Abstract
Background
Over three-fourths of heart failure (HF) patients are aged 65 years and older, and older age is associated with worse symptoms and prognoses than is younger age. Reduced exercise capacity is a chief HF complaint and indicates poorer prognosis, especially among the elderly, but the mechanisms underlying functional decline in older HF patients are largely unknown.
Methods
Baseline cardiopulmonary exercise testing data from the HF-ACTION trial were assessed to clarify age-effects on peak VO2 (peak oxygen consumption) and VE/VCO2 (ventilation-carbon dioxide production) slope.
Results
Among 2331 NYHA class II–IV HF patients, increased age corresponded to decreased peak VO2 (−0.14 mL/kg/min per year above 40 years, p<0.0001) and increased VE/VCO2 slope (0.30 units per year above 70 years, p<0.0001). In a multivariable model with 34 other potential determinants, age was the strongest independent predictor of peak VO2 (partial R2 0.130; total R2 0.392; P < 0.001) and a significant but relatively weaker predictor of VE/VCO2 slope (partial R2 0.037; total R2 0.199; P < 0.001). Blunted peak heart rate (HR) was also a strong predictor of peak VO2. Although peak HR and age were strongly correlated, both were significant independent predictors of peak VO2 when analyzed simultaneously in a model. Aggregate comorbidity increased significantly with age, but did not account for age effects on peak VO2.
Conclusions
Age is the strongest predictor of peak VO2 and a significant predictor of VE/VCO2 slope in the HF-ACTION population. Age-dependent comorbidities do not explain changes in peak VO2. Age-related changes in cardiovascular physiology, potentially magnified by the HF disease state, should be considered a contributor to the pathophysiology and target for more effective therapy for older HF patients.
Keywords: Cardiopulmonary exercise testing, exercise capacity, aging, heart failure, ventilatory efficiency
INTRODUCTION
Over three-fourths of the population with heart failure (HF) is aged 65 years and older, and the proportion of older HF patients is projected to increase as more adults are surviving into old age.(1,2) Aging is associated with both insidious physiological changes and an increased number of comorbid conditions (3), both of which are associated with increased susceptibility to developing HF, complexity of its management, and to poor prognosis.(4) Indeed, older HF patients have greater hospitalization rates, health care expenditures, and mortality (4) as well as symptoms of exercise intolerance that are often more severe than in younger HF patients.(5)
Severely decreased exercise capacity is the main contributor to the primary symptoms of chronic HF, exertional fatigue and dyspnea, and is also an important prognostic indicator and key therapeutic target. However, even in the absence of cardiovascular disease, exercise capacity declines substantially with normal aging due to a variety of physiological changes, including reduced maximal heart rate response.(6–9) In addition, older HF patients have significantly more comorbidities than younger HF patients, on average four to five per patient.(10) It has been hypothesized that aging-related increases in comorbidities lead to worse exercise capacity, symptoms, and quality-of-life in older HF patients.(4, 11, 12) However, it is unclear whether age-related changes alone, independent of the concomitant comorbidities, remain a strong determinant of exercise capacity after the development of heart failure.
Formal assessment of exercise performance with measurement of peak exercise oxygen consumption (peak VO2) and exercise ventilatory efficiency (VE/VCO2 slope) using cardiopulmonary exercise testing (CPX), is commonly used to guide management of HF patients. (13) However, partly due to the above uncertainties, CPX is less often utilized in older HF patients, and it is unknown to what extent peak VO2 and VE/VCO2 slope provide information regarding the underlying disease state of older HF patients as opposed to the extent to which they reflect intrinsic aging physiology and/or comorbidities commonly associated with aging.
The recently completed multi-center HF-ACTION trial provides a unique opportunity to examine these key issues.(14,15) It is the largest study to include CPX testing in HF patients. CPX testing was performed utilizing a highly standardized protocol and strong attention to quality control, including formal field site training and real-time raw data review by a CPX core laboratory. In this study, we analyzed baseline CPX data available on 2229 of the 2331 HF-ACTION enrollees, who were aged 19 to 91 years, including 477 patients aged ≥70. We assessed peak VO2 and VE/VCO2 slope in relationship to age, key comorbidities, and other demographic and clinical characteristics.
METHODS
Details of the HF-ACTION study design have been published previously.(14, 15) The trial adhered to inclusion criteria for systolic HF (ejection fraction ≤35%, median 25%) and New York Heart Association (NYHA) class II to IV symptoms even when nearly all enrollees were receiving optimal HF pharmacological therapy with ACE inhibitors and/or angiotensin II receptor blockers (ACEI/ARB) and beta blockers at stable doses for at least six weeks. Comprehensive demographic and clinical data were systematically gathered. Enrollment included substantial representation of racial minorities (38%) and women (28%), and both ischemic (51%) and non-ischemic etiology of heart failure, all intended to facilitate analyses and conclusions that can be generalized to broad clinical populations.
CPX Testing and Analysis
Symptom-limited exercise testing was completed with concomitant expired gas analysis using a commercially available metabolic cart as previously described.(16) In most cases, exercise was completed using a modified Naughton protocol on a motor driven treadmill. In some cases, an upright cycle ergometer with a ramping protocol (10 watt/minute) was used. Testing supervisors encouraged patients toward an exhaustive endpoint, guided by the respiratory exchange ratio (RER), with a goal of achieving RER >1.10. VE/VCO2 ratios were verified by the CPX Core Laboratory using standard techniques with quality control measures. (16) Peak VO2 was determined in the Core Laboratory as the highest relative oxygen consumption (VO2, mL/kg/min) for a given 15- or 20-second interval within the last 90 seconds of exercise or the first 30 seconds of recovery.(16) The selected relative peak VO2 was also required to fall within 10% of the interval values before and after it, provided the physiologic progression appeared reasonable. Peak RER was defined as the highest recorded value for a 15- or 20-second averaged sampling interval that occurred during the last 90 seconds of exercise. The peak RER selected was required to correspond to appropriate VO2 values and progress in a physiologic fashion from preceding ratios. VE/VCO2 slope was calculated based on VE/VCO2 data across the entire duration of exercise.(17) The 15- or 20-second averaged data for VO2 (mL/min), VCO2 (mL/min) and VE (L/min) were entered into a spreadsheet (Excel; Microsoft). From these data entered, the VE/VCO2 slope was calculated between the resting and peak exercise values, where peak exercise was determined as for peak VO2.
