κB following exposure to SubAB
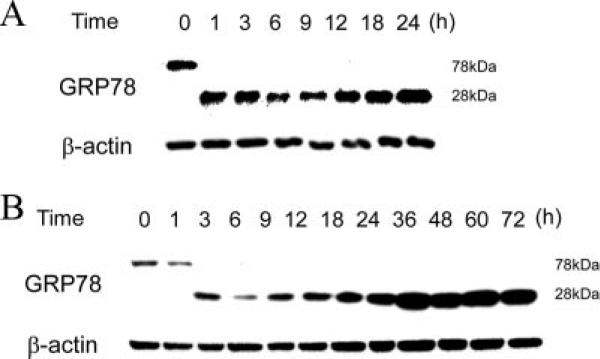
SubAB selectively degrades GRP78 protein by cleavage between two leucine residues at positions 416 and 417 (13). We first examined kinetics of cleavage of GRP78 following exposure to SubAB in NRK-52E cells. Cells were treated with 100 ng/ml SubAB for up to 24 h and subjected to Western blot analysis of the C-terminal region of GRP78. As shown in Fig. 1A, GRP78 protein was rapidly and completely cleaved to the 28 kDa fragment within 1 h. Even after exposure to 10 ng/ml SubAB, complete cleavage of GRP78 was observed within 3 h, and the effect lasted for at least 72 h without additional supply of SubAB (Fig. 1B). Subsequent experiments were performed using 10 ng/ml SubAB, unless otherwise stated.
FIGURE 1.
Kinetics of cleavage of GRP78 by SubAB. NRK-52E cells were treated with 100 ng/ml (A) or 10 ng/ml (B) of SubAB for up to 24 (A) or 72 h (B) and subjected to Western blot analysis of C-terminal region of GRP78. The level of β-actin is shown at the bottom as a loading control.
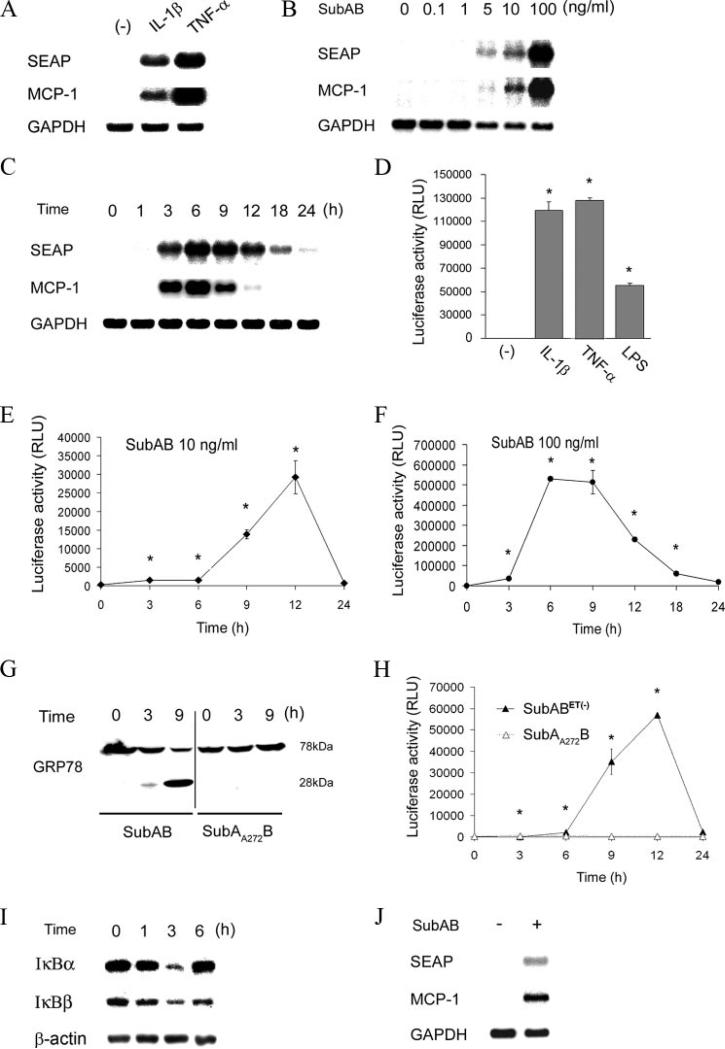
To examine whether SubAB causes activation of NF-κB, we established reporter cells that express SEAP under the control of NF-κB. The established NRK/NFκB-SEAP cells expressed SEAP mRNA in response to activators of NF-κB (IL-1β and TNF-α), and the induction was in parallel with expression of a NF-κB-dependent gene, MCP-1 (Fig. 2A). As shown in Fig. 2B, treatment of the reporter cells with SubAB induced activation of NF-κB and NF-κB-dependent gene expression. The induction of SEAP and MCP-1 by SubAB was dose-dependent and observed at concentrations ≥5 ng/ml. Time-course experiments revealed that the activation of NF-κB and expression of the NF-κB-dependent gene were transient with a peak at 6 h and subsided after 24 h (Fig. 2C).
FIGURE 2.
