Summary
Survival following pancreatic cancer remains poor despite incremental advances in surgical and adjuvant therapy, and new strategies for treatment are needed. Oncolytic virotherapy is an attractive approach for cancer treatment. In this study, we have evaluated the effectiveness of the Lister vaccine strain of vaccinia virus armed with the endostatin-angiostatin fusion gene (VVhEA) as a novel therapeutic approach for pancreatic cancer. The Lister vaccine strain of vaccinia virus was effective against all human pancreatic carcinoma cells tested in vitro, especially those insensitive to oncolytic adenovirus. The virus displayed inherently high selectivity for cancer cells, sparing normal cells both in vitro and in vivo, with effective infection of tumors after both intravenous (IV) and intratumoral (IT) administration. The expression of endostatin-angiostatin fusion protein was confirmed in a pancreatic cancer model both in vitro and in vivo, with evidence of inhibition of angiogenesis. This novel vaccinia virus demonstrated significant antitumor potency in vivo against the Suit-2 model by IT administration. The present study suggests that the novel Lister strain of vaccinia virus armed with the endostatin-angiostatin fusion gene is a potential therapeutic agent for pancreatic cancer.
Keywords: human pancreatic cancer, adenovirus, vaccinia virus, angiogenesis, endostatin, angiostatin
Introduction
Pancreatic cancer is currently a leading cause of cancer-related deaths in Western countries since most patients present with advanced or metastatic disease and few are suitable for curative surgical resection. Palliative chemotherapy has poor results and the overall five-year survival rate is less than 5% 1. New treatment strategies are clearly imperative. With increasing knowledge of the molecular genetics of pancreatic cancer 2, gene therapy has become an attractive option, although clinical trials to date have shown only moderate efficacy 3.
Replication-selective oncolytic viruses are rapidly expanding as a class of therapeutic agents for cancer. Replication-selective oncolytic adenovirus is the most well-researched, and a first generation virus (dl1520, or H101 in China) has been approved as the world's first oncolytic virotherapy for head and neck cancer therapy 4. This virus has been administered by intratumoral (IT) injection into patients with locally advanced pancreatic tumors in phase I/II trials. Although treatments were well tolerated, no objective responses were seen in patients after virus alone, and only two of 21 patients showed objective responses when gemcitabine was used in combination 5. One major hurdle affecting oncolytic adenovirus potency is the genetic make-up in cancer cells, which affects virus infectivity. For example, the expression of adenovirus receptor, CAR (Coxsackie-Adenovirus Receptor) is often low on many tumor types, including pancreatic cancer 6. To make progress, new strategies are needed to overcome this obstacle. One of them could be to use other oncolytic viruses such vaccinia virus.
Oncolytic vaccinia virus represents an attractive alternative, since it has several features that make it suitable for use as an oncolytic agent 7-11. A defining feature of vaccinia virus as an oncolytic agent is that it relies mostly on its own encoded proteins to carry out replication and transcription in the cytoplasm, with few (if any) host proteins required. Also the vaccinia virus has a wide host range and tissue tropism which provides an ability to infect almost all types of mammalian cells. These may overcome the difficulty encountered with adenovirus and allow vaccinia virus to replicate in many tumor types. The vaccinia virus has been safely injected into patients through subcutaneous 12, intramuscular 13, intra-tumoral 14 and intravesical 15 administration. The oncolytic capacity of vaccinia virus, as well as the safety record of this virus, make it a potentially ideal choice for cancer treatment 11,16,17.
Oncolytic vaccinia virus is inherently tumor-selective in part due to the low production of interferons by tumor cells in response to vaccinia infection, while these cytokines inhibit vaccinia replication in normal cells 18. However, tumor selectivity is enhanced due to the overexpression of epidermal growth factor receptor by many tumor types. Vaccinia viruses express vaccinia growth receptor (VGF) early during replication. VGF is homologous to cellular EGF and, therefore, binds to the ErbB family of receptors 19. The signaling through these tyrosine kinase receptors results in DNA synthesis and cell proliferation, including thymidine kinase-induced nucleotide production, thereby increasing vaccinia replication 20. Since pancreatic cancers frequently overexpress EGFR, this tumor type represents a promising model for targeted oncolytic vaccinia therapy 21.
Pancreatic tumors are often well-vascularised and high microvessel density (MVD) has been shown to correlate with poor prognosis after curative resection 22. Therefore, inhibition of angiogenesis is a rational target for the treatment of pancreatic cancer and many angiogenesis inhibitors have been investigated in animal models of pancreatic cancer 23, resulting in recent clinical trials 24,25. Despite these efforts, efficacy has been limited. Endostatin and angiostatin are two potent endogenous angiogenesis inhibitors that have displayed antitumor efficacy in various animal models 26-29, including pancreatic cancer 27,29. They act synergistically when used in combination, which led to the construction of an endostatin-angiostatin fusion gene 30. This has been successfully delivered by both viral 30-33 and non-viral vectors 34.
The combination of an oncolytic vaccinia virus that also expresses the endostatin-angiostatin fusion gene may provide potent, targeted angiogenesis inhibition on a continuous basis in the local tumor environment to slow tumor growth, allowing the virus to spread through and destroy the tumour. We therefore constructed an oncolytic vaccine strain of vaccinia virus expressing a human endostatin-angiostatin fusion gene (VVhEA). In the present report, we tested our hypothesis and demonstrate that VVhEA is a potential therapeutic agent targeting human pancreatic cancer.
