Abstract
Background
Cardiopulmonary exercise testing (CPX) in patients with systolic heart failure (HF) is important for determining HF prognosis and helping guide timing of heart transplantation. Although approximately 20–30% of patients with HF are obese (body mass index [BMI]>30kg/m2), the impact of BMI on CPX results is not well established. The objective of the present study was to assess the relationship between BMI and CPX variables, including peak oxygen uptake, VO2 at ventilatory threshold, O2 pulse, and ventilation / carbon dioxide production ratio.
Methods
Consecutive systolic HF patients (n=2324) enrolled in the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial who had baseline BMI recorded were included in the present study. Subjects were divided into strata based on BMI: underweight (BMI< 18.5 kg/m2), normal weight (BMI 18.5 – 24.9 kg/m2), overweight (BMI 25.0 – 29.9 kg/m2), obese I (BMI 30 – 34.9 kg/m2), obese II (BMI 35–39.9 kg/m2), and obese III (BMI ≥ 40 kg/m2).
Results
Obese III, but not overweight, obese I, or obese II, was associated with decreased peak oxygen uptake (mL/kg/min) compared to normal weight status. Increasing BMI category was inversely related to ventilation / carbon dioxide production (VE/VCO2) ratio (p< 0.0001). On multivariable analysis, BMI was a significant independent predictor of peak oxygen uptake (partial R2 = 0.07, p< 0.0001) and VE/VCO2 slope (partial R2 = 0.03, p< 0.0001) in patients with chronic systolic HF.
Conclusions
BMI is significantly associated with key CPX fitness variables in HF patients. The influence of BMI on the prognostic value of CPX in HF requires further evaluation in longitudinal studies.
Introduction
Heart failure (HF) is a prevalent and highly morbid condition, with over 5 million cases in the United States.1 In patients with advanced systolic HF, cardiopulmonary exercise testing (CPX) plays an important role in determining prognosis and optimal timing of heart transplantation.2,3 Important prognostic CPX variables include peak oxygen uptake (PKVO2), anaerobic threshold (VO2 at ventilatory threshold [VT]), O2 pulse, and ventilation / carbon dioxide production ratio (VE/VCO2). PKVO2 is often used in decision making about listing for heart transplantation; a PKVO2 of 14 mL/kg per minute is often cited as a cut-off point below which risk of mortality is high enough to warrant consideration for heart transplantation and above which heart transplantation can be safely deferred.3–5 However, the prognostic utility of CPX and PKVO2 has been questioned in special populations, including women, patients on beta-blockers, and those with obesity.6–9
Obesity (body mass index [BMI] ≥30 kg/m2) is present in approximately 20–30% of patients with advanced HF. Furthermore, obesity, as indexed by elevated BMI, has been associated with improved, rather than impaired, outcomes in a broad range of HF patients.10–12 Despite the improved HF prognosis observed with obesity, recent studies suggest that HF patients with obesity, as indexed by high BMI, have lower PKVO2 (mL/kg/min) on CPX compared to normal weight subjects.9,10 Since body fat mass, unlike muscle mass, is “metabolically inert”, some have suggested that reporting PKVO2 in mL/kg/min rather than mL/min may lead to misleadingly low values for obese subjects.4,5,13 Given the high prevalence of obesity in HF, it is important to know how BMI may affect CPX variables used in determining HF prognosis. Thus, the objective of this study is to further investigate the impact of BMI on CPX results, including PKVO2, VO2 at VT, O2 pulse, and VE/VCO2.
Methods
Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was a multi-center, randomized trial (1:1) of exercise training vs. usual care in patients with left ventricular systolic dysfunction and symptomatic HF. Major inclusion criteria were left ventricular ejection fraction (LVEF) ≤ 35%, NYHA class II–IV symptoms, stable, optimal medical therapy for 6 weeks prior to randomization, and ability to exercise, as described previously.14 Subjects with baseline BMI (n=2324) recorded were included in the present analysis.