Transient activation of NF-κB and induction of NF-κB-dependent gene expression by SubAB. A, NRK-52E cells were stably transfected with pNFκB-SEAP, and NRK/NFκB-SEAP reporter cells were established. The cells were stimulated with 1 ng/ml IL-1β or 10 ng/ml TNF-α and subjected to Northern blot analysis of SEAP and MCP-1. Expression of GAPDH is shown at the bottom as a loading control. B and C, NRK/NFκB-SEAP cells were stimulated with serial dilutions of SubAB for 6 h (B) or 10 ng/ml SubAB for indicated time periods (C) and subjected to Northern blot analysis. D, NRK-52E cells were stably transfected with pNFκB-Luc, and NRK/NFκB-Luc cells were established. The cells were stimulated with IL-1β, TNF-α, or 1 μg/ml LPS and subjected to chemiluminescent assay to evaluate luciferase activity. RLU, relative light unit. E and F, NRK/NFκB-Luc cells were treated with 10 ng/ml (E) or 100 ng/ml (F) of SubAB for up to 24 h and subjected to luciferase assay. G, Cells were treated with SubAB or its inactive mutant SubAA272B for up to 9 h and subjected to Western blot analysis of GRP78. H, NRK/NFκB-Luc cells were stimulated with endotoxin-free SubABET– (10 ng/ml) or SubAA272B (10 ng/ml) for up to 24 h and subjected to chemiluminescent assay. I, NRK-52E cells were treated with 100 ng/ml SubAB for up to 6 h and subjected to Western blot analysis of IκBα and IκBβ. J, SM/NFκB-SEAP cells were treated with 100 ng/ml SubAB for 9 h and subjected to Northern blot analysis of SEAP and MCP-1. In D–F and H, assays were performed in quadruplicate, and data are expressed as means ± SE. Asterisks indicate statistically significant differences (p < 0.05).
To confirm this observation, we established another reporter cell that expresses luciferase under the control of NF-κB. Induction of luciferase by IL-1β, TNF-α and LPS was confirmed by chemiluminescent assay (Fig. 2D). Using the established NRK/NFκB-Luc cells, we examined kinetics of NF-κB activity following the treatment with SubAB. As shown in Fig. 2E, 10 ng/ml SubAB induced activation of NF-κB within 3 h and peaked at 12 h. Consistent with the result from NRK/NFκB-SEAP cells, the activation was transient and subsided within 24 h. When the cells were exposed to a higher concentration of SubAB (100 ng/ml), the activation occurred more rapidly with a peak at 6–9 h (Fig. 2F). Even under this experimental condition, the activation of NF-κB subsided by 24 h.
To exclude a possibility that the activation of NF-κB by SubAB was caused by contaminating LPS, we purified endotoxin-free SubABET– using a lpxM– E. coli host strain that has penta- rather than hexa-acylated LPS. We also prepared mutant SubAB, SubAA272B, which does not cleave GRP78 (Fig. 2G). NRK/NFκB-Luc cells were treated with SubABET– or SubAA272B, and activation of NF-κB was tested. As shown in Fig. 2H, activation of NF-κB was elicited by SubABET– similarly to that by SubAB. In contrast, the mutant SubAA272B did not activate NF-κB at any time points tested. These results show that the activation of NF-κB by SubAB is independent of LPS, which is caused upon the proteolytic activity of the toxin.
To further confirm activation of NF-κB by SubAB, we examined degradation of IκBα and IκBβ. NRK-52E cells were treated with SubAB for up to 6 h, and the levels of IκBs were evaluated by Western blot analysis. Consistent with the results from reporter assays, SubAB substantially reduced both IκBα and IκBβ within 3 h (Fig. 2I), further confirming activation of NF-κB.
We examined whether or not the activation of NF-κB by SubAB is observed in other cell types. Reporter rat mesangial cells (SM/NFκB-SEAP cells) were treated with SubAB for 9 h and subjected to Northern blot analysis of MCP-1 and SEAP. Like in NRK-52E cells, expression of MCP-1 and SEAP was induced by SubAB in mesangial cells (Fig. 2J). We also tested activity of NF-κB using SubAB-treated murine podocytes. Podocytes were stimulated with SubAB for 7 h and subjected to Northern blot analysis of MCP-1. Podocytes were also transfected with pNFκB-Luc, treated with SubAB and subjected to luciferase assay. The results showed that expression of MCP-1 and activation of luciferase were also induced by SubAB in podocytes (28). These results suggest that activation of NF-κB by SubAB is observed in various cell types.
Transient activation of Akt upstream of NF-κB following exposure to SubAB
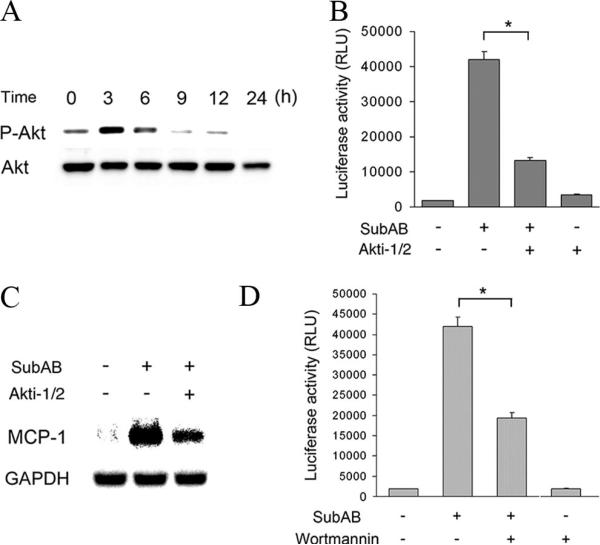
Previous reports suggested that the PI3K-Akt pathway may regulate activity of NF-κB positively or negatively, depending on cellular contexts (29). A previous study also indicated cross-talk between GRP78, Akt, and NF-κB in prostate cancer cells (30). We, therefore, examined involvement of Akt in the transient activation of NF-κB by SubAB. Western blot analysis revealed that, following exposure to SubAB, transient phosphorylation of Akt was observed at 3 h and subsided within 9 h (Fig. 3A). When NRK/NFκB-Luc cells were treated with SubAB in the presence of a selective inhibitor of Akt1/2 (Akti-1/2), activation of NF-κB was significantly suppressed (Fig. 3B). It was associated with attenuated induction of the NF-κB-dependent gene, MCP-1 (Fig. 3C). This result was further confirmed using an inhibitor of PI3K, wortmannin. Reporter assay showed that, like Akti-1/2, wortmannin significantly inhibited SubAB-induced activation of NF-κB (Fig. 3D). These results suggest that activation of NF-κB caused by SubAB is mediated by activation of the PI3K-Akt pathway.
