Abstract
Objective
We aimed to determine whether circulating levels of the biomarkers C-reactive protein (CRP), fibrinogen, plasma viscosity, and haematocrit were associated with cognitive decline in middle-aged to elderly people.
Methods
Subjects consisted of 2,312 men and women aged 50 to 80 years participating in the Aspirin for Asymptomatic Atherosclerosis Trial, all of whom were free of symptomatic cardiovascular disease at baseline. Biomarker levels and cognitive ability were measured at baseline with cognition assessed in all subjects using the Mill Hill Vocabulary Test and in a subgroup of 504 persons using tests of memory, non-verbal reasoning, information processing speed, executive function, and mental flexibility. After 5 years, the five test battery was administered to all participants and scores were used to derive a general cognitive ability factor.
Results
Baseline CRP and fibrinogen levels were negatively associated with age and sex-adjusted follow-up scores on the majority of the cognitive tests, and the general cognitive ability factor (correlations -0.054 to -0.105, P < .05). In analyses adjusting for baseline cognitive scores, asymptomatic atherosclerotic disease, and cardiovascular risk factors, both markers predicted decline in several cognitive domains (excluding memory). Baseline plasma viscosity, but not hematocrit, was negatively associated with follow-up test scores for general cognitive ability, information processing speed and mental flexibility (correlations -0.050 to -0.098, P < .05) and with decline across the same domains (P < .05).
Conclusions
Raised circulating levels of CRP, fibrinogen, and elevated plasma viscosity predicted poorer subsequent cognitive ability and were associated with age-related cognitive decline in several domains, including general ability.
Keywords: inflammation, rheology, cognition, cognitive decline
Introduction
Decline in memory and other cognitive abilities is one of the most feared consequences of growing old. Over and above the problems associated with frank dementia, the prevalence of milder forms of cognitive impairment in older age is large, causing a significant burden on patients and carers alike [1]. Given our generally aging population, the public health consequences of such age-related cognitive decline are likely to increase over the coming years. It has been well established that by the time we observe clinical symptoms of cognitive deficits, much cerebral damage has already been done [2]. There is thus an urgent need to identify markers of future cognitive decline in order to develop effective interventions aimed at reducing neurotoxicity before irreversible neural damage and dementia can occur. One potentially fruitful area in the search for such markers is the inflammatory system, which is thought to be involved in the development of Alzheimer’s disease and may also be important in the development of vascular dementia [3, 4].
Cross-sectional associations between raised levels of peripherally circulating markers of systemic inflammation and reduced cognitive ability in individuals without dementia have already been established [5]. However, whether these markers can actually predict cognitive decline in older age, or indeed whether they may have a causal role in the development of cognitive decline, is unknown. To date, results for the well-recognised inflammatory mediator, C-reactive protein (CRP), are conflicting with well-designed, large-scale studies showing both positive and null associations with follow-up cognitive ability and cognitive decline [6-8]. Furthermore, recent results suggesting a role for plasma fibrinogen in age associated cognitive decline [9] have not been replicated, although they do raise the possibility that differences in blood rheology (such as plasma viscosity) which are influenced by fibrinogen levels and which also show an increase with aging (leading to an increase in overall ‘stickiness’ of the blood with aging) may influence rates of age-associated cognitive decline [10].
In view of these limited findings, we tested the hypothesis that higher levels of the inflammatory markers CRP and fibrinogen and of the rheological factors plasma viscosity and hematocrit are associated with cognitive decline using data from a large prospective study based in central Scotland - part of the Aspirin for Asymptomatic Atherosclerosis (AAA) Trial. This study population had the advantage not only of size (over 2,000 subjects cognitively tested), but also provides detailed cognitive testing within a range of cognitive domains and a 5-year of follow-up period between measurement of inflammatory markers (and pre-morbid cognitive ability) and later life cognitive ability. The population was also free of clinical cardiovascular disease when inflammatory and rheological markers were measured.