Results
Lister strain of vaccinia virus is more potent than adenovirus and replicates in human pancreatic cancer cell lines insensitive to adenovirus
To demonstrate the potential of the Lister vaccine strain of vaccinia virus as a therapeutic agent for human pancreatic cancer, we compared the cytotoxicity of VVLister and Ad5 in a panel of human pancreatic cancer cell lines in vitro (Fig. 1A). VVLister displayed a greater potency than Ad5 in all cell lines tested, including cancer cells insensitive to adenovirus, such as PaTu8988s, Suit-2, HS766T and Capan1. To determine whether vaccinia virus-induced cell killing was indicative of replication induction in cancer cells, replication of VVLister was confirmed in PaTu8988s and Suit-2 (Fig. 1B and C).
Figure 1. Cytotoxicity and replication of Lister strain of vaccinia virus in human cancer cells and normal epithelial cells.
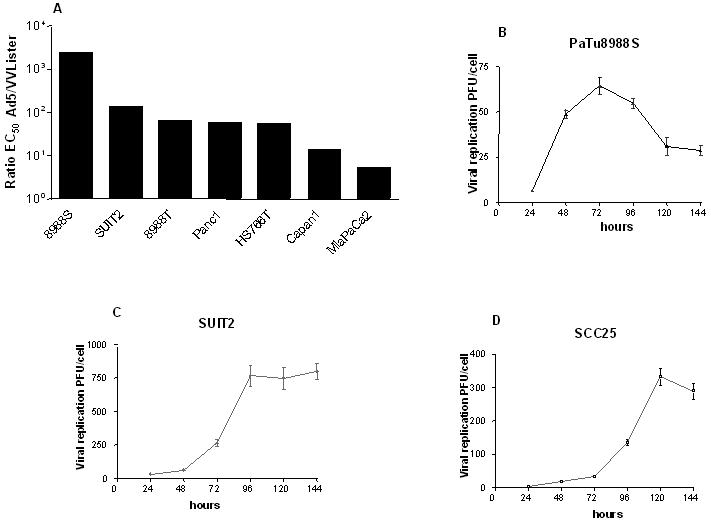
A, Comparison of cytotoxicity of Lister strain vaccinia virus and adenovirus in a panel of human pancreatic cancer cell lines. Mean EC50 values were derived by MTS assay six days after infection of the Lister strain of vaccinia virus and adenovirus, displayed in order of Ad5/VVLister ratio (a value greater than 1.0 indicates that VV Lister was more potent); B and C, The viral replication of vaccinia virus in human pancreatic cancer cells insensitive to adenovirus; D, Viral replication in human squamous epithelial cells. Cells were infected with 1 PFU/cell (5PFU/cell for NHEK) of VVLister and cell lysates harvested over a time course. Mean viral replication values ± SEM were determined by the TCID50 assay.
Lister vaccine strain of vaccinia virus displays selectivity between tumor cells and normal cells in vitro and in vivo
The Lister strain vaccinia virus has been used for visualization of tumors and metastases and cancer treatment 35-37, however, the selective resistance of normal human cells was not definitively demonstrated, especially between normal human cells and cancer cells. Therefore, the tumor selectivity in vitro was evaluated by comparing the replication of VVLister in non-immortalized normal and malignant squamous epithelial cell lines. Despite infecting normal human epithelial keratinocytes (NHEK) cells with five times the dose of VVLister as that used for SCC25 tumor cells, no significant viral replication was seen in NHEK (data not shown) in contrast to the marked viral replication in SCC25 (Fig. 1D).
The VVlister vaccinia virus has shown tumor tropism in vivo in mouse models 35-38, so we investigated whether the virus still has selectivity in our human pancreatic cancer xenograft model by real-time fluorescence imaging and IHC which confirmed the tumor selectivity of the vaccinia virus in vivo. We first confirmed dose-dependent GFP expression in Suit-2 cells infected with VVRG in vitro (supplementary Fig.1) before injecting BALB/c nude mice bearing Suit-2 xenografts with IT or IV injection of VVRG (Fig. 2A and B). GFP expression was seen in all tumors following delivery by either route from 24 through to 240 hours. Expression after IT virus administration increased to a peak at 72 hours, whereas after IV delivery, levels were still rising 10 days later, suggesting that IV delivery of the Lister strain of vaccinia virus may be superior to IT delivery. Only background activity was observed in tumors of control mice injected with PBS. Vaccinia virus displayed excellent tumor selectivity with extra-tumoral fluorescence only observed in the tails of three mice after IV and one after IT delivery, and the paws of two mice after IT delivery, although this resolved by 240 hours.
Figure 2. Biodistribution of vaccinia virus in BALB/c nude mice with Suit-2 subcutaneous tumors.

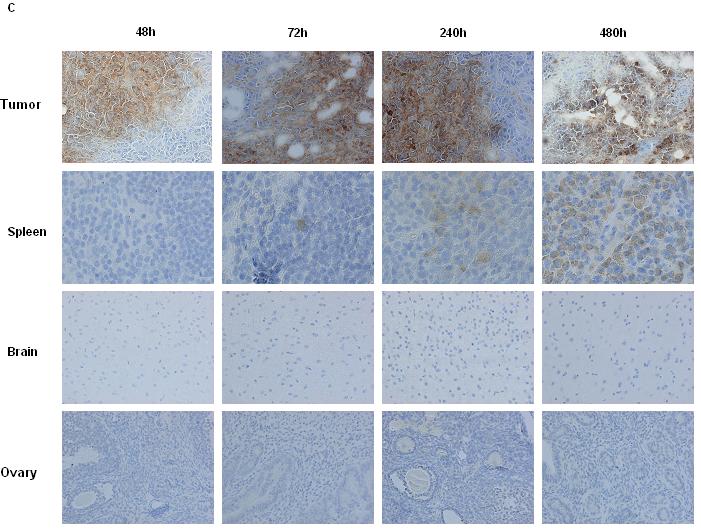
A and B, Biodistribution of VVRG in vivo. Biodistribution of VVRG was ascertained by IVIS imaging under inhalation anaesthesia 24, 48, 72, 120 and 240 hours after virus administration. Representative images are shown at 120 hours (left panel) and combined mean efficiency ± SEM (right panel). 1×106 cells Suit-2 were seeded by subcutaneous injection into the right flank of 10 BALB/c nude mice. When tumors reached 4-5mm in diameter, in A, three mice were injected once IT with 1×107 PFU VVRG and two mice with PBS as controls and in B, four mice were injected once IV with 1×107 PFU VVRG and one mouse with PBS as a control; C, Biodistribution of the Lister strain of vaccinia virus after IV injection of 1×107 PFU VVRG to BALB/c nude mice with Suit-2 tumors by IHC for Lister coat protein in tumors, spleen, brain and ovaries (x200).