FIGURE 3.
Transient activation of Akt upstream of NF-κB following exposure to SubAB. A, NRK-52E cells were treated with SubAB for indicated time periods and subjected to Western blot analysis of phosphorylated Akt (P-Akt). The protein level of total Akt is shown at the bottom as a loading control. B–D, NRK/NFκB-Luc cells were treated with SubAB in the absence (−) or presence (+) of 10 μM Akti-1/2 (B and C) or 100 nM wortmannin (D) and subjected to chemiluminescent assay (B and D) or Northern blot analysis of MCP-1 (C). Data are expressed as means ± SE, and asterisks indicate statistically significant differences (p < 0.05).
Activation of the Akt-NF-κB pathway through UPR triggered by SubAB
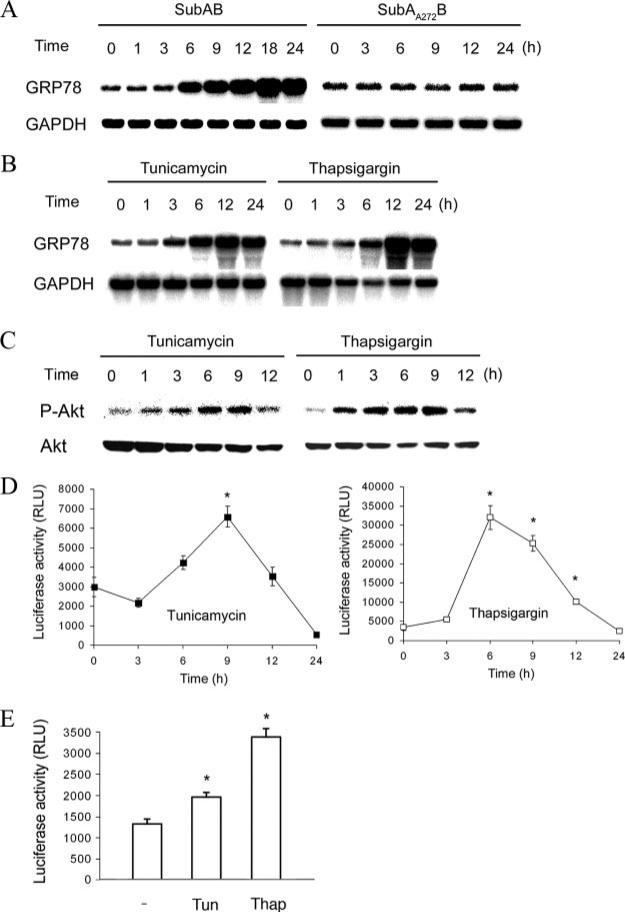
There are three major transducers for the UPR on the membrane of the ER; i.e., PERK, ATF6, and IRE1. All of these molecules associate with GRP78 in their inactive states. Dissociation of GRP78 from these transducers elicits signaling of the UPR (31). Based on this current concept, it is reasonable to speculate that deprivation of GRP78 protein by SubAB triggers the UPR. Indeed, we recently provided evidence that SubAB activates all three branches of the UPR in Vero cells (32). Consistent with this result, treatment of NRK-52E cells with SubAB caused induction of GRP78 mRNA, an indicator of the UPR (Fig. 4A, left). This induction was not observed after treatment with SubAA272B (Fig. 4A, right). The stimulatory effect of SubAB on the UPR was reproduced by known inducers of ER stress, tunicamycin and thapsigargin (Fig. 4B), and treatment with these agents caused transient phosphorylation of Akt (Fig. 4C). Furthermore, like SubAB, treatment with tunicamycin or thapsigargin triggered transient activation of NF-κB, the kinetics of which was closely correlated with that of Akt phosphorylation (Fig. 4D). The activation of NF-κB by the UPR was not specific to NRK-52E cells, because significant activation of NF-κB by tunicamycin and thapsigargin was also observed in Met5A human mesothelial cells (Fig. 4E). These results suggest that the UPR induced by depletion of intact GRP78 is responsible for SubAB-triggered activation of the Akt-NF-κB pathway.
FIGURE 4.
Activation of the Akt-NF-κB pathway through the UPR triggered by SubAB. A–C, NRK-52E cells were treated with SubAB and SubAA272B (A), or tunicamycin (1 μg/ml) and thapsigargin (500 nM) (B and C) for indicated time periods and subjected to Northern blot analysis of GRP78 (A and B) or Western blot analysis of phosphorylated Akt (C). D, NRK/NFκB-Luc cells were treated with tunicamycin or thapsigargin for up to 24 h and subjected to chemiluminescent assay. E, Met5A cells were transfected with pNFκB-Luc, treated with tunicamycin (Tun; 10 μg/ml) or thapsigargin (Thap; 1 μM) for 12 h and subjected to luciferase assay. Data are expressed as means ± SE, and asterisks indicate statistically significant differences (p < 0.05).