Methods
Study Population
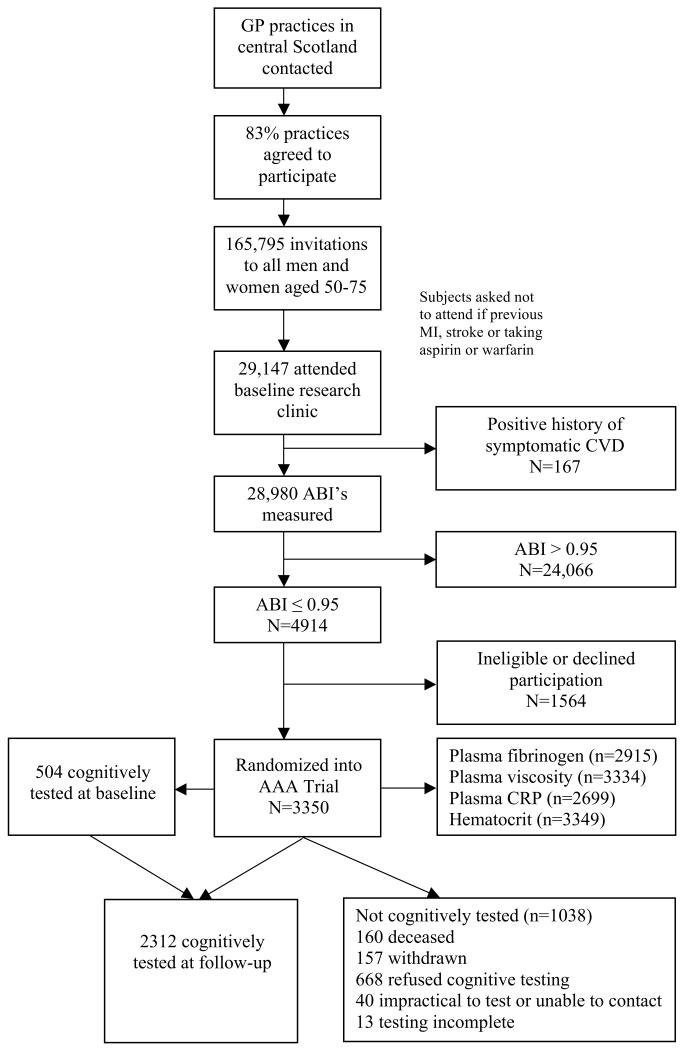
The study population consisted of all subjects recruited into the Aspirin for Asymptomatic Atherosclerosis Trial who underwent cognitive testing after 5 years of follow-up. The AAA Trial is a randomised controlled trial of aspirin for the reduction of cardiovascular events and death in people with asymptomatic atherosclerosis. Details of how potential trial participants were selected from the general population of central Scotland and recruited into the trial, including assessment of asymptomatic atherosclerosis, have been presented previously [11, 12]. Briefly, subjects recruited into the trial were aged over 50 years with no history of cardiovascular disease but with a ratio of systolic blood pressure in the ankle to that in the arm (ankle-brachial index, ABI) of 0.95 or less, indicative of atherosclerotic burden and increased risk of developing symptomatic cardiovascular disease [13, 14]. In addition to symptomatic vascular disease or major illness, subjects were excluded if they had a contra-indication to aspirin therapy, including a haematocrit measurement < 38% for men and < 35% for women. Of the 3,350 subjects recruited into the trial between 1999 and 2001, 2,312 underwent cognitive testing at follow-up between 2003 and 2006 (range 4-7 years, mean 5 years) (Figure 1). A subset had also been targeted for extensive cognitive testing at baseline. Research funding became available to perform these assessments after approximately one third of subjects had been through the clinic. Thereafter, participants were consecutively invited to take the additional cognitive testing until this group totalled a target of 480 in number. In practice, 504 persons ended up partaking in this additional cognitive examination. Approval for the study was granted by the ethical committees of Lanarkshire, Edinburgh and Glasgow, and all subjects gave written informed consent prior to the start of the trial.
Figure 1.
Flow diagram for participation in AAA trial and the cognitively tested study population.