O2 pulse ([mL/kg]/heart beat) was also calculated as the ratio of peak VO2 and heart rate and used as a coarse gauge of stroke volume.
Statistical analyses
Continuous variables are presented as medians with the 25th and 75th percentiles. Categorical data are presented as frequencies and percentages. For descriptive purposes, summary tables present information within broad age groups (<60, 60–69, and ≥70). However, continuous age was used in all model-based analyses. Hypotheses regarding the relationship between age and continuous patient characteristics and outcomes were tested using a normal approximation test for zero correlation. For categorical variables, age was compared between levels by means of t-test or ANOVA. Statistical significance was based on an empirical alpha level of 0.05 in all hypothesis tests. These tests were considered exploratory and no adjustment was made for multiplicity.
Modeling Strategy
Based on assumptions about clinical and cardiac factors that may impact on exercise performance, a comprehensive list of 35 baseline (rest) clinical variables obtained from the case report forms, baseline study clinical assessments and echocardiography were analyzed as candidate covariates of both peak VO2 and VE/VCO2 slope (Table 1). Multivariable linear regression with stepwise variable selection was used to identify key predictors of each of the CPX functional indices. Of the 35 candidate variables, the one with the highest p-value was eliminated first and the model was rerun. This process was repeated iteratively until all remaining covariates had p < 0.05. All variables which remained in the model derived by this stepwise selection process, but which possessed a partial R2 < 0.01 after eliminating non-significant predictors, were then removed to further isolate the factors most significant in determining functional capacity measures.
Table 1.
Candidate variables used to predict peak VO2 and VE/VCO2 slope
|
CABG, coronary artery bypass graft surgery; PCI, percutaneous coronary intervention; MI, myocardial infarction; PAD, peripheral arterial disease; COPD, chronic obstructive pulmonary disease; ACE, angiotensin-converting enzyme; CPX, cardiopulmonary exercise testing; NYHA, New York Heart Association; CCS, Canadian Cardiovascular Society; ECG, electrocardiogram; LBBB, left bundle branch block; RBBB, right bundle branch block; IVCD, intraventricular conduction delay; BP, blood pressure; BMI, body mass index; HR, heart rate; LVEF, left ventricular ejection fraction
Based on plots of each CPX parameter by age, the assumption that these age-relationships were linear in the multiple variable models was re-evaluated and found to be violated. A linear spline was determined to be an appropriate transformation and knots, chosen in order to maximize the resulting model R-square in each case, were located at 40 years in the model of peak VO2 and at 70 years in the model of VE/VCO2.
The variables defined as final CPX model predictors by this methodology were then used to identify age-interaction effects, for both peak VO2 and VE/VCO2 slope. In addition to the independent covariate predictors identified with this analysis, key covariates that were considered clinically relevant for our analysis of functionally-based indices were reentered into the two models. Coronary artery disease (CAD, based on ischemic etiology of HF), chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), left ventricular ejection fraction (LVEF, from the echocardiogram), and resting heart rate (from the CPX test) were reentered into the models of both peak VO2 and VE/VCO2 slope.
Comorbidity Score
To explore the independent impact of aggregate comorbidity on peak VO2 or VE/VCO2 slope, a simple comorbidity score was generated by assigning one point to each member of a broad list of comorbid diagnoses: CAD, DM, COPD, peripheral arterial disease (PAD), hypertension, atrial fibrillation and/or flutter, and excessively low or high body mass index (<18 or >30). Results were presented as a percent of possible morbidity (based on the number of comorbid conditions for each patient relative to the available accounts of comorbidity for each patient in the denominator).
Impact of peak exercise heart rate on peak VO2 and VE/VCO2 slope
The impact of heart rate at peak exercise (peak HR) was assessed as a key indicator of age-related physiological changes that may impact on CPX assessments of peak VO2 and VE/VCO2 slope. Peak HR was determined from the electrocardiogram at peak exercise.
RESULTS
The key demographic and clinical characteristics in the three age groups are shown in Tables 2a and 2b. Weight, body mass index, and resting heart rate were reduced while systolic blood pressure was increased with increasing age; the proportion of those with ischemic HF etiology was increased with increasing age. Average age was higher among patients with ischemic etiology of HF, but lower among users of ACEI/ARB and beta-adrenergic blockers. Exercise performance results stratified by the three age groups are shown in Table 3. The median peak VO2 for the entire population was 14.4 mL/kg/min, verifying that as a group the HF-ACTION subjects had considerable exercise intolerance. Notably, peak respiratory exchange ratio (RER) was similar in all three groups (median of 1.09), consistent with the observation that individuals in all age groups achieved a similar percentage of peak efforts on the exercise test.