Roles of individual branches of UPR in SubAB-triggered activation of Akt and NF-κB
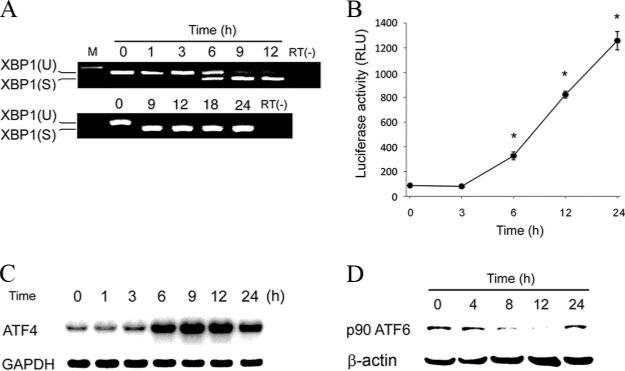
To examine roles of individual branches of the UPR in SubAB-triggered activation of NF-κB, we evaluated induction of the IRE1, PERK, and ATF6 pathways. Activated IRE1 catalyzes removal of a small intron from the XBP1 mRNA. This splicing event creates a translational frameshift to produce an active transcription factor. XBP1 subsequently binds to UPRE, leading to expression of target genes (33). We first examined the splicing of XBP1 mRNA in NRK-52E cells following exposure to SubAB. RT-PCR analysis revealed that the spliced form of XBP1 was detectable within 6 h (Fig. 5A, top) and lasted for at least 24 h (Fig. 5A, bottom). Consistently, reporter assay showed that significant activation of UPRE was observed within 6 h and sustained for at least 24 h (Fig. 5B), confirming induction of the IRE1-XBP1 pathway by SubAB. Activation of PERK leads to phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α), which causes induction of a transcription factor ATF4 (33). Northern blot analysis revealed that treatment of NRK-52E cells with SubAB caused induction of ATF4 within 6 h and sustained for at least 24 h (Fig. 5C). This result suggests that the PERK pathway is also induced by SubAB. In response to ER stress, p90ATF6 transits to the Golgi where it is cleaved by the proteases S1P and S2P, yielding a free cytoplasmic domain that is an active transcription factor p50ATF6 (33). Western blot analysis revealed that treatment with SubAB caused cleavage of p90ATF6 within 8 h, suggesting induction of the ATF6 pathway (Fig. 5D). In contrast to sustained activation of the IRE1 and PERK pathways, activation of ATF6 was transient, peaked at 12 h and subsided within 24 h.
FIGURE 5.
Activation of individual branches of the UPR by SubAB. A, NRK-52E cells were treated with SubAB for 1–12 h (top) or 9–24 h (bottom) and subjected to RT-PCR analysis of XBP1 mRNA. XBP1(U), the unspliced form of XBP1; XBP1(S), the spliced form of XBP1. RT(-), without reverse transcriptase. B, NRK/UPRE-Luc cells were treated with SubAB for indicated time periods and subjected to luciferase assay to evaluate activation of UPRE. Data are expressed as means ± SE, and asterisks indicate statistically significant differences (p < 0.05). C, NRK-52E cells were treated with SubAB, and expression of ATF4 was examined by Northern blot analysis. D, NRK-52E cells were transiently transfected with FLAG-ATF6, treated with SubAB, and subjected to Western blot analysis of p90ATF6 using an anti-FLAG Ab.
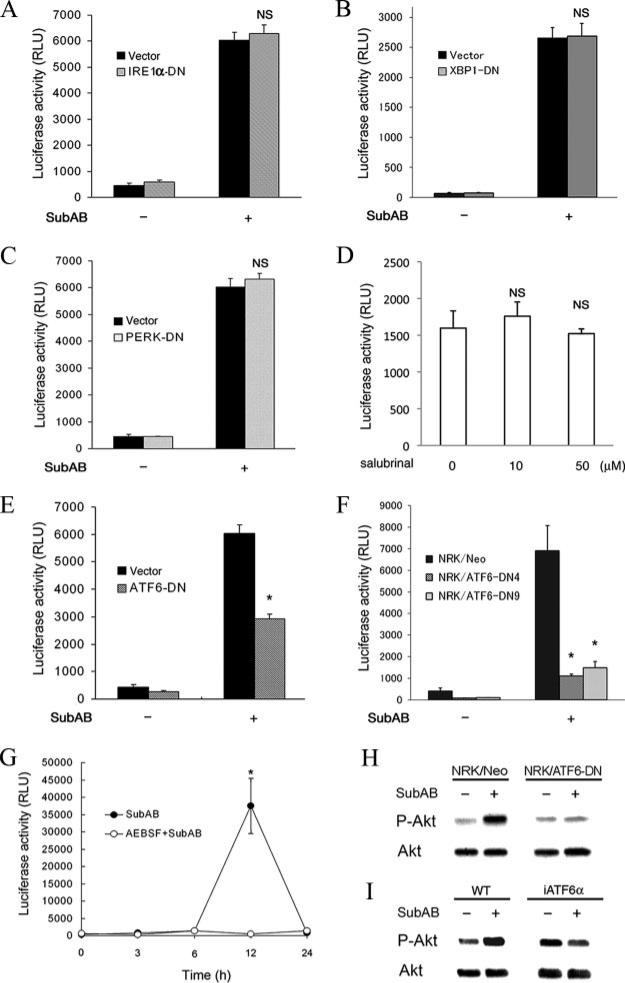
As described above, deprivation of GRP78 by SubAB triggered induction of the major branches of the UPR. To identify pathway(s) responsible for activation of NF-κB, reporter assays were performed using dominant-negative mutants of individual transducers. NRK-52E cells were transiently cotransfected with pNFκB-Luc together with a gene encoding a dominant-negative mutant of IRE1α, XBP1, PERK, or ATF6, stimulated by SubAB and subjected to chemiluminescent assay to evaluate luciferase activity. As shown in Fig. 6, A and B, transfection with IRE1α-DN or XBP1-DN did not affect activation of NF-κB by SubAB. Similarly, transfection with PERK-DN also did not influence the NF-κB activation (Fig. 6C). Consistent with this result, treatment of NRK/NFκB-Luc cells with salubrinal, a selective activator of the eIF2α pathway (34), did not induce activation of NF-κB (Fig. 6D). In contrast, overexpression of ATF6-DN significantly attenuated SubAB-triggered activation of NF-κB, suggesting involvement of the ATF6 pathway (Fig. 6E). To confirm this result, we created stable transfectants expressing ATF6-DN. The established cells were transiently transfected with pNFκB-Luc and stimulated with SubAB. Reporter assay revealed that both NRK/ATF6-DN4 cells and NRK/ATF6-DN9 cells exhibited blunted responses of NF-κB to SubAB when compared with mock-transfected NRK/Neo cells (Fig. 6F). We also tested an effect of a chemical inhibitor of ATF6, AEBSF, which inhibits S1P and S2P proteases (35). NRK/NFκB-Luc cells were stimulated with SubAB in the absence or presence of AEBSF, and kinetics of NF-κB activation was evaluated. Consistent with the results from the transfection studies, AEBSF abrogated activation of NF-κB by SubAB (Fig. 6G), confirming the crucial role of ATF6.