Cognitive and physical assessment
Details of the baseline physical examinations and of the cognitive assessments have been reported previously [15]. Briefly, five individual cognitive tests were administered to a subset of 504 persons at baseline and to 2,312 persons at follow-up. The tests in this battery included: the Auditory Verbal Learning Test - AVLT (immediate and delayed memory) [16]; Raven’s Standard Progressive Matrices - RAVENS (non-verbal reasoning) [17]; the Digit Symbol Test - DST (processing speed) [18]; the Verbal Fluency Test - VFT (executive function) [16]; and the Trail-Making Test, Part B - TMT (mental flexibility and processing speed) [16]. Principal components analysis was used to ascertain the presence of a general cognitive factor (traditionally called g) at both baseline and follow-up using the information from the five-test battery. The scores on the first unrotated principal component were saved for both measurements of g. Scree slope analyses indicated a single principal component at both baseline and follow-up. At baseline, g accounted for 52.6% of the variance from the full cognitive battery compared with 56.9% at follow-up. Each of the five cognitive tests loaded strongly on g. A measurement of pre-morbid cognitive ability was also estimated for all participants at baseline using the Mill Hill Vocabulary Test (MHVT). As results on vocabulary tests such as the MHVT vary little with aging [19], especially in a relatively young and healthy cohort like the AAA Trial, they can be used to estimate peak prior cognitive ability [20]. Ankle brachial index, systolic and diastolic brachial blood pressure, plasma total cholesterol levels and smoking history were measured using standard techniques and strict quality control measures.
Biomarker measurements
Baseline venous blood samples were used to measure plasma viscosity, haematocrit, fibrinogen, and CRP. Assays were performed in the University Department of Medicine, Glasgow Royal Infirmary. Plasma viscosity was assayed in a fresh blood sample anticoagulated with K2EDTA in a capillary viscometer (Coulter) at 37°C [21]. In the same sample, haematocrit was measured using a microcentrifuge and optical reader (Hawksley) [21]. In stored plasma anticoagulated with trisodium citrate, fibrinogen was assayed by the automated Clauss assay (MDA-180 coagulometer, Organon Teknika), and CRP using a high-sensitivity immunonephelometric assay [21].
Statistical analysis
Age and sex-adjusted Pearson correlations were calculated between the cognitive scores and biomarker levels. The TMT scores and CRP levels were log-transformed due to positive skew in their distributions. The associations identified by the correlations were analyzed further via a series of linear regression analyses of biomarker levels against follow-up cognition, actual 5 year cognitive change across individual tests, and estimated cognitive change (follow-up scores adjusted for baseline vocabulary scores). In each of the models, age and sex were entered as covariates with further adjustments being made when modelling 5 year change (baseline cognitive scores) and estimated change (MHVT). Baseline scores were entered as covariates as opposed to calculating change scores as this method reduces the chance of spurious correlations between baseline and change scores. Analyses were repeated to account for the role of cardiovascular disease (CVD) risk factors by adjusting for ABI, diastolic blood pressure, cholesterol level, and smoking (estimated as the number of 20-cigarette packs smoked per day times the number of years as a smoker, with a zero value entered for lifelong non-smokers). Plasma viscosity calculations were also repeated after adjusting for its main constituent, fibrinogen, to enable an investigation of its residual component. Analyses were performed using SPSS 14.0 for Windows [22].
Results
Table 1 gives the baseline characteristics of the entire AAA Trial population, the cognitively tested study population and the cognitive change subset. Compared with the entire AAA Trial population, there was little difference in the demographics of the group of subjects who went on to be cognitively tested. Overall the two cognitively tested groups were very similar, with the subset approximately 1 year older and with very slightly higher baseline CRP and fibrinogen levels.
Table 1.