The tumor selectivity of the parental VVLister was also confirmed by IHC of tumors and organs harvested from nude mice bearing Suit-2 xenografts after single IV virus injection (Fig. 2C). VVLister was seen in all tumors from 24 to 480 hours after delivery. Selectivity over normal tissues was confirmed, as only monocytes in the spleen were positive for VV coat protein in one of three mice at 24 and 72 hours and all three mice from 120 to 480 hours. Ovaries, brains, liver, kidneys, lungs and adrenal glands were all negative, including PBS-treated controls (not shown).
Construction, potency, and replication of a novel vaccinia virus expressing human endostatin-angiostatin fusion protein
In order to enhance the efficacy of oncolytic vaccinia virus, the endostatin-angiostatin fusion gene was inserted at the Not1 restriction site of the Lister vaccine strain of vaccinia virus (VVhEA, Fig. 3A). Another recombinant virus (VVlacZ) was constructed with the marker gene at the same genomic site serving as a control (Fig. 3A). The sequences of inserted genes were confirmed by PCR and sequencing (data not shown). The potency and replication of both recombinant viruses in vitro were consistently less than that of the parental VVLister (Fig. 3B, C and D). The potency of VVhEA was also less than that of VVlacZ in all cell lines tested, but there was no significant difference in the levels of peak replication between VVhEA and VVlacZ in PaTu8988s or Suit-2 cells (Fig. 3 C and D).
Figure 3. Potency and replication of recombinant vaccinia viruses armed with human endostatin-angiostatin fusion gene and LacZ gene.

A, VVlacZ and VVhEA construction by insertion of the lacZ and human endostatin-angiostatin fusion genes respectively into the Not1 restriction site of VVLister; B, Potency of VVhEA and VVLacZ in human pancreatic cancer cell lines. EC50 values + SEM calculated by MTS assay 144 hours after infection with different vaccinia viruses; C and D, Replication of recombinant viruses in pancreatic cell lines infected with 1 PFU/cell VVlacZ or VVhEA. Mean viral replication ± SEM was determined by TCID50 assay.
The endostatin-angiostatin fusion protein is expressed in Suit-2 cells infected with VVhEA and inhibits HUVEC cell tube formation and proliferation in vitro
The expression of the human endostatin-angiostatin fusion gene in Suit-2 cells infected with VVhEA was confirmed by Western blotting (Fig. 4A). The anti-angiogenic function of the endostatin-angiostatin fusion protein was confirmed by the inhibition of HUVEC tube formation and proliferation (Fig. 4B, C and D). HUVEC tube formation was significantly inhibited by supernatant from Suit-2 cells infected with VVhEA when compared to VVlacZ (p<0.001) or mock-infected controls (Fig. 4C). The inhibition of HUVEC proliferation was also significantly greater when treated with VVhEA supernatant than either VVlacZ (p<0.0001) or mock-infected controls (Fig. 4D).
Figure 4. Expression and angiogenesis inhibition by the human endostatin-angiostatin fusion protein in Suit-2 cells infected with VVhEA in vitro.
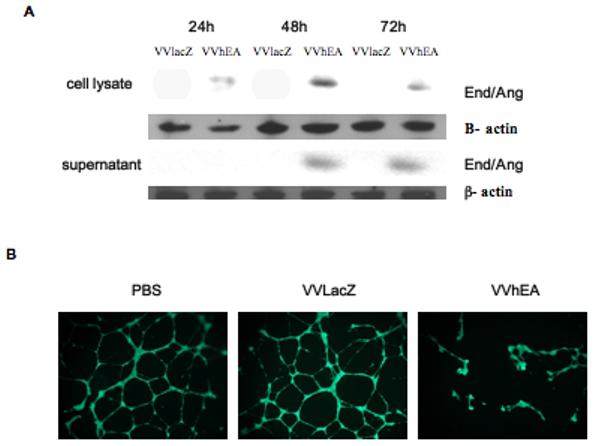

A, Endostatin-angiostatin fusion protein expression in Suit-2 cells. Suit-2 cells were infected with 1 PFU/cell of VVhEA or VVlacZ. Cells and supernatant were harvested 24, 48 and 72 hours later. Western blots were performed as described in Materials and Methods. All fusion protein bands were between 70 and 80 kDa and β actin around 45 kDa. Although intracellular transgene expression was found in the cells by 24 hours following infection, endostatin-angiostatin fusion protein was detected in the supernatant only after 48 hours; B and C, Inhibition of HUVEC tube formation. HUVEC were seeded in Matrigel with 10μg of supernatant harvested and concentrated 72 hours after Suit-2 cells were infected with 1 PFU/cell of VVhEA or VVlacZ. 16 hours later, 400 μg Calcein AM was added and fluorescence microscopy used to assess the number of fully formed HUVEC tubes, which were compared to mock-infected controls and each other by one-way ANOVA; D, Inhibition of HUVEC proliferation, Mean cell survival ± SEM as a percentage of uninfected HUVEC by MTS assay 96 hours post-treatment with 10μg/ml of supernatant harvested from Suit-2 cells infected with 1 PFU/cell of VVhEA or VVlacZ for 72 hours. Mean results were compared by one-way ANOVA with Bonferonni post-hoc testing.