FIGURE 6.
Roles of individual branches of the UPR in SubAB-triggered activation of Akt and NF-κB. A–C, and E, NRK-52E cells were transiently cotransfected with pNFκB-Luc and pcDNA3.1 (Vector), pCAG-hIRE1αK699A (IRE1α-DN) (A), pcDNA3.1-dnXBP (XBP1-DN) (B), pcDNA3-hPERK.K621M (PERK-DN) (C), or pcDNA3.1-ATF6α(171–373)ΔAD (ATF6-DN) (E), stimulated with or without SubAB for 12 h and subjected to chemiluminescent assay to evaluate luciferase activity. D, NRK/NFκB-Luc cells were treated with 10–50 μM salubrinal for 12 h and subjected to luciferase assay. F, Mock-transfected NRK/Neo, NRK/ATF6-DN4, and NRK/ATF6-DN9 cells were transiently transfected with pNFκB-Luc, stimulated with SubAB, and subjected to luciferase assay. G, NRK/NFκB-Luc cells were treated with SubAB in the absence or presence of 300 μM AEBSF and subjected to chemiluminescent assay. In A–G, assays were performed in quadruplicate. Data are expressed as means ± SE, and asterisks indicate statistically significant differences (p < 0.05). NS, not significant. H and I, NRK/Neo and NRK/ATF6-DN cells (H) or wild-type MEF (WT) and iATF6α MEF (I) were stimulated by SubAB, and phosphorylation of Akt was evaluated by Western blot analysis.
To examine a role of ATF6 in the SubAB-induced activation of Akt, the event upstream of NF-κB activation, NRK/Neo cells and NRK/ATF6-DN cells were stimulated by SubAB, and phosphorylation of Akt was evaluated. As shown in Fig. 6H, activation of Akt was blunted in NRK/ATF6-DN cells. We also confirmed this result using iATF6α MEF stably expressing ATF6α siRNA. In wild-type MEF, treatment with SubAB induced phosphorylation of Akt. In contrast, the Akt phosphorylation was not observed in iATF6α MEF (Fig. 6I). Taken together, these results demonstrate that SubAB induces activation of the Akt-NF-κB pathway through the ATF6 branch of the UPR.
Discussion
In the present report, we demonstrated that the subtilase cytotoxin SubAB caused transient phosphorylation of Akt and consequent activation of NF-κB. Subsequent experiments revealed that loss of GRP78 by SubAB triggered the UPR and that the UPR was responsible for the stimulatory effects of SubAB on Akt and NF-κB. Among the three major branches of the UPR, the ATF6 pathway, but not others, was shown to be responsible for activation of the Akt-NF-κB signaling. Our results revealed a novel mechanism by which SubAB causes activation of NF-κB, a crucial pathological event involved in HUS and renal injury.
GRP78 is a molecule induced by ER stress. A previous report indicated that, under inflammatory situations, GRP78 was required for activation of NF-κB by TNF-α via binding to IκB kinase (36). Another report also showed that, in cancer cells, binding of activated forms of the proteinase inhibitor α2-macroglobulin to cell surface-associated GRP78 induced NF-κB activation, and silencing of GRP78 suppressed this process (30). In contrast to these previous reports, we provided experimental evidence that GRP78 is essential to maintain an inactive state of NF-κB under unstimulated conditions. We tested effects of GRP78 overexpression on the basal and cytokine-inducible NF-κB activity in NRK-52E cells, but we could not detect any significant effects on NF-κB activity (our unpublished data). This result indicates that basal levels of GRP78 are sufficient to inhibit NF-κB. ER stress is known to activate NF-κB (37), and GRP78 is the major chaperone that attenuates ER stress. It is, therefore, reasonable to speculate that GRP78 suppresses activation of NF-κB via halting the UPR.