There were 2329 of 2331 HF-ACTION study subjects who underwent baseline CPX testing prior to randomization. The primary method used for exercise testing was a modified Naughton treadmill protocol. For patients unable to exercise on a treadmill or at those testing sites that routinely used this modality for exercise testing, a leg ergometer was used (20 watts/ 2 minute stage or 10 watts / min ramp). Respiratory gas exchange was recorded during exercise testing, in addition to blood pressure and continuous EKG recordings. Patients were strongly encouraged to exercise to a sign and symptom-limited maximal exertion.14 Multiple physiologic variables obtained via CPX testing were determined, including PKVO2, VO2 at VT, O2 pulse, and VE/VCO2, maximum heart rate, and exercise time. PKVO2 is defined as oxygen uptake at peak exercise, and can be described as an absolute value (mL/min) or relative to body weight (mL/kg/min). Ventilatory-derived anaerobic threshold (VO2 at VT), the VO2 at which ventilation increases disproportionately relative to VO2 and work, also known as the lactate threshold, was determined by the modified v-slope method by two blinded reviewers (mL/kg/min). O2 pulse is defined as PKVO2 in mL/min divided by peak heart rate and is expressed as mL/kg/beat. VE/VCO2 is the slope of ventilation to carbon dioxide output 5. Furthermore, 6-minute walk tests were performed at baseline to determine sub-maximal exercise capacity (meters). All CPX data were analyzed by a core laboratory.
BMI (weight in kg / height in m2), which strongly correlates with body fat mass, was recorded at baseline in all subjects included in the present analysis. Subjects were divided into groups based on BMI as defined by the International Obesity Taskforce: underweight (BMI< 18.5 kg/m2), normal weight (BMI 18.5 – 24.9 kg/m2), overweight (BMI 25.0 – 29.9 kg/m2), obese I (BMI 30 – 34.9 kg/m2), obese II (BMI 35–39.9 kg/m2), and obese III (BMI ≥ 40 kg/m2).15 Baseline demographic data, clinical data, and CPX data were analyzed by BMI category. Data are expressed as median and interquartile range. The correlation between continuous variables and BMI was assessed by Pearson’s r correlation coefficient. Separate multivariable linear regression models were fit for PKVO2 and VE/VCO2, respectively. For each multivariable model, a list of 33 candidate predictor variables was considered for inclusion in the multivariable model. Those variables are listed in Table 4. From the 33 variables, those with the highest p-values were sequentially eliminated according to the partial F-test from inclusion in the multivariable model until all remaining variables had multivariable p<0.05. From the remaining variables, those variables with partial R-square <0.01 were eliminated from inclusion in the final multivariable model. The variables which were included in the final multivariable model all have partial R-square ≥ 0.01.
Table 4.
Candidate Predictor Variables for the Peak VO2 and VE/VCO2 Multivariable Models
|
Statistical analyses were performed using SAS version 9.0 (SAS Institute, Inc, Cary, North Carolina) and R version 2.7.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were 2-tailed with statistical significance defined to be at the 0.05 level.
Results
Of 2331 subjects randomized, 2324 had complete baseline BMI data and were included in the present analysis. Median LVEF in the study cohort was 25% (25th–75th percentiles: 20% – 30%). The NYHA II, III, and IV class representation were 63%, 36%, and <1%, respectively; 94% of the patients were on an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, 94% were on a beta-blocker, and 18% had biventricular pacemakers. The baseline characteristics of the cohort stratified by BMI category are displayed in Table 1. Higher BMI category was associated with younger age and higher likelihood of black race (p<0.0001). Elevated BMI was associated with a lower overall score on the Kansas City Cardiomyopathy Questionnaire, suggesting a lower disease specific health status16
Table 1.
Baseline Characteristics of the Study Cohort Stratified by Body Mass Index Category
| Underweig ht* N = 10 |
Normal weight N = 448 |
Overweight N = 724 |
Obese I N = 551 |
Obese II N = 330 |
Obese III N = 261 |
Pearson’s r coef.** |
|
|---|---|---|---|---|---|---|---|
| NYHA II/III/IV % |
30/70/0 | 63/36/1 | 69/30/1 | 63/35/2 | 62/37/1 | 51/49/0 | |
| Left ventricular ejection fraction, % |
29 (24, 34) | 24 (20, 30) | 25 (20, 30) | 25 (21, 30) | 25 (20, 31) | 25 (20, 30) | 0.00 (p=0.85) |
| Ischemic Etiology, % |
40% | 52% | 60% | 57% | 44% | 25% | |
| Beck Depression Inventory II score |
14 (5, 16) | 7 (4, 13) | 8 (4, 13) | 8 (5, 15) | 10 (5, 17) | 10 (6, 17) | 0.12 |
| Kansas City Cardiomyo- pathy Question- naire Overall Score |
67 (49, 83) | 72 (55, 88) | 71 (55, 86) | 66 (50, 82) | 61 (47, 80) | 60 (43, 76) | −0.18 |
| EuroQOL | 56 (50, 80) | 70 (50, 80) | 70 (56, 80) | 70 (50, 80) | 66 (50, 80) | 65 (50, 80) | −0.07 |
| Age (y) | 61 (56, 75) | 63 (55, 74) | 63 (54, 70) | 59 (51, 67) | 56 (49, 62) | 50 (40, 57) | −0.37 |
| Sex F/M% |
50/50 | 32/68 | 22/78 | 27/73 | 32/68 | 37/63 | |
| Race Black/White/ other % |
10/70/20 | 29/65/6 | 25/69/6 | 31/65/4 | 40/55/5 | 57/39/4 | |
| Serum creatinine, mg/dL |
0.8 (0.6, 0.9) |
1.2 (1.0, 1.5) |
1.2 (1.0, 1.5) |
1.2 (1.0, 1.5) |
1.1 (1.0, 1.4) |
1.1 (0.9, 1.3) |
−0.06 (p=0.01) |
| Beta-blocker, % |
90% | 94% | 93% | 95% | 97% | 96% | |
| Angiotensin- Converting Enzyme Inhibitor or Angiotensin II Receptor Blocker, % |
90% | 93% | 94% | 94% | 96% | 94% |
Data are recorded as median (interquartile range) for continuous variables and % total for categorical variables.