The endostatin-angiostatin fusion protein is expressed and inhibits angiogenesis in Suit-2 xenografts treated with VVhEA in vivo
The distribution of VVhEA and VVlacZ and human endostatin-angiostatin fusion protein expression were confirmed by IHC in Suit-2 tumors harvested from nude mice after IV or IT virus injection. The expression of human endostatin-angiostatin in animals after IT (Fig 5A) and IV (not shown, similar to IT) therapy was confirmed from 48 hours onwards, with high intensity immunoreactivity correlated with Lister coat protein staining in spatially similar areas in serial sections. PBS-treated tumors were negative for both proteins (not shown). Fusion protein expression was also confirmed in plasma after IV (Fig 5B) and IT delivery (supplementary Figure 2). The level of endostatin expression in plasma after IV injection of VVhEA was significantly higher and lasted longer than those after IT injection of VVhEA, consistent with the result of reporter gene expression in tumour tissues (Fig.2A and 2B). The microvessel density (MVD) in tumors from VVhEA-treated mice was significantly lower than those from VVlacZ-treated mice from 120 hours onwards after IT treatment (Fig 5C) and 72 hours onwards after IV delivery (Fig. 5D), indicating the inhibition of angiogenesis by the fusion protein.
Figure 5. Expression and angiogenesis inhibition by the human endostatin-angiostatin fusion protein after treatment of Suit-2 tumor model in vivo.
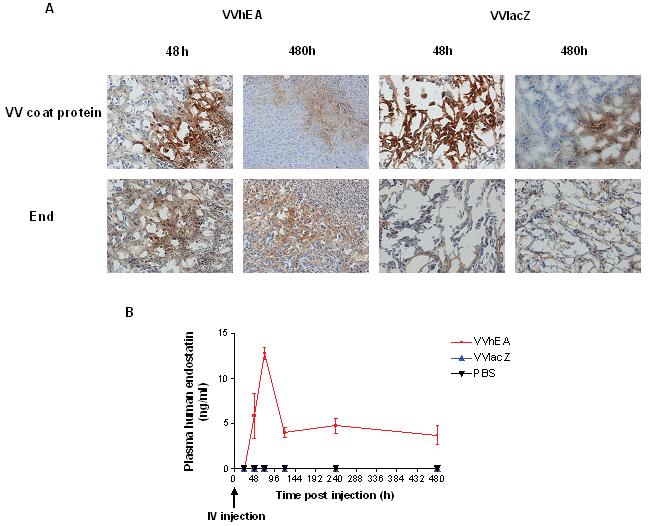

A, IHC for Lister vaccinia virus coat protein and human endostatin was performed on Suit-2 tumors; B, ELISA for human endostatin performed on plasma harvested from animals after 1×107 PFU IV VVhEA or VVlacZ with representative images at 48 and 480 hours (X200); C and D, IHC for murine CD31 to assess mean microvessel density± SEM in 5 random x200 fields of IT (C) or IV (D) VVhEA-, VVlacZ- or PBS-treated tumors compared by t tests.
VVhEA prolongs survival of nude mice bearing Suit-2 tumor xenografts after IT delivery
The Suit-2 xenograft human pancreatic cancer model was established in BALB/c nude mice and treated with IT injections of vaccinia viruses to assess the antitumor potency of the novel vaccinia virus in vivo (Fig. 6). Tumors regressed in three of five mice treated with low dose (three doses at 1×107 PFU, Fig. 6A) VVhEA therapy, which resulted in significantly longer survival than mice in other groups (Fig. 6B). Two of these mice remained alive at the end of the experiment 91 days after treatment.
Figure 6. Antitumoral efficacy of different vaccinia viruses in established Suit-2 tumors in vivo.
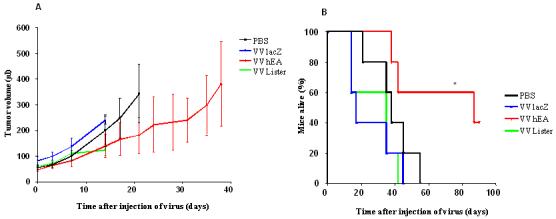
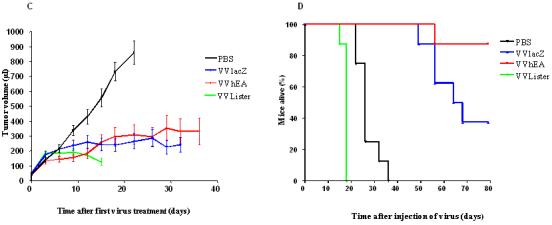
Suit-2 human pancreatic carcinoma xenografts implanted subcutaneously in BALB/c nu/nu mice as described in Materials and Methods. A and B, PBS or 1×107 PFU of different viruses were injected on days 0, 2 and 4 (n=5/group); C and D, 5×107 PFU of different viruses and PBS were injected on days 0, 3, 6, 9, 12 and 15 (n=8/group); A and C show the tumor growth curves (until death or sacrifice due to tumor ulceration or weight loss of first animal in each group); B and D show the survival rate of nude mice by Kaplan-Meier survival analysis (log rank test for statistical significance).
High dose (six doses of 5×107 PFU) therapy with all vaccinia viruses resulted in significantly slower rates of tumor growth than in PBS-treated controls (Fig. 6C). However, VVLister-treated mice survived for a significantly shorter period as all mice had to be killed as a result of weight loss and sickness by 18 days after their first treatment. Post-mortem analysis found pock lesions on their feet, tails and oral cavities. Mice treated with VVhEA or VVlacZ did not suffer from the same severity of systemic side effects and survived longer than controls (Fig. 6D). The VVhEA-treated mice survived significantly longer compared to the VVLacZ-treated mice although the rate of tumor growth was no different between two groups.