In the present study, we showed that SubAB causes activation of NF-κB via phosphorylation of Akt. A previous report indicated that ER stress induced by tunicamycin and thapsigargin up-regulated Akt phosphorylation in glial cells (38). The authors found that Akt was localized in the ER and was activated when the cells were treated with the ER stress-inducing reagents. Consistent with our results, they also observed that LY294002 or wortmannin inhibited ER stress-induced Akt activation, suggesting that the UPR triggers Akt activation via PI3K. Activation of PI3K is mediated through the binding of the p85 subunit to tyrosine-phosphorylated proteins (39). Previous reports showed that, following ER stress, Akt and p85 translocate to the ER (39, 40). This molecular event may be involved in the SubAB-triggered activation of NF-κB through the PI3K-Akt pathway.
Previous studies suggested that ER stress has the potential to activate NF-κB through IRE1/TNF-associated factor 2 (TRAF2) or eIF2α (41–44). For example, in response to thapsigargin and tunicamycin, IκB kinase may form a complex with IRE1α through TRAF2, and knockdown or knockout of either IRE1α or TRAF2 could impair activation of NF-κB by ER stress (41, 42). Other reports also showed that phosphorylation of eIF2α may be necessary and sufficient to activate NF-κB (43, 44), but the molecular mechanisms involved have not been fully elucidated. In contrast to these previous reports, we demonstrated that SubAB-triggered, UPR-mediated activation of NF-κB is dependent on the ATF6 pathway, but not on the IRE1 and PERK pathways. This conclusion was also supported by the facts that only activation of the ATF6 branch was transient following the exposure to SubAB and that activation of other branches were sustained for at least 24 h (Fig. 6). To our knowledge, this is the first study to demonstrate a selective involvement of ATF6 in UPR-mediated NF-κB activation. Molecular events involved in the activation of Akt by ATF6 remains to be elucidated, and further investigation is required to identify mechanisms involved.
Recently, Hu et al. (45) reported that XBP1 may induce phosphorylation of Akt in zebrafish embryonic cells. They showed that Akt phosphorylation was induced in cells stably overexpressing the spliced form of XBP1. However, involvement of XBP1 in the activation of the Akt-NF-κB pathway by SubAB is unlikely in our experimental setting, because 1) transfection with XBP1-DN did not affect activation of NF-κB by SubAB; 2) similarly, transfection with IRE1α-DN did not affect NF-κB activation; 3) following exposure to SubAB, activation of NF-κB was transient and subsided within 24 h, whereas splicing of XBP1 mRNA was sustained for at least 24 h; and 4) activation of UPRE triggered by XBP1 progressed for at least 24 h. Another recent report also indicated a role for the PERK pathway in activation of Akt. Kazemi et al. (46) showed that a conditionally active form of PERK acted upstream of PI3K and turned on the Akt pathway. They also showed that induction of the PI3K pathway was impaired in PERK-null mutant cells in response to various stimuli that activate PERK and that PI3K activation by PERK was indirect and required inhibition of protein synthesis by eIF2α (46). In the present study, we found that transfection with PERK-DN did not affect activation of NF-κB by SubAB. Activation of the eIF2α pathway by salubrinal also did not induce NF-κB activation. However, expression of ATF4, an indicator for activation of the PERK pathway, was partially attenuated 24 h after the exposure to SubAB. Furthermore, our preliminary result indicated that transfection with eIF2α-DN modestly attenuated activation of NF-κB by SubAB (our unpublished data). We, therefore, cannot exclude a possibility that the PERK pathway may play some minor role in the activation of the Akt-NF-κB pathway by SubAB.
In the present investigation, we observed early, low levels of NF-κB activation at 0–6 h and progressive activation of NF-κB at 6–12 h, following exposure to SubAB. Previous reports suggested that activation of the ATF6, PERK, and IRE1 pathways show different time courses in response to ER stress (47) and that the PERK and IRE1 pathways may also be involved in the activation of NF-κB by ER stress in some cell types (41–44). The early, low levels of NF-κB activation could be caused via UPR pathways other than ATF6.
In this report, we demonstrated that exposure to SubAB caused transient activation of NF-κB via the UPR in the early phase. However, in the later phase, the UPR may rather suppress basal and inducible activation of NF-κB (28, 48–51). Interestingly, regulation of Akt by the UPR may also be biphasic. Hosoi et al. reported that Akt phosphorylation was up-regulated in response to short-term exposure to ER stress, whereas with long-term exposure to ER stress, Akt phosphorylation was down-regulated (38). Consistent with this result, we found in the present study that, following exposure to SubAB, Akt was transiently phosphorylated in the early phase, whereas the basal level of Akt phosphorylation was rather suppressed in the later phase (Fig. 3A). The fact that the biphasic kinetics of Akt precedes the biphasic kinetics of NF-κB further supports our conclusion that Akt acts upstream of NF-κB.
Endothelial cell activation, subsequent recruitment of leukocytes, and consequent injury of the vasculature underlie the development of HUS (3, 4). Previous reports suggested that Shiga toxin, the causal factor of HUS, induces leukocyte adhesion to endothelial cells by up-regulation of NF-κB-dependent adhesion proteins and chemokines (5–7). SubAB is produced by certain highly virulent Shiga toxigenic E. coli strains and has the potential to induce HUS (12). Our current results indicate a mechanism underlying the development of HUS. The UPR-mediated activation of the Akt-NF-κB pathway may be a possible target for therapeutic intervention in pathologies caused by subtilase cytotoxin.