Baseline characteristics of AAA Trial population, cognitively tested study population and cognitive change subset
| AAA Trial population (maximum n=3350) | Cognitively tested population (maximum n = 2,312) | Cognitive change subset (maximum n = 504) | |
|---|---|---|---|
| Age (years) | 61.9 (6.7) | 61.7 (6.55) | 63.0 (6.85) |
| Female - n (%) | 2,396 (72) | 1,689 (73) | 369 (73) |
| Ankle brachial index | 0.86 (0.09) | 0.86 (0.09) | 0.87 (0.09) |
| Systolic BP (mmHg) | 147.9 (21.59) | 147.4 (21.44) | 142.7 (19.41) |
| Diastolic BP (mmHg) | 83.7 (10.74) | 83.7 (10.71) | 80.5 (9.76) |
| Cholesterol (mmol/L) | 6.18 (1.09) | 6.27 (1.08) | 6.20 (1.16) |
| Smoking Status - n (%) | |||
| Never Smoked | 1087 (32.4) | 733 (31.7) | 153 (30.4) |
| Current Smoker | 1101 (32.9) | 776 (33.6) | 183 (36.3) |
| Ex-smoker | 1162 (34.7) | 803 (34.7) | 168 (33.3) |
| Fibrinogen (g/L) | 3.29 (0.71) | 3.27 (0.71) | 3.36 (0.72) |
| CRP (mg/L) a | 2.00 (0.92, 4.39) | 1.85 (0.89, 4.08) | 1.98 (0.89, 4.18) |
| Plasma Viscosity (mPa.s) | 1.27 (0.07) | 1.27 (0.07) | 1.27 (0.07) |
| Hematocrit - % (s.d.) | 42.3 (3.25) | 42.2 (3.16) | 42.6 (3.15) |
Values are means (standard deviations) except where stated
Median and inter-quartile range
AAA = Aspirin for Asymptomatic Atherosclerosis, CRP = C-reactive protein, BP = blood pressure
Age and sex-adjusted Pearson correlations between biomarker levels and follow-up cognitive test scores are shown in Table 2. Small but significant correlations were found for both fibrinogen and CRP with RAVENS, DST, TMT, and g (P <0.01). Plasma viscosity associated significantly with scores on the DST, TMT, and with g (P <0.01). The latter associations became non-significant after adjustment for fibrinogen levels with the exception of the TMT correlation. CRP and plasma viscosity also correlated significantly with the baseline MHVT scores (P < 0.001 and P < 0.05, respectively). Inverse correlations for all tests except the TMT, in which high scores reflect lower cognitive ability, indicated that higher levels of the biomarkers were associated with poorer cognitive performance. No significant correlations were found between haematocrit levels and any of the cognitive tests. Of the subset tested at baseline the correlations between the cognitive test scores and the MHVT pre-morbid estimate of IQ ranged from 0.19 to 0.39 (the correlation with the g factor was 0.37).
Table 2.
Age and sex-adjusted Pearson correlations between baseline marker levels and follow-up cognitive test scores
| AVLT | RAVENS | VFT | DST | ln(TMT) | g | MHVT | |
|---|---|---|---|---|---|---|---|
| Fibrinogen | -0.039 | -0.060** | -0.025 | -0.082*** | 0.091*** | -0.077** | -0.038 |
| ln(CRP) | -0.021 | -0.088*** | -0.054* | -0.105*** | 0.097*** | -0.100*** | -0.097*** |
| Plasma Viscosity | -0.032 | -0.035 | -0.036 | -0.069** | 0.098*** | -0.069** | -0.050* |
| Plasma Viscosity (fib adjusted) | -0.008 | -0.006 | -0.025 | -0.036 | 0.068** | -0.034 | -0.029 |
| Hematocrit | 0.024 | 0.019 | -0.006 | 0.003 | 0.029 | 0.006 | 0.006 |
| Mean (s.d.) | 63.0 (16.8) | 34.7 (9.3) | 37.4 (12.8) | 40.0 (11.7) | 4.6 (0.4) | 0.0 (1.0) | 31.0 (4.7) |
P < .05
P < .01
P < .001
Number of subjects with complete data varied by test: fibrinogen (1,797-2,124); ln(CRP) (1,669-1,976); plasma viscosity (1,987-2,331); plasma viscosity adjusted for fibrinogen (1,787-2,113); haematocrit (1,997-2,342).
AVLT = Auditory Verbal Learning Task, RAVENS = Raven’s Standard Progressive Matrices, VFT = Verbal Fluency Test, DST = Digit Symbol Test, TMT = Trail Making Test, MHVT = Mill Hill Vocabulary Test, CRP = C-reactive protein, fib = fibrinogen.
Results of the models investigating marker levels and estimated cognitive decline are shown in Table 3. After age, sex, and MHVT-adjustment, plasma fibrinogen, CRP, and viscosity were significantly associated with poorer functioning on the DST (P < 0.01), TMT (P < 0.01), and g (P < 0.05). All of these associations remained significant after further adjustment for CVD risk factors, with the exception of CRP with g. Fibrinogen and CRP were also associated with decreased performance on RAVENS (p < 0.05) although these disappeared after CVD adjustments. Fibrinogen-adjusted plasma viscosity was significantly associated with TMT scores, both before and after adjustment for baseline cardiovascular risk factors (P < 0.01). There was one weakly significant association between haematocrit and cognition or cognitive change and this was for age, sex, and MHVT-adjusted TMT scores (P < 0.05). Overall, the maximum standardised effect size found was -0.095 for CRP using the DST to assess age and sex-adjusted cognition. This implies that for every standard deviation change in CRP levels the DST scores change by -0.095 standard deviations. As the standard deviation on the DST was approximately 11 (Table 2) the effect size can be roughly interpreted as a 1.0 point decrease on DST performance for a three-fold increase in CRP level.