Table 2.
| Table 2a : Characteristics of the HF population | ||||||
|---|---|---|---|---|---|---|
| Parameter | All Subjects | ----------------------Age---------------------- | Linear Correlation | P-value | ||
| <60 | 60–69 | ≥70 | ||||
| Age at Randomization (yrs) | 2331 59 (51, 68) |
1214 51.5 (44.8, 56.1) |
640 64.4 (62.2, 67.2) |
477 75.3 (72.5, 78.7) |
||
| Weight (kg) | 2331 90 (76, 106) |
1214 97 (79, 114) |
640 88 (77, 102) |
477 82 (70, 93) |
−0.35 | <0.001 |
| Height (cm) | 2324 173 (166, 180) |
1211 173 (165, 180) |
637 173 (168, 180) |
476 173 (168, 179) |
−0.04 | 0.044 |
| BMI (kg/m2) | 2324 30 (26, 35) |
1211 32 (27, 38) |
637 29 (26, 33) |
476 27 (24, 31) |
−0.37 | <0.001 |
| HR at rest, clinic visit (bpm) | 2326 70 (63, 77) |
1212 72 (64, 80) |
638 68 (62, 75) |
476 68 (60, 75) |
−0.22 | <0.001 |
| Systolic BP (mmHg) | 2327 111 (100, 126) |
1213 110 (100, 122) |
639 112 (102, 127) |
475 118 (104, 130) |
0.12 | <0.001 |
| Diastolic BP (mmHg) | 2326 70 (60, 78) |
1213 70 (62, 80) |
638 70 (60, 78) |
475 68 (60, 76) |
−0.13 | <0.001 |
| LVEF (%) | 2327 25 (20, 30) |
1210 24 (20, 30) |
640 25 (20, 30) |
477 26 (21, 31) |
0.05 | 0.025 |
| Comorbidity Score | 2331 29 (14, 43) |
1214 29 (14, 43) |
640 33 (15, 46) |
477 43 (29, 43) |
0.23 | <0.001 |
| Table 2b: Characteristics of HF-ACTION patient population | ||||||
|---|---|---|---|---|---|---|
| Parameter | All Subjects | ----------------------Age---------------------- | P value | |||
| <60 | 60–69 | ≥70 | ||||
| Overall Group Size (N) | 2331 | 1214 | 640 | 477 | ||
| Female | 661 (28.4) | 393 (32.4) | 166 (25.9) | 102 (21.4) | <0.001 | |
| NYHA class | ||||||
| • II | 1477 (63.4) | 808 (66.6) | 393 (61.4) | 276 (57.9) | <0.001 | |
| • III | 831 (35.7) | 399 (32.9) | 241 (37.7) | 191 (40.0) | ||
| • IV | 23 (1.0) | 9 (0.6) | 6 (0.9) | 10 (2.1) | ||
| Race | ||||||
| • Black or African American | 749 (32.6) | 502 (42.2) | 158 (25.0) | 89 (18.7) | <0.001 | |
| • White | 1426 (62.1) | 619 (52.0) | 434 (68.8) | 373 (78.5) | ||
| • Other | 121 (5.3) | 69 (5.8) | 39 (6.2) | 13 (2.7) | ||
| Therapy | ||||||
| • ACEI/ARB | 2199(94.3) | 1159 (95.6) | 603 (94.2) | 437 (91.6) | 0.010 | |
| • Beta Blocker | 2203 (94.5) | 1163 (95.8) | 604 (94.4) | 436 (91.4) | <0.001 | |
| • Bi-V pacemaker | 419 (18.0) | 195 (16.1) | 120 (18.8) | 104 (21.8) | <0.001 | |
| • Pacemaker | 414 (17.8) | 149 (12.3) | 134 (20.9) | 131 (27.5) | <0.001 | |
| Ischemic HF Etiology | 1197 (51.4) | 456 (37.6) | 396 (61.9) | 345 (72.3) | <0.001 | |
| Comorbidity | ||||||
| • DM | 748 (32.1) | 352 (29.0) | 246 (38.4) | 150 (31.5) | <0.001 | |
| • COPD | 249 (10.8) | 89 (7.4) | 89 (13.4) | 71 (15.0) | <0.001 | |
| • PAD | 157 (7) | 43 (4) | 57 (9) | 57 (12) | <0.001 | |
In each cell, data presented are N followed by median (25th percentile, 75th percentile). P-value is for the hypothesis test that the linear correlation (of the parameter with age as a continuous variable) is zero. Abbreviations: BMI, body mass index; HR, heart rate; BP, blood pressure; LVEF, left ventricular ejection fraction
In each cell, data presented are n followed by percentage.
Abbreviations: NYHA, New York Heart Association; ACEI/ARB, ACE inhibitor or Angiotensin II receptor blocker; Bi-V pacemaker, biventricular pacemaker; HF, heart failure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease.
Table 3.
Cardiopulmonary testing assessments
| Parameter | Total | ----------------------Age---------------------- | P value | ||
|---|---|---|---|---|---|
| <60 | 60–69 | ≥70 | |||
| Totals (N) | 2260 | 1181 | 614 | 465 | |
| Resting HR (bpm) | 70 (63–79) | 72 (64–80) | 68 (61–75) | 69 (61–76) | <0.001 |
| Max HR (bpm) | 120 (104–134) | 125 (111–141) | 115 (102–130) | 110 (95–122) | <0.001 |
| Peak VO2 (mL/kg/min) | 14.4 (11.5–17.7) | 15.6 (12.3–18.8) | 14.1 (11.5–17.2) | 12.8 (10.0–15.4) | <0.001 |
| VE/VCO2 slope | 32.6 (28.1–38.5) | 31.0 (26.9–35.8) | 32.9 (28.9–38.8) | 36.9 (31.8–42.9) | <0.001 |
| RER | 1.09 (1.02–1.16) | 1.09 (1.02–1.16) | 1.09 (1.01–1.16) | 1.09 (1.02–1.16) | 0.91 |
| O2 pulse (mL/heart beat) | 0.12 (0.10–0.15) | 0.12 (0.10–0.15) | 0.13 (0.10–0.15) | 0.12 (0.10–0.14) | 0.03 |
In each cell, data presented are median, followed by 25th and 75th percentiles.