Acknowledgments
We appreciate Dr. Laurie H. Glimcher (Harvard Medical School), Dr. Kazutoshi Mori (Kyoto University), Dr. Ron Prywes (Columbia University), Dr. Masayuki Miura (University of Tokyo), Dr. Ronald C. Wek (Indiana University School of Medicine), Dr. Kazunori Imaizumi (University of Miyazaki), and Dr. David Ron (New York University School of Medicine) for providing us with plasmids. We also thank Dr. Laurie H. Glimcher for kind gifts of wild-type MEF and iATF6 MEF.
Footnotes
This work was supported, in part, by Grant-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No.16390243, No.17651026, No.19651024; to M.K.), and by Grant No. R01AI-068715 from the National Institutes of Health (to A.W.P. and J.C.P.).
Abbreviations used in this paper: HUS, hemolytic uremic syndrome; SubAB, subtilase cytotoxin; GRP78, 78 kDa glucose-regulated protein; ER, endoplasmic reticulum; UPR, unfolded protein response; ATF6, activating transcription factor 6; AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride; SEAP, secreted alkaline phosphatase; MEF, mouse embryonic fibroblasts; UPRE, UPR element; DN, dominant-negative mutant; IRE1α, inositol-requiring ER-to-nucleus signal kinase 1α; XBP1, X-box binding protein 1; PERK, RNA-dependent protein kinase-like ER kinase; eIF2α, eukaryotic translation initiation factor 2α; IKK, IκB kinase; TRAF2, TNF-associated factor 2.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Tesh VL, O'Brien AD. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 1991;5:1817–1822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Ruggenenti P. The hemolytic uremic syndrome. Kidney Int. 1995;47:2–19. doi: 10.1038/ki.1995.261. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth KD, Simpson AC, Fitzpatrick MM, Barratt TM, Levinsky RJ. Neutrophil-mediated endothelial injury in haemolytic uraemic syndrome. Lancet. 1989;ii:411–414. doi: 10.1016/s0140-6736(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick MM, Shah V, Trompeter RS, Dillon MJ, Barratt TM. Interleukin-8 and polymorphoneutrophil leukocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 1992;42:951–956. doi: 10.1038/ki.1992.372. [DOI] [PubMed] [Google Scholar]
- 5.Morigi M, Micheletti G, Figliuzzi M, Imberti B, Karmali MA, Remuzzi A, Remuzzi G, Zoja C. Verotoxin-1 promotes leukocyte adhesion to cultured endothelial cells under physiologic flow conditions. Blood. 1995;86:4553–4558. [PubMed] [Google Scholar]
- 6.Collins T, Cybulsky MI. NF-κB: pivotal mediator or innocent bystander in atherogenesis? J. Clin. Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, Imberti B, te Loo M, Monnens L, Remuzzi G, Morigi M. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-κB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 2002;62:846–856. doi: 10.1046/j.1523-1755.2002.00503.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura A, Johns EJ, Imaizumi A, Yanagawa Y, Kohsaka T. Activation of β2-adrenoceptor prevents Shiga toxin 2-induced TNF-α gene transcription. J. Am. Soc. Nephrol. 2001;12:2288–2299. doi: 10.1681/ASN.V12112288. [DOI] [PubMed] [Google Scholar]
- 9.Fan E, Merritt EA, Verlinde CLMJ, Hol WGJ. AB5 toxins: structures and inhibitor design. Curr. Opin. Struct. Biol. 2000;10:680–686. doi: 10.1016/s0959-440x(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 10.Paton AW, Srimanote P, Talbot UM, Wang H, Paton JC. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 2004;200:35–46. doi: 10.1084/jem.20040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton AW, Paton JC. Multiplex PCR for direct detection of Shiga toxigenic Escherichia coli producing the novel subtilase cytotoxin. J. Clin. Microbiol. 2005;43:2944–2947. doi: 10.1128/JCM.43.6.2944-2947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Paton JC, Paton AW. Pathologic changes in mice induced by subtilase cytotoxin, a potent new Escherichia coli AB5 toxin that targets the endoplasmic reticulum. J. Infect. Dis. 2007;196:1093–1101. doi: 10.1086/521364. [DOI] [PubMed] [Google Scholar]
- 13.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 14.Duncan EL, Whitaker NJ, Moy EL, Reddel RR. Assignment of SV40-immortalized cells to more than one complementation group for immortalization. Exp. Cell Res. 1993;205:337–344. doi: 10.1006/excr.1993.1095. [DOI] [PubMed] [Google Scholar]
- 15.Schiwek D, Endlich N, Holzman L, Holthöfer H, Kriz W, Endlich K. Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int. 2004;66:91–101. doi: 10.1111/j.1523-1755.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- 16.Meng Y, Kasai A, Hiramatsu N, Hayakawa K, Takeda M, Shimizu F, Kawachi H, Yao J, Kitamura M. Real-time monitoring of mesangial cell-macrophage cross-talk using SEAP in vitro and ex vivo. Kidney Int. 2005;68:886–893. doi: 10.1111/j.1523-1755.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen J, Prywes R. ER stress signaling by regulated proteolysis of ATF6. Methods. 2005;35:382–389. doi: 10.1016/j.ymeth.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J, Kitamura M. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J. Biol. Chem. 2008;283:4252–4260. doi: 10.1074/jbc.M705951200. [DOI] [PubMed] [Google Scholar]
- 21.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 22.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. USA. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez J, Yaman I, Sarnow P, Snider MD, Hatzoglou M. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2α. J. Biol. Chem. 2002;277:19198–19205. doi: 10.1074/jbc.M201052200. [DOI] [PubMed] [Google Scholar]