Table 3.
Multivariate associations between biomarkers and late-life cognition, and estimated cognitive change. a
| AVLT | RAVENS | VFT | DST | ln(TMT) | g | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Biomarker + adjustments |
Unstandardised β (Standard Error) | Standardised β | |||||||||||
| Fibrinogen | ||||||||||||
| + Age and Sex | -0.90 (0.52) |
-0.037 | -0.77 (0.30) ** b |
-0.058 | -0.46 (0.41) |
-0.025 | -1.25 (0.33) *** |
-0.075 | 0.05 (0.01) *** |
0.087 | -0.10 (0.03) ** |
-0.072 |
| + MHVT | -0.67 (0.49) |
-0.028 | -0.57 (0.26) * |
-0.043 | -0.29 (0.36) |
-0.016 | -1.18 (0.31) *** |
-0.073 | 0.05 (0.01) *** |
0.081 | -0.08 (0.03) ** |
-0.058 |
| + MHVT and CVD risk factors | -0.60 (0.50) |
-0.026 | -0.46 (0.26) |
-0.035 | -0.32 (0.37) |
-0.018 | -0.85 (0.32) ** |
-0.052 | 0.04 (0.01) ** |
0.064 | -0.07 (0.02) * |
-0.046 |
| ln(C-reactive Protein) | ||||||||||||
| + Age and Sex | -0.30 (0.34) |
-0.019 | -0.72 (0.19) *** |
-0.083 | -0.63 (0.27) * |
-0.053 | -1.01 (0.22) *** |
-0.095 | 0.03 (0.01) *** |
0.092 | -0.09 (0.02) *** |
-0.093 |
| + MHVT | 0.24 (0.33) |
0.016 | -0.38 (0.17) * |
-0.045 | -0.19 (0.24) |
-0.017 | -0.73 (0.21) *** |
-0.069 | 0.02 (0.01) ** |
0.064 | -0.04 (0.02) * |
-0.048 |
| + MHVT and CVD risk factors | 0.31 (0.33) |
0.020 | -0.29 (0.17) |
-0.034 | -0.14 (0.25) |
-0.012 | -0.50 (0.21) * |
-0.047 | 0.01 (0.01) * |
0.045 | -0.03 (0.02) |
-0.032 |
| Plasma Viscosity | ||||||||||||
| + Age and Sex | -7.49 (5.01) |
-0.031 | -4.50 (2.85) |
-0.033 | -6.71 (3.96) |
-0.036 | -10.63 (3.25) ** |
-0.063 | 0.56 (0.12) *** |
0.093 | -0.95 (0.31) ** |
-0.065 |
| + MHVT | -5.45 (4.75) |
-0.023 | -3.48 (2.48) |
-0.026 | -5.64 (3.53) |
-0.031 | -9.21 (3.04) ** |
-0.055 | 0.51 (0.11) *** |
0.087 | -0.87 (0.26) ** |
-0.060 |
| + MHVT and CVD risk factors | -3.61 (4.90) |
-0.015 | -2.30 (2.55) |
-0.017 | -4.79 (3.65) |
-0.026 | -7.47 (3.13) * |
-0.045 | 0.45 (0.11) *** |
0.077 | -0.68 (0.27) * |
-0.047 |
Cognitive test scores adjusted for MHVT.
Nonsignificant after CVD adjustments.
P < .05
P < .01
P < .001
AVLT = Auditory Verbal Learning Task, RAVENS = Raven’s Standard Progressive Matrices, VFT = Verbal Fluency Test, DST = Digit Symbol Test, TMT = Trail Making Test, MHVT = Mill Hill Vocabulary Test, CVD risk factors = cardiovascular disease risk factors (ABPI, cholesterol, smoking, and diastolic blood pressure).
When cognitive decline was measured in the subset of study participants who undertook detailed cognitive testing at both baseline and follow-up (Table 4), plasma fibrinogen was found to be significantly and inversely associated with change in VFT scores (standardised β -0.066, P = 0.046), plasma CRP with RAVENS scores (standardised β -0.069, P = 0.037) and plasma viscosity with poorer performance on the TMT (standardised β 0.092, P = 0.011). Again these associations remained significant after further adjustment for baseline CVD risk factors. Interestingly, higher plasma CRP levels appeared to be associated with an improvement in performance on the AVLT (standardised β 0.081, P = 0.029).