underweight (BMI< 18.5 kg/m2), normal weight (BMI 18.5 – 24.9 kg/m2), overweight (BMI 25.0 – 29.9 kg/m2), obese I (BMI 30 – 34.9 kg/m2), obese II (BMI 35–39.9 kg/m2), obese III (BMI ≥ 40 kg/m2)
Pearson's r correlation coefficient for all of the continuous variables correlated with BMI as a continuous variable. Unless otherwise indicated, all variables significant at the p < 0.0001 level.
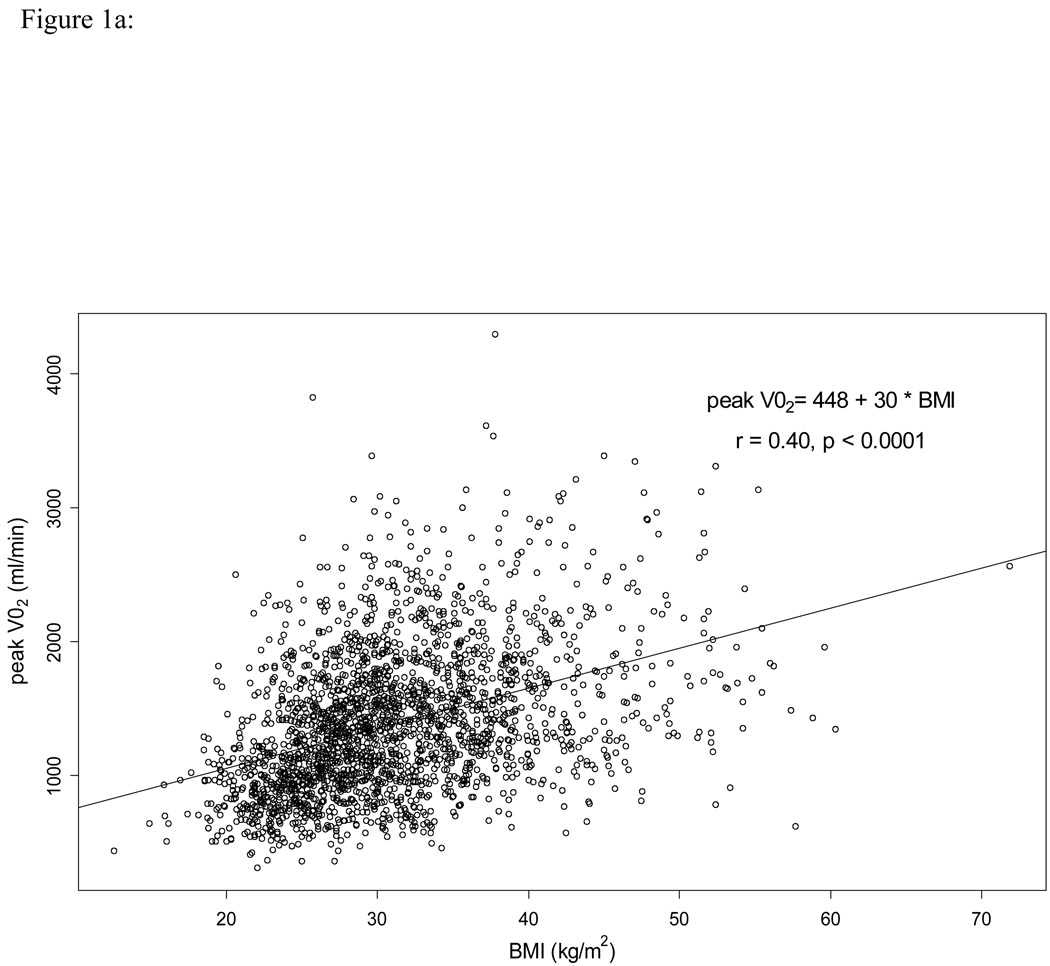
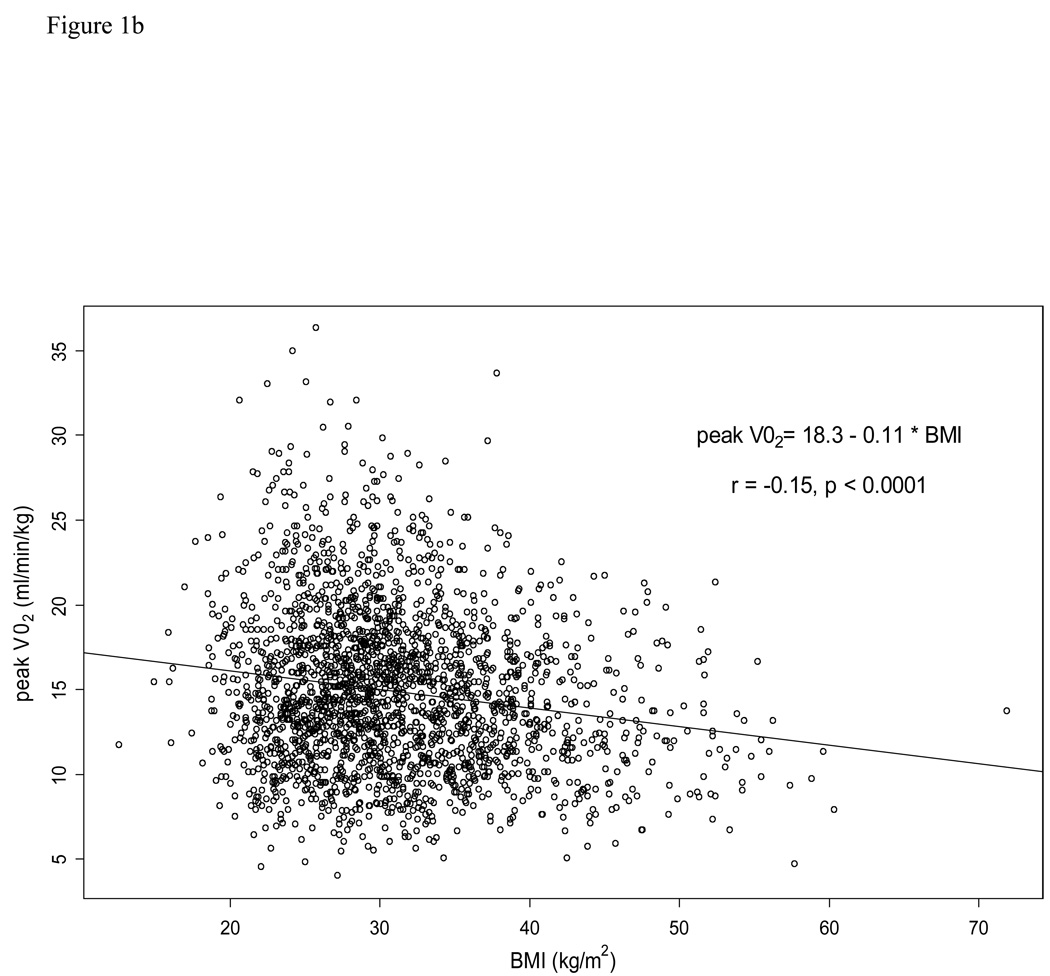
Table 2 outlines the physiologic exercise variables by BMI category. For all of the exercise variables besides VO2 at VT, data was non-missing in over 96% of the patients. For VO2 at VT, data was non-missing in 83% of the patients. Absolute PKVO2 (mL/min) increased with increasing BMI category. However, relative PKVO2 (mL/kg/min) was lowest in the Obese III category and not qualitatively different among the normal weight, overweight, and Obese I categories. Scatterplots depicting the relationship between BMI and PKVO2 (ml/min) and between BMI and PKVO2 (ml/kg/min) are displayed in Figures 1a and 1b, respectively. VO2 at VT, O2 pulse, VE/VCO2 slope, and exercise time all decreased with increasing BMI category. Distance on six-minute walk test also decreased with increasing BMI category.
Table 2.