- 24.Hiramatsu N, Kasai A, Hayakawa K, Nagai K, Kubota T, Yao J, Kitamura M. Secreted protein-based reporter systems for monitoring inflammatory events: critical interference by endoplasmic reticulum stress. J. Immunol. Methods. 2006;315:202–207. doi: 10.1016/j.jim.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa K, Meng Y, Hiramatsu N, Kasai A, Yamauchi K, Yao J, Kitamura M. Priming of glomerular mesangial cells by activated macrophages causes blunted responses to proinflammatory stimuli. J. Immunol. 2006;176:2529–2537. doi: 10.4049/jimmunol.176.4.2529. [DOI] [PubMed] [Google Scholar]
- 26.Rollins BJ, Morrison ED, Stiles CD. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc. Natl. Acad. Sci. USA. 1988;85:3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katayama T, Imaizumi K, Honda A, Yoneda T, Kudo T, Takeda M, Mori K, Rozmahel R, Fraser P, George-Hyslop PS, Tohyama M. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer's disease-linked presenilin-1 mutations. J. Biol. Chem. 2001;276:43446–43454. doi: 10.1074/jbc.M104096200. [DOI] [PubMed] [Google Scholar]
- 28.Okamura M, Takano Y, Hiramatsu N, Hayakawa K, Yao J, Paton AW, Paton JC, Kitamura M. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am. J. Physiol. 2008;295:F1495–F1503. doi: 10.1152/ajprenal.00602.2007. [DOI] [PubMed] [Google Scholar]
- 29.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol. Pharm. Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 30.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-κB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J. Biol. Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 32.Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 2008;10:1775–1786. doi: 10.1111/j.1462-5822.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell. Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 35.Okada T, Haze K, Nadanaka S, Yoshida H, Seidah NG, Hirano Y, Sato R, Negishi M, Mori K. A serine protease inhibitor prevents endoplasmic reticulum stress-induced cleavage but not transport of the membrane-bound transcription factor ATF6. J. Biol. Chem. 2003;278:31024–31032. doi: 10.1074/jbc.M300923200. [DOI] [PubMed] [Google Scholar]
- 36.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosoi T, Hyoda K, Okuma Y, Nomura Y, Ozawa K. Akt up- and down-regulation in response to endoplasmic reticulum stress. Brain Res. 2007;1152:27–31. doi: 10.1016/j.brainres.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 39.Otsu M, Hiles I, Gout I, Fry MJ, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan J, Totty N, et al. Characterization of two 85 kD proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991;65:91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- 40.Daniele N, Rajas F, Payrastre B, Mauco G, Zitoun C, Mithieux G. Phosphatidylinositol 3-kinase translocates onto liver endoplasmic reticulum and may account for the inhibition of glucose-6-phosphatase during refeeding. J. Biol. Chem. 1999;274:3597–3601. doi: 10.1074/jbc.274.6.3597. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 42.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor-α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol. Cell. Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor-κB by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu MC, Gong HY, Lin GH, Hu SY, Chen MH, Huang SJ, Liao CF, Wu JL. XBP-1, a key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line. Biochem. Biophys. Res. Commun. 2007;359:778–783. doi: 10.1016/j.bbrc.2007.05.183. [DOI] [PubMed] [Google Scholar]
- 46.Kazemi S, Mounir Z, Baltzis D, Raven JF, Wang S, Krishnamoorthy JL, Pluquet O, Pelletier J, Koromilas AE. A novel function of eIF2α kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol. Biol. Cell. 2007;18:3635–3644. doi: 10.1091/mbc.E07-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayakawa K, Hiramatsu N, Okamura M, Yao J, Paton AW, Paton JC, Kitamura M. Blunted activation of NF-κB and NF-κB-dependent gene expression by geranylgeranylacetone: involvement of unfolded protein response. Biochem. Biophys. Res. Commun. 2008;365:47–53. doi: 10.1016/j.bbrc.2007.10.115. [DOI] [PubMed] [Google Scholar]
- 49.Takano Y, Hiramatsu N, Okamura M, Hayakawa K, Shimada T, Kasai A, Yokouchi M, Shitamura A, Yao J, Paton AW, et al. Suppression of cytokine response by GATA inhibitor K-7174 via unfolded protein response. Biochem. Biophys. Res. Commun. 2007;360:470–475. doi: 10.1016/j.bbrc.2007.06.082. [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa K, Hiramatsu N, Okamura M, Yamazaki H, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Acquisition of anergy to proinflammatory cytokines in non-immune cells through endoplasmic reticulum stress response: a mechanism for subsidence of inflammation. J. Immunol. 2009;182:1182–1191. doi: 10.4049/jimmunol.182.2.1182. [DOI] [PubMed] [Google Scholar]
- 51.Du S, Hiramatsu N, Hayakawa K, Kasai A, Okamura M, Huang T, Yao J, Takeda M, Araki I, Sawada N, et al. Suppression of NF-κB by cyclosporine A and tacrolimus (FK506) via induction of the C/EBP family: implication for unfolded protein response. J. Immunol. 2009;182:7201–7211. doi: 10.4049/jimmunol.0801772. [DOI] [PubMed] [Google Scholar]