HR, heart rate; Peak VO2, maximal exercise capacity; VE/VCO2 slope, ventilatory efficiency; RER, peak respiratory exchange ratio
Peak VO2 and maximal heart rate were significantly reduced and VE/VCO2 slope was increased in the older age stratum relative to younger strata. Results of stepwise selection of independent variables to predict peak VO2 are shown in Table 4. Eleven covariates remained in the final model and the total model R2 was 0.392. Thus, the total model explained 39% of the variability in peak VO2. Age was the strongest predictor of peak VO2 (accounting for 13% of variability in peak VO2, after controlling for other variables in the model). For every seven year increment in age there was approximately a 1 mL/kg/min lower peak VO2. However, this relationship existed primarily in patients over 40 years of age only (see below and Figure 1). Age was followed by body mass index (BMI), sex, race, and NYHA HF classification in magnitude of predicting peak VO2 (i.e., after controlling for other variables in the model, these covariates accounted for 7%, 6%, 6% and 6% of the peak VO2 variability, respectively). Men had a mean adjusted peak VO2 that was approximately two units greater than women. African Americans had lower peak VO2 than whites. NYHA class II subjects had a higher peak VO2 (by two units, on average) than class III or IV subjects.
Table 4.
Covariates predicting baseline Peak VO2 in the model
| Variable | Coefficient | 95% CI | P-value | Partial R2 |
|---|---|---|---|---|
| Age | -------- | -------- | <0.0001 (overall) |
0.13 (overall) |
| <=40 years | 0.03 | (−0.05, 0.10) | ||
| >40 years | −0.14 | (−0.16, −0.13) | ||
| BMI | −0.16 | (−0.18, −0.13) | <0.0001 | 0.07 |
| Sex | −2.08 | (−2.43, −1.72) | <0.0001 | 0.06 |
| Race -White | -------- | -------- | <0.0001 | 0.06 |
| Black or African American | −2.16 | (−2.53, −1.80) | ||
| Other | −1.10 | (−1.81, −0.39) | ||
| NYHA Class (III/IV vs. II) | −2.02 | (−2.35, −1.69) | <0.0001 | 0.06 |
| Vent. Conduction Abnormality | -------- | -------- | <0.0001 (overall) |
0.028 (overall) |
| -Normal | ||||
| LBBB | −0.56 | (−1.03, −0.10) | ||
| RBBB | −1.62 | (−2.50, −0.77) | ||
| IVCD | −1.25 | (−1.75, −0.75) | ||
| Paced | −1.52 | (−1.95, −1.10) | ||
| Geographic Region | -------- | -------- | <0.0001 (overall) |
0.026 (overall) |
| -South USA | ||||
| Northeast USA | −0.75 | (−1.29, −0.21) | ||
| Midwest USA | 0.49 | (0.11, 0.87) | ||
| West USA | 0.70 | (0.15, 1.24) | ||
| Canada | −1.13 | (−1.76, −0.50) | ||
| France | 2.01 | (0.81, 3.38) | ||
| CPX mode (bike vs. TM) | −2.45 | (−3.13, −1.78) | <0.0001 | 0.023 |
| Diabetes | −1.21 | (−1.56, −0.87) | <0.0001 | 0.022 |
| LVEF | 0.07 | (0.05, 0.09) | <0.0001 | 0.021 |
| PAD | −1.96 | (−2.59, −1.33) | <0.0001 | 0.017 |
Multiple R-Square = 0.392
Reference categories: sex = male, diabetes = non-diabetics, PAD = non-PAD, region = South USA, race = White, NYHA Class = Class II, CPX mode = treadmill, vent. conduction abnormality = normal. Abbreviations: BMI, body mass index; NYHA, New York Heart Association; Vent. conduction abnormality, ventricular conduction abnormalities; LBBB, Left bundle branch block; RBBB, Right bundle branch block; IVCD, intraventricular conduction delay; Paced, pacemaker; CPX mode (bike vs. TM), cardiopulmonary exercise testing mode (bike ergometer vs. treadmill); LVEF, left ventricular ejection fraction; PAD, peripheral arterial disease
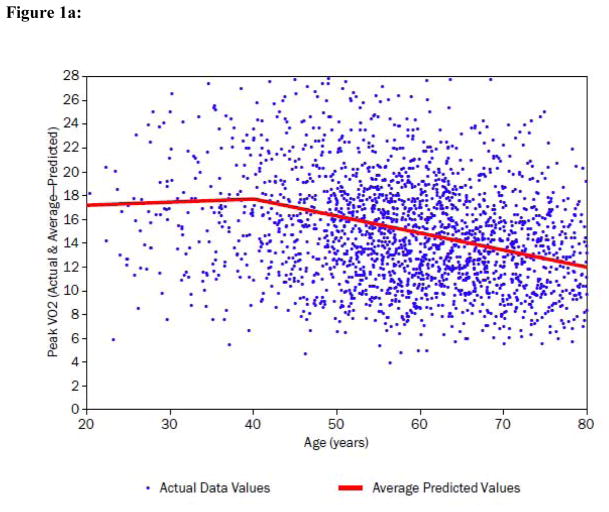
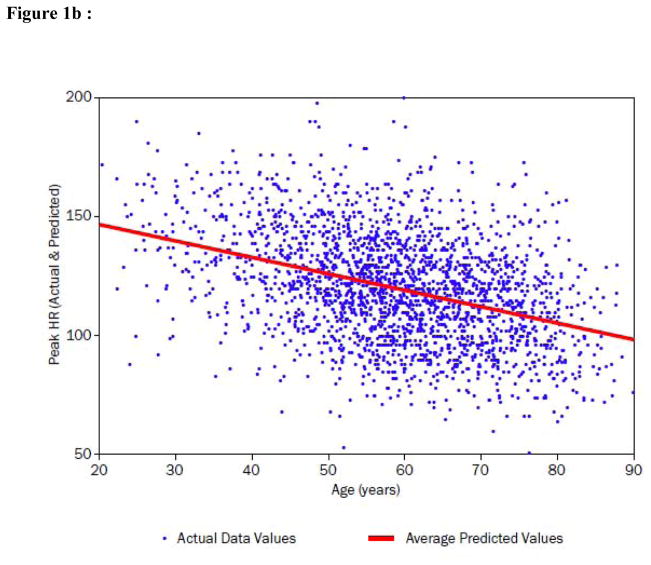
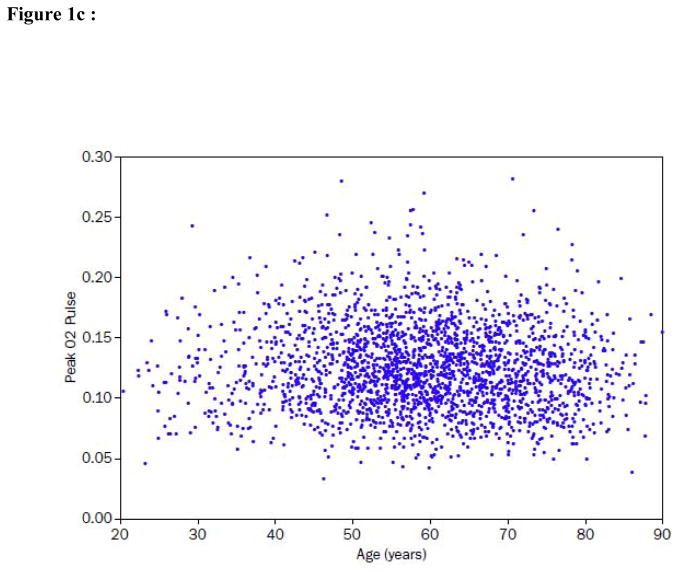
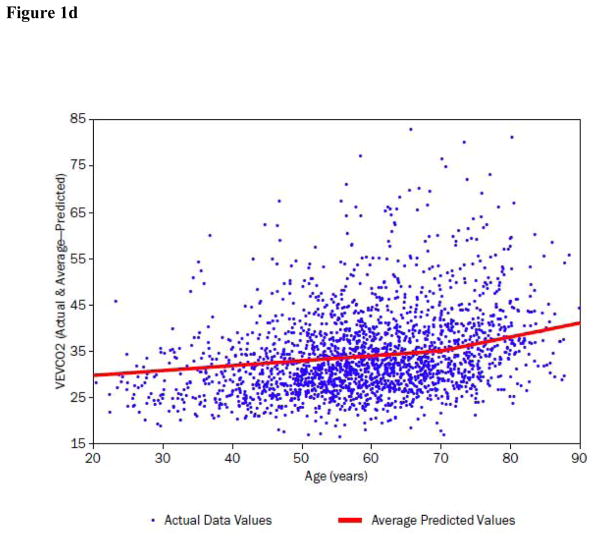