Baseline Exercise Variables Stratified by Body Mass Index Categories.
| Underweight N = 10 |
Normal weight N = 448 |
Over- weight N = 724 |
Obese I N = 551 |
Obese II N = 330 |
Obese III N = 261 |
Pearson’s r coef.** |
|
|---|---|---|---|---|---|---|---|
| Peak VO2 (mL/ min) |
655 (628, 921) |
962 (772, 1205) |
1278 (981, 1575) |
1439 (1071, 1763) |
1523 (1242, 1858) |
1676 (1279, 2117) |
0.40 |
| Peak VO2 (mL/kg/min) |
15.4 (11.8, 18.3) |
14.4 (11.6, 18.0) |
15.1 (12.4, 18.4) |
15.0 (11.2, 17.8) |
13.9 (11.3, 16.6) |
12.4 (10.1, 15.9) |
−0.17 |
| VO2 at VT (mL/kg/min) |
13.0 (10.0, 13.6) |
10.9 (9.3, 13.0) |
10.8 (9.3, 12.6) |
10.7 (8.7, 12.7) |
10.0 (8.4, 11.6) |
9.5 (8.1, 11.4) |
−0.18 |
| Peak respiratory exchange ratio |
1.12 (1.00, 1.21) |
1.09 (1.02, 1.17) |
1.10 (1.03, 1.17) |
1.09 (1.02, 1.16) |
1.08 (1.02, 1.14) |
1.07 (1.00, 1.13) |
−0.12 |
| O2 pulse (mL/ kg/beat) |
0.13 (0.12, 0.15) |
0.13 (0.10, 0.16) |
0.13 (0.11, 0.15) |
0.13 (0.10, 0.15) |
0.11 (0.10, 0.13) |
0.10 (0.08, 0.12) |
−0.27 |
| VE/VCO2 | 36 (33, 42) | 36 (30, 43) |
33 (29, 37) |
32 (29, 37) |
30 (27, 35) |
29 (25, 34) |
−0.25 |
| Exercise time (min) |
7.1 (6.0, 10.5) |
9.4 (6.7, 12.0) |
10.3 (8.0, 13.0) |
9.8 (7.1, 12.0) |
9.1 (6.6, 11.6) |
8.0 (5.7, 10.3) |
−0.15 |
| 6 minute walk distance (m) |
362 (269, 402) |
366 (296, 430) |
387 (317, 446) |
372 (297, 443) |
362 (294, 426) |
335 (274, 407) |
−0.12 |
| HR at peak exercise (beats/min) |
123 (94, 139) | 115 (98, 131) |
119 (105, 132) |
120 (103, 133) |
122 (108, 136) |
123 (111, 141) |
0.13 |
Data are recorded as median (interquartile range) for continuous variables and % total for categorical variables.
underweight (BMI< 18.5 kg/m2), normal weight (BMI 18.5 – 24.9 kg/m2), overweight (BMI 25.0 – 29.9 kg/m2), obese I (BMI 30 – 34.9 kg/m2), obese II (BMI 35–39.9 kg/m2), obese III (BMI ≥ 40 kg/m2)
Pearson's r correlation coefficient for all of the continuous variables correlated with BMI as a continuous variable. All r values are significant at the p < 0.0001 level.
Figure 1.
1a.Scatterplot of the relationship between body mass index (BMI) and peak oxygen uptake (PKVO2, mL/min).
1b.Scatterplot of the relationship between body mass index (BMI) and peak oxygen uptake (PKVO2, mL/kg/min).