In the term of clinical application, IV injection may be more feasible than IT. Therefore, 1×107 PFU of VvhEA and VVLacZ were injected intravenously into the Suit-2 bearing nude mice. Unfortunately, all animals treated with recombinant vaccinia viruses developed tumor ulceration and pock lesions on their tails, and had to be sacrificed before showing efficacy.
Discussion
In this study, we first demonstrated that the Lister vaccine strain of vaccinia virus was more potent at tumor cell killing than Ad5 in all cell lines tested, supporting our hypothesis that vaccinia virus represents a promising alternative to adenovirus for cancer gene therapy. The WR strain of vaccinia virus has been used most commonly for the construction of oncolytic vaccinia viruses, based on its superior lytic activity in vitro 17. However, it is a laboratory-based strain, without the advantage of clinical experience as a vaccine. The Lister vaccine strain is an attractive oncolytic virus as its good safety profile has already been proved in humans during the vaccination program against smallpox in Europe and Asia. The recent complete sequencing of the Lister strain of vaccinia virus facilitated its further development and construction of more advanced vectors for gene therapy 39.
In addition to its wide tissue tropism, oncolytic vaccinia virus must display tumor selectivity. The Lister vaccine strain does not cause any known human disease 23. However, 80 per million vaccinia vaccinations produced lysis of skin epithelial cells, especially in immunosuppressed individuals, resulting in spreading tissue necrosis known as vaccinia necrosum 40,41. We therefore selected normal squamous cells NHEK (more likely than other tissue origin to support vaccinia virus replication) and compared VVLister replication to SCC25, a human tumor cell line also of squamous cell origin. Only SCC25 supported the replication of vaccinia virus, confirming the selectivity of the Lister stain in vitro.
In a human pancreatic cancer model, we have also demonstrated that the Lister vaccinia virus is inherently tumor-selective after delivery in vivo, evidenced by live fluorescence imaging of VVRG and the biodistribution of IV VVLister by IHC. This selectivity was also shown in murine tumor xenograft models (Tysome et al. unpublished data). The Lister vaccine strain vaccinia virus presents a superior safety profile in vivo as the Lister strain, in contrast to the WR strain 9,18, has not been detected in the ovaries or brains of mice. Most surprisingly, we, for the first time, demonstrated that IV delivery of the Lister strain vaccinia virus might be superior to IT delivery. This finding may have important implications for the future use of oncolytic vaccinia virus as IV therapy could target not only primary tumors, but also metastases. This observation may be strain-dependent, as tumor-specific transgene expression by a TK-deleted WR strain of vaccinia virus expressing cytosine deaminase 7 and a Wyeth strain virus expressing GM-CSF 42, did not show this feature. Unfortunately, tumor specificity following IV delivery in our study did not translate into antitumor efficacy using the standard dose of 1×107pfu, since the routine dose used for oncolytic vaccinia virus in this study was limited by tumor ulceration and pock lesions on the tail, paws and in oral cavities of the nude mice. These side effects may result from the huge viral replication in the “factory” of tumor cells as we have observed that reporter gene expression and endostatin-angiostatin expression were significantly higher after IV injection than IT injection (Fig 2 B and Fig 5B). Further experiments are needed to achieve the optimal regime. In addition, while pock lesions have been reported following vaccinia virus administration to nude mice 43-45, they have not been observed in immunocompetent mice 44-47. Therefore, further studies are needed to clarify our findings using immunocompetent tumor models, where the potential for systemic treatment may not be hampered by systemic side effects.
Anti-angiogenic agents, which target activated endothelial cells, represent a promising strategy for the treatment of tumors. However, their development has been hampered by several factors including manufacturing difficulties, stability and solubility issues 48. In addition, the majority of these inhibitors of angiogenesis are not directly cytotoxic to tumor cells so they need to be expressed on a continuous basis. Given the tumor-specific replication of oncolytic vaccinia virus, it is timely to explore their use to engineer inhibition of angiogenesis. We constructed a novel oncolytic Lister strain of vaccinia virus armed with a human endostatin-angiostatin fusion gene targeting the process of neovascularisation. The continuous expression and angiogenesis inhibition of the endostatin-angiostatin fusion protein was confirmed in the Suit-2 pancreatic cancer model both in vitro and up to 20 days after one treatment in vivo. We found that the potency and replication of both F14.5L-deleted viruses (VVhEA and VvlacZ) were attenuated when compared to the parental VVLister virus. We anticipate that this was due to insertional inactivation at the NotI site of the hypothetical protein F3 encoded by a small gene located between F14L (List049) and F15L (List050) of the genome 39. The vaccinia F3 protein may be involved in immune evasion, accounting for viral attenuation after deletion. This concept was recently confirmed by others using VVRG (described by the authors as GLV-ld27) 38.
VVhEA therapy resulted in significantly longer survival when compared to VVlacZ-treated mice in vivo, with regression in three of five tumors after low dose, and all tumors after high dose therapy. However, tumor regression was also seen after high dose treatment of all vaccinia viruses, although survival was only prolonged with VVhEA and VVlacZ when compared to PBS. The attenuation of both recombinant viruses resulted in fewer side effects in immunodeficient mice in vivo, as VVLister-treated mice developed more pock lesions and rapidly lost weight, requiring their sacrifice even before PBS-treated controls. The survival advantage of VVhEA over VVlacZ after high dose therapy may have been due to the VVhEA-mediated inhibition of angiogenesis affecting intratumoral propagation of the virus and thus spread and distribution of the virus throughout the mice, which may have reduced the side-effect. In addition, VVhEA may reverse VEGF-induced cancer-associated systemic syndrome (CASS) and prevent death in tumor-bearing mice without significantly compromising tumor growth 49. Although the added benefit of the fusion gene in VVhEA over VVlacZ was not as great as might have been expected, these results obviously warrant further investigation in an immunocompetent model as anti-angiogenesis therapy can overcome endothelial cell anergy, promote leukocyte-endothelium interactions and inhibit tumor growth and microvessel density significantly, with remarked infiltration of leukocytes (CD45), as well as the number of CD8+ cytotoxic T lymphocytes in tumors 50.