Figure 1. Peak VO2, peak heart rate (HR), O2 pulse and ventilatory efficiency (VE/VCO2) in relation to age in the HF-ACTION subject population.
In each plot individual data points represent actual data and regression lines of best fit are shown for some variables.
a) Peak VO2 (mL/kg/min) is displayed as a function of age. The regression line of best fit demonstrates a spline at approximately age 40 where the individual best fit regression lines have characteristics: <40 years: coefficient +0.03, partial r2 = 0.00, p=0.494; >40 years: coefficient −0.14, partial r2 = 0.12; p<0.0001 (Table 4).
b) Peak exercise HR (beats per minute) is displayed as a function of age. The best fit regression line is shown and displays an inverse relationship to age across the age distribution.
c) Peak O2 pulse (mL/kg/heart beat) is displayed as a function of age. There is a statistically significant but small correlation (r = −0.04, p = 0.03) between the two variables.
d) VE/VCO2 slope is displayed as a function of age. The regression line of best fit demonstrates a spline at approximately age 70 where the individual best fit regression lines have characteristics: <70 years: coefficient +0.11, partial r2 = 0.014, p<0.0001; >70 years: coefficient +0.30, partial r2 = 0.011; p<0.0001 (Table 5).
Figure 1a demonstrates the inverse association between peak VO2 and age. Although the relationship between peak VO2 and age is strong, there is also clearly a non-linear aspect to it. Adjusting for this departure from linearity was determined to have only a minor impact on the overall fit of the final model of peak VO2 (R2=0.392 in Table 4 vs. R2=0.388 with age a simple linear predictor).
Figure 1b demonstrates that there was a significant age-related decline in peak HR. Table 3 similarly demonstrates that peak heart rates were significantly lower in the higher age strata. Investigating the relative importance of peak HR to the prediction of peak VO2, we observed that, by adding peak heart rate to the model from Table 4, the total R2 of the peak VO2 model was increased from 0.392 to 0.494. For the purpose of investigating the interaction of peak HR with age in the final model, an F-test with two degrees of freedom showed a statistically significant interaction between age overall and peak HR (p<0.0001). The linear spline corresponding to age >40 interacted significantly with peak HR (p<0.0001), whereas the interaction of peak HR with the linear spline for age ≤40 was non-significant (p=0.925).
The importance of the age-related decline in peak HR to the age-related decline in peak VO2 was further evaluated by examining the relation between age and peak O2 pulse. O2 pulse correlated inversely with age in a small but statistically significant relationship.(Figure 1c).
In contrast to the strong age-interaction of peak HR on peak VO2 (p<0.0001), the analysis of other age interactions in relation to peak VO2 were more modest. PAD, diabetes, and BMI had such modest but significant age interactions. For both PAD (p=0.004) and diabetes (0.030), significant age interactions were only evident beyond age 40 years. In HF patients with PAD and diabetes aged over 40, average peak VO2 was lower than in age-matched HF patients without each of those comorbidities. The interaction manifests itself as follows: the relative reductions with age in average peak VO2 among patients with PAD and diabetes were less pronounced than among those without the comorbidities. The age by BMI interaction (p=0.0004) was evidenced by a more pronounced age-related reduction in average peak VO2 among the set of patients with lower BMI values than among patients with higher BMI values.