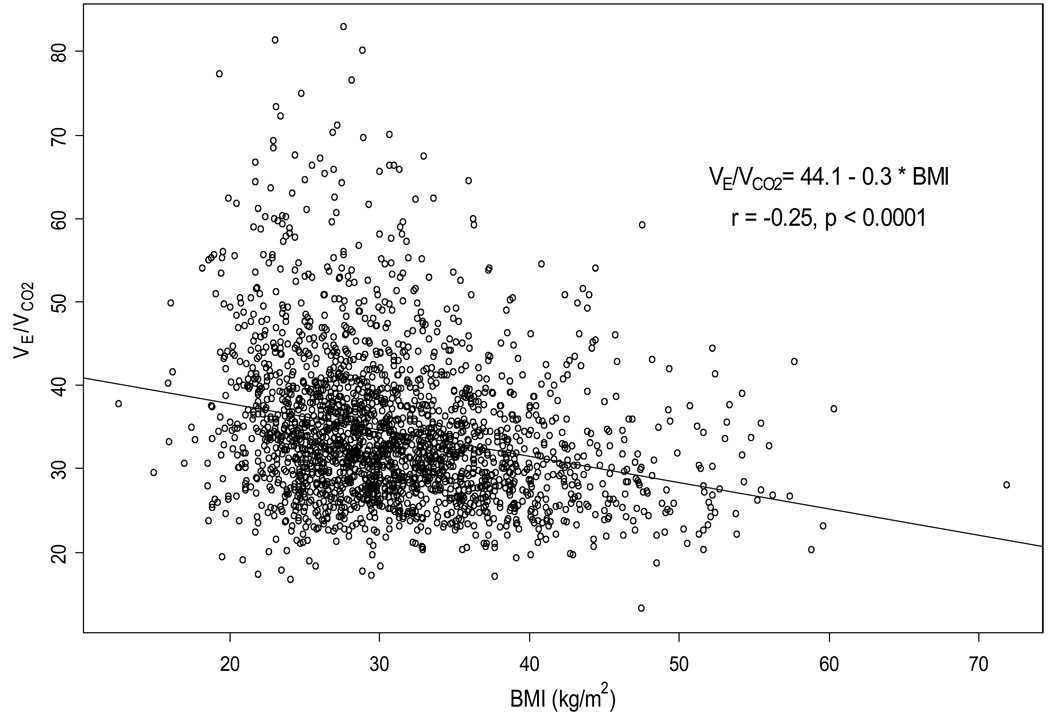
In multivariable linear regression analysis for prediction of PKVO2 (mL/kg/min) as a continuous variable (Table 3), BMI was a strong predictor of PKVO2, second only to age among the variables examined from Table 4. The other significant variables associated with PKVO2 in multivariable analysis were history of diabetes, gender, geographic region, LVEF, mode of CPX, NYHA class, history of peripheral vascular disease, race, and ventricular conduction on EKG. When PKVO2 was analyzed as a categorical variable on logistic regression analysis, the same variables were found to predict low PKVO2 (≤ 14 mL/kg/min). BMI was also found to be a significant predictor of VE/VCO2 slope (Table 5, Figure 2).
Table 3.
Multivariable Analysis of Peak VO2 (mL/kg/min) as a Continuous Variable.
| Variable | Coefficient | 95% Confidence Interval |
Partial R-square |
P-value |
|---|---|---|---|---|
| Age | −0.13 | (−0.14, −0.11) | 0.12 | <0.0001 |
| BMI | −0.16 | (−0.18, −0.13) | 0.07 | <0.0001 |
| CPX mode (treadmill vs. bike) |
−2.5 | (−3.2, −1.9) | 0.02 | <0.0001 |
| Diabetes | −1.2 | (−1.5, −0.8) | 0.02 | <0.0001 |
| ECG vent. cond. |
0.03 (overall) |
<0.0001 (overall) |
||
| IVCD | −1.3 | (−1.8, −0.8) | ||
| LBBB | −0.5 | (−1.0, −0.1) | ||
| Paced | −1.5 | (−1.9, −1.1) | ||
| RBBB | −1.6 | (−2.5, −0.8) | ||
| Gender | −2.1 | (−2.5, −1.7) | 0.06 | <0.0001 |
| LVEF | 0.07 | (0.05, 0.09) | 0.02 | <0.0001 |
| NYHA Class (II vs. III/IV) |
−2.0 | (−2.4, −1.7) | 0.06 | <0.0001 |
| PVD | −2.0 | (−2.6, −1.4) | 0.02 | <0.0001 |
| Race | 0.06 (overall) |
<0.0001 (overall) |
||
| African Amer. | −2.2 | (−2.5, −1.8) | ||
| Other | −1.1 | (−1.8, −0.4) | ||
| Region | 0.03 (overall) |
<0.0001 (overall) |
||
| West USA | 0.7 | (0.1, 1.2) | ||
| Midwest USA | 0.5 | (0.2, 0.9) | ||
| Northeast USA | −0.8 | (−1.3, −0.2) | ||
| Canada | −1.1 | (−1.7, 0.5) | ||
| France | 2.3 | (1.0, 3.6) | ||
Reference categories for categorical variables: sex = male, diabetes = non-diabetics, PVD = non-PVD, region = South USA, race = Caucasian, NYHA Class = Class II, CPX mode = treadmill, Overall model R2 = 0.39.
Table 5.
Multivariable Analysis of VE/VCO2 on CPX.
| Variable | Coefficient | 95% Confidence Interval |
Partial R-square |
P-value |
|---|---|---|---|---|
| Age | 0.14 | (0.11, 0.17) | 0.04 | <0.0001 |
| BMI | −0.22 | (−0.27, −0.17) | 0.03 | <0.0001 |
| ECG vent. cond. |
0.01 (overall) |
<0.0001 (overall) |
||
| IVCD | 2.2 | (1.1, 3.3) | ||
| LBBB | 1.3 | (0.3, 2.3) | ||
| Paced | 1.9 | (0.9, 2.8) | ||
| RBBB | 3.3 | (1.5, 5.2) | ||
| LVEF | −0.16 | (−0.20, −0.11) | 0.02 | <0.0001 |
| NYHA Class (II vs. III/IV) |
3.4 | (2.8, 4.2) | 0.04 | <0.0001 |
Reference categories for categorical variables: NYHA Class = Class II, ECG ventricular conduction = normal conduction
Overall model R2 = 0.19.