In conclusion, we demonstrate the superior antitumor potency of the Lister strain of vaccinia virus over wild-type adenovirus against pancreatic cancer, especially for tumor cell lines insensitive to adenovirus. The virus also displays inherently high selectivity for cancer cells, sparing normal cells both in vitro and in vivo. Through arming the Lister strain with the human endostatin-angiostatin fusion gene, we combined the antiangiogenic properties of the fusion protein with the oncolytic properties of the Lister virus. This novel recombinant virus has shown efficacy in terms of tumor regression and prolonged survival in a human pancreatic cancer xenograft model and shows potential for translation to clinical therapy in the future.
Materials and methods
Cell lines
CV1, the African Green Monkey normal kidney cell line was obtained from the American Type Culture Collection (ATCC, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Human umbilical vein epithelial cells (HUVEC) and normal human epithelial keratinocytes (NHEK) were obtained from Cambrex (Cambridge, UK) and maintained in EGM-2 and KGM respectively.
Human pancreatic carcinoma cell lines Suit-2, PaTu8988S, PaTu8988T, MiaPaCa2, PANC1 and Capan1 were maintained in DMEM with 10% FCS. The human HNSCC cell line SCC25 was obtained from CRUKCCS and maintained in 1 DMEM : 1 HAMSF12 with 10% FCS and 400ng/ml hydrocortisone (Sigma-Aldrich, MO, USA).
Viruses
The Lister vaccine strain (LIVP) of vaccinia virus was used as a parental virus for the construction of recombinant viruses 43. The fused human endostatin and angiostatin genes were inserted following the method previously described 51 into the non-essential Not I restriction site, located between F14L and F15L of the LIVP and encoding a hypothetical protein F3. VVlacZ was constructed in the same way after insertion of the E. coli lacZ gene, to serve as a control for VVhEA. PCR and DNA sequencing were used to verify recombinant viruses. In a previous study, the Renilla luciferase-GFP fusion gene was inserted into the same region to create VVRG 43. Wild-type adenovirus Ad5 was described previously 52.
Evaluation of viral cytotoxicity in vitro
Cells were seeded at 1 and 5×103 cells/well, depending on growth rates, in 96-well plates, and infected with viruses 16-18 hours later. Cell survival on day 6 after viral infection was determined by MTS assay and EC50 value (viral dose killing 50% of tumor cells) was calculated as previously described 52. All assays were performed at least three times.
Viral replication
Cells were seeded at 2 to 4 ×105 cells/well, depending on growth rates, in three wells of 6-well plates in media with 10% FCS, and infected with 1 PFU/cell of vaccinia viruses 16-18 hours later. Samples were harvested in triplicate at 24-hour intervals up to 144 hours. Viral replication was detected by TCID50 (50% tissue culture infective dose) as previously described 52.
Biodistribution of VVRG by fluorescence imaging in vivo
1×106 Suit-2 cells were implanted subcutaneously (SC) into the right flank of 10 female BALB/c nude mice (Harlan UK Ltd., Bicester, UK). When tumors reached 0.4-0.5cm in diameter, mice received 100μl IT or IV tail vein injections of 1.0×108 PFU VVRG or PBS. The biodistribution of VVRG was determined in mice anaesthetized (2% halothane by inhalation in O2 1 l/min and NO 1 l/min) 1, 2, 3, 5 and 10 days following treatment by measuring fluorescence with the IVIS camera in tumors defined as ROI after imaging. All animal experiments were performed in accordance with the regulations of the UK Home Office and the local animal ethics committee.
Western-blotting
2×105 Suit-2 cells were seeded in 60mm dishes in conditional media and infected with 1 PFU/cell VVhEA or VVlacZ in DMEM with 2% FCS after 16-18 hours. Cells and supernatant were harvested separately 24, 48 and 72 hours following infection and supernatants concentrated using Centricon 10kDa columns (Millipore, MA, USA). 30μg aliquots of total protein were used to detect endostatin-angiostatin fusion protein expression by Western Blotting as previously described 53.
HUVEC proliferation and tube formation assay
1×104 HUVEC cells were seeded in 96-well plates in EGM-2 and treated 16-18 hours later with 10μg/ml of supernatant concentrated from Suit-2 cells infected with VVlacZ or VVhEA for 72 hours. After another 96 hours, cell viability was measured by MTS assay to determine HUVEC proliferation.
The inhibition of HUVEC tube formation was observed using the BD Biocoat™ angiogenesis system (BD Biosciences, MA, USA) according to the manufacturer's instructions. Tube formation by HUVEC treated with supernatant was compared to mock-infected HUVEC alone by counting the number of completely formed tubes/well by fluorescence microscopy.
Histopathology and immunohistochemistry staining
When established Suit-2 tumors reached 0.4-0.5cm in diameter, mice were stratified into groups of 18. Each received one 100μl intravenous (IV) or intratumoral (IT) injection of 1.0×107 PFU of VVLister, VVhEA, VVlacZ or PBS. Groups of three mice in each cohort were sacrificed 24, 48, 72, 120, 240 and 480 hours later, tumors removed, snap-frozen in isopentane and stored at −80°C. Blood was collected into a heparinized tube and plasma stored at −80°C. Where animals had received IV VVLister or PBS, the spleen, liver, kidneys, brain, ovaries, lungs and adrenal glands were also collected and stored. All tissues were processed for histopathology and IHC analysis for viral coat protein (1:2000 rabbit anti-vaccinia virus coat protein polyclonal antibody (MorphoSys UK Ltd, Bath, UK), endostatin expression (1:250 rabbit anti-human endostatin polyclonal antibody (Abcam, Cambridge, UK)) and CD31 (1:200 rat anti-murine CD31 antibody (BD Biosciences, Oxford, UK)) to determine MVD as described previously 54.