Results of a similar analysis as for peak VO2, using stepwise selection of independent covariates to predict VE/VCO2 slope are shown in Table 5 and Figure 1d. Six covariates remained in a final model, but the total model R2 was only 0.199. While age was a significant predictor for VE/VCO2 slope, it had relatively less impact than was demonstrated for peak VO2; age only accounted for 3.7% of the variability in VE/VCO2 slope, after accounting for the other variables in the model. Age did not interact significantly with any of the other covariates in the model of VE/VCO2 slope.
Table 5.
Covariates predicting baseline VE/VCO2 slope in the model
| Variable | Coefficient | 95% Confidence Interval | P-value | Partial R2 |
|---|---|---|---|---|
| Age | -------- | -------- | <0.0001 (overall) |
0.037 (overall) |
| ≤70 years | 0.11 | (0.07, 0.14) | ||
| >70 years | 0.30 | (0.18, 0.42) | ||
| NYHA Class (II vs. III/IV) | 3.44 | (2.72, 4.79) | <0.0001 | 0.039 |
| BMI | −0.22 | (−0.27, −0.17) | <0.0001 | 0.030 |
| LVEF | −0.15 | (−0.20, −0.11) | <0.0001 | 0.019 |
| Vent. Conduction Abnormality | -------- | -------- | <0.0001 (overall) |
0.016 (overall) |
| -Normal | ||||
| LBBB | 1.35 | (0.35, 2.35) | ||
| RBBB | 3.41 | (1.58, 5.24) | ||
| IVCD | 2.43 | (1.36, 3.52) | ||
| Paced | 1.94 | (1.03, 2.85) | ||
| Geographic Region | --- | --- | 0.0004 (overall) |
0.010 (overall) |
| -South USA | ||||
| Northeast USA | 0.49 | (−0.67, 1.65) | ||
| Midwest USA | −0.40 | (−1.22, 0.42) | ||
| West USA | −0.49 | (−1.64, 0.65) | ||
| Canada | 2.72 | (1.39, 4.05) | ||
| France | −0.65 | (−3.08, 1.78) |
Multiple R-Square = 0.199
Reference categories: NYHA Class = Class II; vent. Conduction abnormality = normal; region = south USA
Abbreviations: BMI, body mass index; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; Vent. conduction abnormality, ventricular conduction abnormalities; LBBB, Left bundle branch block; RBBB, Right bundle branch block; IVCD, intraventricular conduction delay; Paced, pacemaker
While peak heart rate added to the predictive value of the VE/VCO2 model, the effects were relatively modest compared to the impact of peak HR on the model to predict peak VO2. Adding peak heart rate to the VE/VCO2 slope model increased the R2 from 0.199 to 0.210, and peak HR interacted significantly with both linear age-related splines. Up to age 70, higher peak HR corresponded to lower VE/VCO2 slope; however, this relationship between HR and VE/VCO2 slope was relatively less marked for patients aged over 70.
The aggregate comorbidity score was significantly higher with greater age (Table 2a). After adjusting for the final model clinical and demographic covariates, when adding the comorbidity score into the multivariable regression model, peak VO2 was inversely related to increasing aggregate comorbidity (p<0.0001). R2 increased from 0.392 to 0.399 when the comorbidity score was added to the model, but there was no age-interaction between the comorbidity score and peak VO2. Examining the other endpoint, the comorbidity score was positively related to VE/VCO2 in the multivariable model (p<0.0001). For the VE/VCO2 slope model, the R2 increased from 0.199 to 0.208 when the comorbidity score was added, but the interaction between age and the comorbidity score in relation to VE/VCO2 slope was not significant.
DISCUSSION
We used the detailed demographic, clinical, and CPX testing data available from the large HF-ACTION cohort of NYHA class II–IV HF patients at baseline to examine the relationships between age and two key physiologically and prognostically independent exercise performance measures, peak VO2 and VE/VCO2 slope. We observed strong age-associated effects on these outcomes even when age competed in models with 34 other demographic and clinical covariates. Age was the strongest independent predictor of peak VO2 and was also one of the strongest predictors for VE/VCO2 slope. Thus, even in the presence of established HF and severely reduced LV systolic function and after accounting for multiple comorbidities that are common in older patients, age remains a strong, independent determinant of exercise capacity and ventilatory efficiency in an optimally medicated HF population.
Age-related decline in peak exercise heart rate was also a strong contributor to diminished exercise capacity with age-related decline in peak VO2 after age 40 but was not a contributor to age-related changes in VE/VCO2 slope. It has been well established that the normal, expected age-related decline in maximal heart rate during exercise is the primary contributor to the age-related decline in peak exercise VO2.(8,9) It is remarkable that even after the development of HF due to severe LV systolic dysfunction, the aging-heart rate effect remains a powerful determinant of exercise capacity even in optimally medically treated patients on significant doses of beta-adrenergic blocking agents. We believe this seemingly simple but strong effect on the important outcome of exercise capacity has not been fully appreciated, and has obvious clinical implications. It may, for instance, help explain why older persons with heart failure have more severe symptoms, lower exercise capacity, and overall poorer prognosis, when all other variables appear equal.