Figure 2.
Scatterplot of the relationship between body mass index (BMI) and ventilation / carbon dioxide production ratio (VE/VCO2).
Discussion
Historically, PKVO2 has been the most widely used CPX variable in determining HF prognosis and timing of transplant; however, other variables determined by CPX, including percent predicted PKVO2, VE/VCO2 slope, and O2 pulse, are also prognosticators in chronic systolic HF.3,4,17–20 CPX results are also used to evaluate functional status, guide exercise prescriptions, and assess efficacy of therapy in HF.21 Given the prevalence of obesity in HF, the impact of BMI on physiologic variables obtained from CPX is important to determine. This is the largest study, to our knowledge, to systematically explore the relationship between BMI and results of CPX in patients with chronic HF and LVEF≤35%. We have found that although elevated BMI is associated with higher absolute PKVO2 (mL/min), elevated BMI is an independent predictor of low relative PKVO2 (mL/kg/min). Higher BMI is also associated with lower O2 pulse, VO2 at VT, and VE/VCO2. On multivariable analysis, BMI remains a significant predictor of VE/VCO2 slope, although the relationship between BMI and VE/VCO2 is weaker than that between BMI and PKVO2.
Although obesity is associated with increased risk of new onset HF 22, a surprising relationship between BMI and outcomes in those with established HF has been observed. Counter to expectations, high BMI, or overweight/obesity, is consistently associated with better HF outcomes. This “obesity paradox” in HF has been confirmed in diverse HF populations, including advanced HF patients awaiting transplant, stable outpatients with HF enrolled in clinical trials, and hospitalized patients with acute decompensated HF.10–12,23 Recent studies suggest that despite better prognosis with obesity, HF patients with high BMI have lower PKVO2 (mL/kg/min).9,10 Furthermore, in apparently healthy subjects and coronary artery disease patients, a graded inverse relationship between BMI and PKVO2 (mL/kg/min) has been demonstrated.24,25 The PKVO2 of morbidly obese subjects without apparent heart disease has been shown to be in the same low range as that for NYHA III–IV HF patients.25
It has been suggested that reporting PKVO2 in mL per kg of total body weight may result in misleadingly low values for obese subjects, since a large percentage of body weight may be fat mass, which does not metabolize O2. 4,5,13 In fact, several studies of HF patients have demonstrated PKVO2 adjusted for lean body mass (as determined by skin-fold thickness or DEXA scan) may be a better discriminator of prognosis in chronic HF compared to PKVO2 adjusted for total body weight.26–28 However, valid and reliable measures of body composition may not be available during routine CPX. Other studies have shown that O2 pulse or VE/VCO2 slope may be better predictors of prognosis compared to PKVO2 in patients with high BMI or obesity.20,29 Of note, lower VE/VCO2, which was associated with higher BMI in the current analysis, corresponds to better prognosis in subjects with chronic HF.
Limitations
A limitation of this study is that BMI is our only index of obesity; there are no measures of body composition or fat mass. Measures of pulmonary function which may affect CPX variables were not available. This is a cross-sectional study of baseline variables in a large randomized-controlled trial; a future report will assess the interaction between BMI and CPX variables on prognosis.
Conclusions
In this large, well-characterized cohort of HF patients undergoing CPX, BMI is a strong independent predictor of lower relative PKVO2. Higher BMI is also independently associated with lower VE/VCO2 slope. Longitudinal studies are needed to assess which CPX variables are the best predictors of prognosis in obese HF patients.