ELISA
Human endostatin levels in the plasma of BALB/c nu/nu mice bearing Suit-2 xenografts treated with IV VVhEA, VVlacZ or PBS were obtained using the Duoset® ELISA for human endostatin (R and D Systems, Abingdon, UK unless otherwise stated) according to the manufacturers' instructions.
In vivo efficacy experiments
As described above, when established Suit-2 xenografts of 20 mice reached 0.4-0.5cm in diameter, mice were regrouped by tumor size and received 100μl IT injections of 1.0×107 PFU VVLister, VVlacZ, VVhEA or PBS on days 0, 2 and 4. Tumor volumes were estimated (Volume = (length × width2 × π)/6) twice weekly until mice were sacrificed when tumor volume reached 1.00 cm3 or had been present for three months. This was repeated in experiments where 36 mice with Suit-2 xenografts received 5.0×107 PFU IT injections on days 0, 3, 6, 9, 12 and 15, and 20 mice with Suit-2 xenografts received 1.0×107 PFU IV injection on day 0 when tumor xenografts reached 0.8-0.5cm.
Statistical analysis
All statistical analyses were performed as described in figure legends or results. Differences were considered significant where p<0.05.
Supplementary Material
Acknowledgement
This project is supported by Cancer Research UK (C633-A6253/A6251), Nature Sciences Foundation of China (30530800) and The Royal College of Surgeons of England (JT), and Barts and The London Research Advisory Board. I. F was sponsored by the U.S. Army/National Medical Technology Testbed, Inc. We appreciate the critical and insightful comments from Dr. Gunnel Hallden. We are grateful to Mr. John Overton for his pilot experiments in vitro. We also appreciate Vipul Bhakta, Keyur Trivedi and Mohamed Ikram for IHC staining.
Footnotes
Supplementary information is available at Gene Therapy's Website.
Financial disclosures: None of the authors have any financial arrangement nor involvement with commercial organizations producing competing products.
References
- 1.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Michalski C, Friess H, Buchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111–118. doi: 10.1097/01.mpa.0000229010.62538.f2. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya M, Lemoine NR. Gene therapy developments for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:285–298. doi: 10.1016/j.bpg.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 5.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 6.Rein DT, Breidenbach M, Curiel DT. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2006;2:137–143. doi: 10.2217/14796694.2.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnant MF, Puhlmann M, Alexander HR, Jr., Bartlett DL. Systemic administration of a recombinant vaccinia virus expressing the cytosine deaminase gene and subsequent treatment with 5-fluorocytosine leads to tumor-specific gene expression and prolongation of survival in mice. Cancer Res. 1999;59:3396–3403. [PubMed] [Google Scholar]
- 8.Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- 9.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 10.Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR, et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 11.Thorne SH, Bartlett DL, Kirn DH. The use of oncolytic vaccinia viruses in the treatment of cancer: a new role for an old ally? Curr Gene Ther. 2005;5:429–443. doi: 10.2174/1566523054546215. [DOI] [PubMed] [Google Scholar]
- 12.Slobod KS, Lockey TD, Howlett N, Srinivas RV, Rencher SD, Freiden PJ, et al. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004;23:106–110. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 13.Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5:690–699. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 14.Mastrangelo MJ, Maguire HC, Jr., Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 15.Gomella LG, Mastrangelo MJ, McCue PA, Maguire HJ, Mulholland SG, Lattime EC. Phase i study of intravesical vaccinia virus as a vector for gene therapy of bladder cancer. J Urol. 2001;166:1291–1295. [PubMed] [Google Scholar]
- 16.Alemany R. A smart move against cancer for vaccinia virus. Lancet Oncol. 2008;9:507–508. doi: 10.1016/S1470-2045(08)70136-0. [DOI] [PubMed] [Google Scholar]
- 17.Guo ZS, Bartlett DL. Vaccinia as a vector for gene delivery. Expert Opin Biol Ther. 2004;4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 18.Luker KE, Hutchens M, Schultz T, Pekosz A, Luker GD. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005;341:284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 19.Stein RA, Staros JV. Evolutionary analysis of the ErbB receptor and ligand families. J Mol Evol. 2000;50:397–412. doi: 10.1007/s002390010043. [DOI] [PubMed] [Google Scholar]
- 20.Tzahar E, Moyer JD, Waterman H, Barbacci EG, Bao J, Levkowitz G, et al. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. Embo J. 1998;17:5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemoine NR, Hughes CM, Barton CM, Poulsom R, Jeffery RE, Kloppel G, et al. The epidermal growth factor receptor in human pancreatic cancer. J Pathol. 1992;166:7–12. doi: 10.1002/path.1711660103. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, et al. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–1563. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Hypoxia and angiogenesis in pancreatic cancer. ANZ J Surg. 2006;76:830–842. doi: 10.1111/j.1445-2197.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari V, Valcamonico F, Amoroso V, Simoncini E, Vassalli L, Marpicati P, et al. Gemcitabine plus celecoxib (GECO) in advanced pancreatic cancer: a phase II trial. Cancer Chemother Pharmacol. 2006;57:185–190. doi: 10.1007/s00280-005-0028-1. [DOI] [PubMed] [Google Scholar]