Blunted exercise heart rate response has been a frequent observation in patients with HF, though its impact on exercise capacity and outcomes has not been fully appreciated.(18–21) The present study suggests that aging effects on HR should also be taken into account. It is noteworthy that the mechanism that has been hypothesized for the blunted heart rate response to exercise in HF is the same as that independently hypothesized to account for the age-related decline in maximal heart rate: blunted beta-adrenergic responsiveness despite increased circulating catecholamines (22–26). Recent observations by Borlaug et al (27) suggest that the blunted heart rate response in heart failure patients extends to those with HF and preserved ejection fraction and also negatively impacts exercise capacity in these patients, suggesting that it may be a universal phenomenon of HF. Brubaker et al. reported that nearly 30% of older patients with heart failure due to LV systolic dysfunction meet formal criteria for chronotropic incompetence (28). In addition, by including a healthy, age-matched control group, the Brubaker et al. data indicate that the blunted heart rate response in older heart failure patients was not due to normal aging alone. Our present data confirm that the blunted heart rate response to exercise in systolic HF is aggravated in older patients and indicate that the relationship is non-linear with a significant inflection point at about 40 years of age. The finding of non-linearity with age is consistent with the body of aging-exercise physiology literature. Fleg et al. reported a non-linear relationship between age and peak VO2 in healthy men and women.(6) Similarly, heart rate, vascular stiffness and cardiac function have been demonstrated to undergo non-linear age-related changes, particularly during an exercise stimulus.(29–31)
The present study significantly expands upon prior literature by demonstrating the relationship between age and VE/VCO2 slope in HF. While VE/VCO2 slope does not change significantly as function of age per se among healthy adults (32), it becomes relatively steeper in association with diastolic and systolic heart failure.(33,34) In our study of systolic HF patients, age was a significant predictor of VE/VCO2 slope, but only explained 3.7% of the variance, after accounting for the other model covariates, i.e., approximately equal to the effects of BMI and NYHA functional class in the model.
We observed that, while peak heart rate also predicted VE/VCO2 slope (increasing R2 from 0.197 to 0.207), there were no age interactions. This is expected; while peak VO2 depends primarily on variables pertaining to the underlying Fick formula physiology (heart rate, stroke volume, and arteriovenous O2 difference), VE/VCO2 slope depends on different physiology properties, including ventilatory physiology including metabolism, skeletal muscle, lung function, and autonomic regulation (35) to which HR is not related.
PAD and BMI were also significant predictors of peak VO2 and had significant statistical interactions with age. Notably, higher BMI had a significant age-related impact on predictions of peak VO2 (lower peak VO2 values in young HF patients with high BMI, with less relative declines in older adults with high BMI). Increased BMI has been associated with a possible survival advantage in older adults (37) and to some extent this seems consistent with CPX data showing less marked decreases in peak VO2 in relation to age (38). Still, these age interactions are primarily related to the fact that peak VO2 was relatively reduced among younger HF patients with high BMIs, properties that do not seem consistent with any likely health benefit. However, the main effect of decreased VE/VCO2 slope as BMI increases (see Table 5) seems somewhat consistent with a health benefit. Our data also indicate that the effect of BMI on exercise performance is at least partly independent of the effect of advancing age.
We observed that the number of comorbidities, as assessed by the aggregate comorbidity score, increased substantially with age and was inversely related to peak VO2. However, given its negligible effect on variance in our model, peak VO2 appeared to be relatively independent of the aggregate comorbidity. Aggregate comorbidity score was positively related to VE/VCO2 slope in the multivariable model (p<0.0001) but again had little effect on the overall R2, and there was no age-interaction.
VE/VCO2 slope is regarded as a useful prognostic index partly because it is less dependent on maximal exertion than is peak VO2. It is notable that in our cross-sectional assessment of the HF-ACTION study population, respiratory exchange ratios were equally high in all age strata, supporting the validity of the test results and feasibility of reasonably exhaustive exercise functional assessment in all age patients with properly trained staff, equipment, and quality control. HF-ACTION utilized a low-intensity (modified Naughton) treadmill exercise modality that was both safe (see accompanying manuscript in this supplement) and effective for achieving a peak or near-maximal endpoint in older HF patients. These data significantly extend the prior observation by Marburger et al., which was a single site study that studied 10 patients to show safety, feasibility, and reliability of cardiopulmonary exercise testing in an older HF population. (39)
We acknowledge that age explained only 12% of the variance in peak VO2. In addition, even though we had a wide range of other clinical and demographic variables to consider in the model, when all significant covariates were added to the model, less than 40% of the variation in peak VO2 was explained. This suggests that unmeasured factors are important in explaining exercise performance in heart failure patients. Indeed, it is relatively well-accepted that peripheral factors – such as muscle mass, mitochondrial energetics, blood flow, and blood hemoglobin – are likely important contributors.(5, 40) Differences in patient motivation and intrinsic test within-patient variability may also impact on the peak VO2 assessments.(16)
The strengths of the present study include the very large sample size, multiple performance sites, standardized CPX protocol, extensive quality control via a core laboratory, and prospective capture of detailed data, including key data on comorbidities, medications, and demographics that contributed to the modeling described in this manuscript.
There are several limitations. Some clinical parameters that might be related to exercise functional capacity were not included in the modeling due to the proportion of missing data that would have substantially reduced the sample size for this study and limited power to detect significant effects, in addition to possibly introducing bias. The most notable of these are hematocrit and serum creatinine. Since this is a cross-sectional study, we can only draw inferences about relationships and hypothesize regarding mechanisms. Even though HF-ACTION was successful in enrolling many older HF patients with substantial comorbidity, there is a possibility of selection bias. Patients who volunteered for a multi-year exercise training study may have had exercise performance, clinical, and/or personal characteristics that could differ from HF patients who would not enroll in such a trial.
CONCLUSION
Among patients with systolic HF, age seems to be the strongest clinical or demographic correlate of peak VO2 and one of the strongest correlates of ventilatory efficiency (VE/VCO2). Age-related effects are nonlinear and show splines at age 40 for peak VO2 and age 70 for VE/VCO2. These effects do not appear to be mediated via the increased comorbidities that are usually present in older HF patients. In contrast, the age-dependent decline in peak VO2 is significantly related to the age-related decline in peak HR at ages over 40 years. Thus, age is a key consideration in understanding the pathophysiology and the clinical management of reduced exercise performance in older HF patients.
Acknowledgments
A complete list of the HF-ACTION investigators is available as the last item in this supplement. Supported by National Institutes of Health grants: 5U01HL063747, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494,5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, 5U01HL064264, R37AG18915, P60AG10484.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel E. Forman, Cardiovascular Division, Brigham and Women’s Hospital, and Geriatric Research, Education, and Clinical Center (GRECC), VA Boston Healthcare System, Boston, MA.