Acknowledgments
Grant Support: HF-ACTION is funded by the NHLBI; grant numbers 5U01-HL063747, 5U01-HL066461, HL068973, HL068973, HL066501, HL066482, HL064250, HL066494, HL064257, HL066497 HL068980, HL064265, HL066491, HL064264
HF-ACTION is registered: www.clinicaltrials.gov, study number NCT00047437
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y for the American Heart Association Statistics Committee and Stroke Statistics S. Heart Disease and Stroke Statistics--2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Likoff MJ, Chandler SL, Kay HR. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol. 1987;59:634–638. doi: 10.1016/0002-9149(87)91183-0. [DOI] [PubMed] [Google Scholar]
- 3.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 4.Milani RV, Lavie CJ, Mehra MR. Cardiopulmonary exercise testing: how do we differentiate the cause of dyspnea? Circulation. 2004;110:e27–e31. doi: 10.1161/01.CIR.0000136811.45524.2F. [DOI] [PubMed] [Google Scholar]
- 5.Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part I: Interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil. 2006;13:150–164. doi: 10.1097/01.hjr.0000209812.05573.04. [DOI] [PubMed] [Google Scholar]
- 6.Tabet JY, Metra M, Thabut G, Logeart D, Cohen-Solal A. Prognostic value of cardiopulmonary exercise variables in chronic heart failure patients with or without beta-blocker therapy. Am J Cardiol. 2006;98:500–503. doi: 10.1016/j.amjcard.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Elmariah S, Goldberg LR, Allen MT, Kao A. Effects of Gender on Peak Oxygen Consumption and the Timing of Cardiac Transplantation. Journal of the American College of Cardiology. 2006;47:2237. doi: 10.1016/j.jacc.2005.11.089. [DOI] [PubMed] [Google Scholar]
- 8.Zugck C, Haunstetter A, Krüger C, Kell R, Schellberg D, Kübler W, Haass M. Impact of beta-blocker treatment on the prognostic value of currently used risk predictors in congestive heart failure. Journal of the American College of Cardiology. 2002;39:1615. doi: 10.1016/s0735-1097(02)01840-5. [DOI] [PubMed] [Google Scholar]
- 9.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–894. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 10.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 11.Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LAM, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, Granger CB, McMurray JJV, Solomon SD for the CI. Body Mass Index and Prognosis in Patients With Chronic Heart Failure: Insights From the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Program. Circulation. 2007;116:627–636. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Barker D, Artis N, Tan LB. Correcting data for body size may confound results. Chest. 2006;129:493–494. doi: 10.1378/chest.129.2.493. author reply 493–4. [DOI] [PubMed] [Google Scholar]
- 14.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Pina IL. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 16.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic Value of Health Status in Patients With Heart Failure After Acute Myocardial Infarction. Circulation. 2004;110:546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 17.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 18.Stelken AM, Younis LT, Jennison SH, Miller DD, Miller LW, Shaw LJ, Kargl D, Chaitman BR. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol. 1996;27:345–352. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 19.Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, Poole-Wilson PA, Coats AJ. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1997;29:1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV, Mehra MR. Peak exercise oxygen pulse and prognosis in chronic heart failure. The American Journal of Cardiology. 2004;93:588. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: Interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil. 2006;13:485–494. doi: 10.1097/01.hjr.0000201518.43837.bc. [DOI] [PubMed] [Google Scholar]
- 22.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 23.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Shubair MM, Kodis J, McKelvie RS, Arthur HM, Sharma AM. Metabolic profile and exercise capacity outcomes: their relationship to overweight and obesity in a Canadian cardiac rehabilitation setting. J Cardiopulm Rehabil. 2004;24:405–413. doi: 10.1097/00008483-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher MJ, Franklin BA, Ehrman JK, Keteyian SJ, Brawner CA, deJong AT, McCullough PA. Comparative impact of morbid obesity vs heart failure on cardiorespiratory fitness. Chest. 2005;127:2197–2203. doi: 10.1378/chest.127.6.2197. [DOI] [PubMed] [Google Scholar]
- 26.Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol. 2000;36:2126–2131. doi: 10.1016/s0735-1097(00)00985-2. [DOI] [PubMed] [Google Scholar]
- 27.Cicoira M, Davos CH, Francis DP, Doehner W, Zanolla L, Franceschini L, Piepoli MF, Coats AJ, Zardini P, Poole-Wilson PA, Anker SD. Prediction of mortality in chronic heart failure from peak oxygen consumption adjusted for either body weight or lean tissue. J Card Fail. 2004;10:421–426. doi: 10.1016/j.cardfail.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 28.LeMaitre JP, Harris S, Hannan J, Fox KA, Denvir MA. Maximum oxygen uptake corrected for skeletal muscle mass accurately predicts functional improvements following exercise training in chronic heart failure. Eur J Heart Fail. 2006;8:243–248. doi: 10.1016/j.ejheart.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Chase P, Arena R, Myers J, Abella J, Peberdy MA, Guazzi M, Bensimhon D. Relation of the Prognostic Value of Ventilatory Efficiency to Body Mass Index in Patients With Heart Failure. Am J Cardiol. 2008;101:348–352. doi: 10.1016/j.amjcard.2007.08.042. [DOI] [PubMed] [Google Scholar]