- 25.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebert C, Siavash H, Norris K, Nikitakis NG, Sauk JJ. Endostatin inhibits nitric oxide and diminishes VEGF and collagen XVIII in squamous carcinoma cells. Int J Cancer. 2005;114:195–201. doi: 10.1002/ijc.20692. [DOI] [PubMed] [Google Scholar]
- 27.Nie SL, Yuan SZ. Experimental study of gene therapy with angiostatin gene in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2002;1:452–457. [PubMed] [Google Scholar]
- 28.Schmitz V, Wang L, Barajas M, Gomar C, Prieto J, Qian C. Treatment of colorectal and hepatocellular carcinomas by adenoviral mediated gene transfer of endostatin and angiostatin-like molecule in mice. Gut. 2004;53:561–567. doi: 10.1136/gut.2003.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Xu J, Lawler J, Terwilliger E, Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin Cancer Res. 2007;13:3968–3976. doi: 10.1158/1078-0432.CCR-07-0245. [DOI] [PubMed] [Google Scholar]
- 30.Scappaticci FA, Contreras A, Smith R, Bonhoure L, Lum B, Cao Y, et al. Statin-AE: a novel angiostatin-endostatin fusion protein with enhanced antiangiogenic and antitumor activity. Angiogenesis. 2001;4:263–268. doi: 10.1023/a:1016067717433. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Liu YH, Lee SJ, Gardner TA, Jeng MH, Kao C. Prostate-restricted replicative adenovirus expressing human endostatin-angiostatin fusion gene exhibiting dramatic antitumor efficacy. Clin Cancer Res. 2008;14:291–299. doi: 10.1158/1078-0432.CCR-07-0867. [DOI] [PubMed] [Google Scholar]
- 32.Raikwar SP, Temm CJ, Raikwar NS, Kao C, Molitoris BA, Gardner TA. Adenoviral vectors expressing human endostatin-angiostatin and soluble Tie2: enhanced suppression of tumor growth and antiangiogenic effects in a prostate tumor model. Mol Ther. 2005;12:1091–1100. doi: 10.1016/j.ymthe.2005.07.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CT, Lin YC, Lin CL, Lu J, Bu X, Tsai YH, et al. Oncolytic herpesvirus with secretable angiostatic proteins in the treatment of human lung cancer cells. Anticancer Res. 2005;25:2049–2054. [PubMed] [Google Scholar]
- 34.Weiss JM, Shivakumar R, Feller S, Li LH, Hanson A, Fogler WE, et al. Rapid, in vivo, evaluation of antiangiogenic and antineoplastic gene products by nonviral transfection of tumor cells. Cancer Gene Ther. 2004;11:346–353. doi: 10.1038/sj.cgt.7700686. [DOI] [PubMed] [Google Scholar]
- 35.Fodor I, Timiryasova T, Denes B, Yoshida J, Ruckle H, Lilly M. Vaccinia virus mediated p53 gene therapy for bladder cancer in an orthotopic murine model. J Urol. 2005;173:604–609. doi: 10.1097/01.ju.0000143196.37008.2c. [DOI] [PubMed] [Google Scholar]
- 36.Kelly KJ, Woo Y, Brader P, Yu Z, Riedl C, Lin SF, et al. Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum Gene Ther. 2008;19:774–782. doi: 10.1089/hum.2008.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 39.Garcel A, Crance JM, Drillien R, Garin D, Favier AL. Genomic sequence of a clonal isolate of the vaccinia virus Lister strain employed for smallpox vaccination in France and its comparison to other orthopoxviruses. J Gen Virol. 2007;88:1906–1916. doi: 10.1099/vir.0.82708-0. [DOI] [PubMed] [Google Scholar]
- 40.Gurvich EB, Gomes LA, Yartsev MN, Khakhalin LN, Grigor'eva LB, Dmitrieva NG, et al. Vaccinia virus persistence in a child against the background of immune deficiency. J Hyg Epidemiol Microbiol Immunol. 1986;30:177–183. [PubMed] [Google Scholar]
- 41.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–2081. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Denes B, Gridley DS, Fodor N, Takatsy Z, Timiryasova TM, Fodor I. Attenuation of a vaccine strain of vaccinia virus via inactivation of interferon viroceptor. J Gene Med. 2006;8:814–823. doi: 10.1002/jgm.907. [DOI] [PubMed] [Google Scholar]
- 44.Hutt LM. The immune response to infection with vaccinia virus in mice. I. Infection and the production of antibody neutralizing cell-associated and cell-free virus. J Hyg (Lond) 1975;74:301–314. doi: 10.1017/s0022172400046829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ober BT, Bruhl P, Schmidt M, Wieser V, Gritschenberger W, Coulibaly S, et al. Immunogenicity and safety of defective vaccinia virus lister: comparison with modified vaccinia virus Ankara. J Virol. 2002;76:7713–7723. doi: 10.1128/JVI.76.15.7713-7723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermiston TW, Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002;9:1022–1035. doi: 10.1038/sj.cgt.7700542. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y, Religa P, Cao R, Hansen AJ, Lucchini F, Jones B, et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci U S A. 2008;105:18513–18518. doi: 10.1073/pnas.0807967105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dirkx AE, oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. Faseb J. 2006;20:621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 51.Timiryasova TM, Chen B, Fodor N, Fodor I. Construction of recombinant vaccinia viruses using PUV-inactivated virus as a helper. Biotechniques. 2001;31:534, 536, 538–540. doi: 10.2144/01313st07. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Xue SA, Hallden G, Francis J, Yuan M, Griffin BE, et al. Virus-associated RNA I-deleted adenovirus, a potential oncolytic agent targeting EBV-associated tumors. Cancer Res. 2005;65:1523–1531. doi: 10.1158/0008-5472.CAN-04-3113. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman HL, Deraffele G, Mitcham J, Moroziewicz D, Cohen SM, Hurst-Wicker KS, et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest. 2005;115:1903–1912. doi: 10.1172/JCI24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