Robert Clare, Duke Clinical Research Institute, Durham, NC.
Dalane W. Kitzman, Sections on Cardiology and Geriatrics, Department of Internal Medicine, Wake Forest University Health Sciences, Winston-Salem, NC.
Stephen J. Ellis, Duke Clinical Research Institute, Durham, NC.
Jerome L. Fleg, Division of Cardiovascular Disease, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Toni Chiara, Malcom Randall VA Medical Center, Department of Physical Therapy, College of Public Health and Health Professions, University of Florida, Gainesville.
Gerald Fletcher, Mayo Clinic Jacksonville, Jacksonville, FL.
William E. Kraus, Division of Cardiology, Department of Medicine, Duke University Medical Center, Durham, NC.
References
- 1.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–24. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–73. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 2007;3:381–7. doi: 10.1016/j.hfc.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitzman DW, Groban L. Exercise intolerance. Heart Fail Clin. 2008;4:99–115. doi: 10.1016/j.hfc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–82. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 7.Hollenberg M, Yang J, Haight TJ, et al. Longitudinal changes in aerobic capacity: implications for concepts of aging. J Gerontol A Biol Sci Med Sci. 2006;61:851–8. doi: 10.1093/gerona/61.8.851. [DOI] [PubMed] [Google Scholar]
- 8.Higginbotham MB, Morris KG, Williams RS, et al. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. 1986;57:1374–9. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa T, Spina RJ, Martin WH, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 11.Kitzman DW, Daniel KR. Diastolic heart failure in the elderly. Clin Geriatr Med. 2007;23:83–106. doi: 10.1016/j.cger.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Bench T, Burkhoff D, O’Connell JB, et al. Heart failure with normal ejection fraction: Consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57–64. doi: 10.1007/s11897-009-0010-z. [DOI] [PubMed] [Google Scholar]
- 13.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–69. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 14.Whellan D, O’Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007 Feb;153(2):201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102:712–7. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arena R, Myers J, Aslam SS, Varughese EB, et al. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124:720–7. doi: 10.1378/chest.124.2.720. [DOI] [PubMed] [Google Scholar]
- 18.Vallebona A, Gigli G, Orlandi S, et al. Heart rate response to graded exercise correlates with aerobic and ventilatory capacity in patients with heart failure. Clin Cardiol. 2005;28:25–9. doi: 10.1002/clc.4960280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92:481–486. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark AL, Coats AJ. Chronotropic incompetence in chronic heart failure. Int J Cardiol. 1995;49:225–231. doi: 10.1016/0167-5273(95)02316-o. [DOI] [PubMed] [Google Scholar]
- 21.Jorde UP, Vittorio TJ, Kasper ME, et al. Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: time to pace? Eur J Heart Fail. 2008;10:96–101. doi: 10.1016/j.ejheart.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Fleg JL, Schulman S, O’Connor F, et al. Effects of acute beta-adrenergic receptor blockade on age-associated changes in cardiovascular performance during dynamic exercise. Circulation. 1994;90:2333–41. doi: 10.1161/01.cir.90.5.2333. [DOI] [PubMed] [Google Scholar]
- 23.Brubaker PH, Kitzman DW. Prevalence and management of chronotropic incompetence in heart failure. Curr Cardiol Rep. 2007;9:229–35. doi: 10.1007/BF02938355. [DOI] [PubMed] [Google Scholar]
- 24.Fleg JL, O’Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 25.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 26.DeMaria AN, Neumann A, Schubart PJ, et al. Systematic correlation of cardiac chamber size and ventricular performance determined with echocardiography and alterations in heart rate in normal persons. Am J Cardiol. 1979;43:1–9. doi: 10.1016/0002-9149(79)90036-5. [DOI] [PubMed] [Google Scholar]
- 27.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker PH, Joo KC, Stewart KP, et al. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–9. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Lakatta EG. Hemodynamic adaptations to stress with advancing age. Acta Med Scand Suppl. 1986;711:39–52. doi: 10.1111/j.0954-6820.1986.tb08930.x. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, Parise H, Benjamin EF, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 31.Kovács SJ, Meisner JS, Yellin EL. Modeling of diastole. Cardiol Clin. 2000;18:459–87. doi: 10.1016/s0733-8651(05)70156-9. [DOI] [PubMed] [Google Scholar]
- 32.Sun XG, Hansen JE, Garatachea N, et al. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–8. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 33.Moore B, Brubaker PH, Stewart KP, et al. VE/VCO2 slope in older heart failure patients with normal versus reduced ejection fraction compared with age-matched healthy controls. J Card Fail. 2007;13:259–62. doi: 10.1016/j.cardfail.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46:1883–90. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Arena R, Myers J, Aslam SS, et al. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147:354–60. doi: 10.1016/j.ahj.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Makowsky MJ, McAlister FA, Galbraith PD, et al. Lower extremity peripheral arterial disease in individuals with coronary artery disease: prognostic importance, care gaps, and impact of therapy. Am Heart J. 2008;155:348–55. doi: 10.1016/j.ahj.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116:627–36. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 38.Chase P, Arena R, Myers J, et al. Relation of the prognostic value of ventilatory efficiency to body mass index in patients with heart failure. Am J Cardiol. 2008;101:348–52. doi: 10.1016/j.amjcard.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 39.Marburger CT, Brubaker PH, Pollock WE, et al. Reproducibility of cardiopulmonary exercise testing in elderly patients with congestive heart failure. Am J Cardiol. 1998;82:905–9. doi: 10.1016/s0002-9149(98)00502-5. [DOI] [PubMed] [Google Scholar]
- 40.Duscha BD, Schulze PC, Robbins JL, et al. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev. 2008;13:21–37. doi: 10.1007/s10741-007-9056-8. [DOI] [PubMed] [Google Scholar]