Table 4.
Multivariate associations between biomarkers and 5 year cognitive change
| AVLT | RAVENS | VFT | DST | ln(TMT) | g | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Biomarker + adjustments |
Unstandardised β (Standard Error) | Standardised β | |||||||||||
| Fibrinogen | ||||||||||||
| + Baseline cognition | 0.56 (0.80) |
0.026 | -0.53 (0.38) |
-0.044 | -1.04 (0.52) * |
-0.066 | -0.43 (0.42) |
-0.028 | 0.03 (0.02) |
0.052 | -0.05 (0.03) |
-0.036 |
| + CVD risk factors | 0.77 (0.81) |
0.035 | -0.56 (0.39) |
-0.046 | -1.15 (0.53) * |
-0.073 | -0.38 (0.43) |
-0.025 | 0.02 (0.02) |
0.042 | -0.04 (0.03) |
-0.032 |
| ln(C-reactive Protein) | ||||||||||||
| + Baseline cognition | 1.18 (0.54) * |
0.081 | -0.56 (0.27) * |
-0.069 | -0.60 (0.36) |
-0.058 | -0.48 (0.29) |
-0.047 | 0.01 (0.01) |
0.034 | -0.02 (0.02) |
-0.018 |
| + CVD risk factors | 1.37 (0.55) * |
0.094 | -0.63 (0.27) * |
-0.078 | -0.64 (0.37) |
-0.062 | -0.47 (0.29) |
-0.046 | 0.01 (0.01) |
0.030 | -0.02 (0.02) |
-0.018 |
| Plasma Viscosity | ||||||||||||
| + Baseline cognition | -6.48 (7.87) |
-0.029 | 1.32 (3.85) |
0.010 | -0.83 (5.32) |
-0.005 | -4.50 (4.27) |
-0.028 | 0.50 (0.20) * |
0.092 | -0.36 (0.32) |
-0.027 |
| + CVD risk factors | -4.24 (8.22) |
-0.019 | 1.22 (4.03) |
0.010 | -2.25 (5.55) |
-0.014 | -2.92 (4.45) |
-0.018 | 0.43 (0.21) * |
0.079 | -0.25 (0.33) |
-0.019 |
P < .05.
AVLT = Auditory Verbal Learning Task, RAVENS = Raven’s Standard Progressive Matrices, VFT = Verbal Fluency Test, DST = Digit Symbol Test, TMT = Trail Making Test, CVD risk factors = cardiovascular disease risk factors (ABPI, cholesterol, smoking, and diastolic blood pressure).
In a final model, subjects with a CRP measurement greater than 10 mg/l were excluded from the analyses. CRP values above this threshold are regarded as being indicative of acute inflammation [23] and abnormally large values may have obscured any potential relationships. However, the number of subjects with CRP levels > 10 mg/l was only 112 (4.8% of the original population of 2,312) and after exclusion of these subjects from the dataset, the results remained similar (data not shown).
Discussion
In the present study, raised levels of plasma fibrinogen and CRP were associated with poorer general cognitive ability, non-verbal reasoning, executive function (CRP only), processing speed, and mental flexibility after 5 years of follow-up and following adjustment for age and sex. All but one of these associations remained significant after adjustment for baseline MHVT scores, suggesting an association with estimated lifetime cognitive decline. Despite smaller numbers of subjects, we also found significant associations between CRP and fibrinogen and 5 year decline in executive function and non-verbal reasoning, respectively. The size of these associations were again small, although this may be an effect of assessing cognitive change in a relatively young cohort (mean age 62 years at baseline). There is also a strong possibility of a practice effect on the cognitive tests skewing these results. Whilst a five year follow-up is comparable to the other studies discussed [7, 9] an assessment of cognitive change over a longer timeframe would have been ideal. Overall, our findings were most pronounced for tests associated with processing speed and mental flexibility, possibly reflecting the increased sensitivity of these tests to age-related cognitive decline compared with tests for alternative domains such as memory [24]. However, one previous study found that plasma fibrinogen was also associated with estimated lifetime decline in memory [9].
Of the rheological factors tested, baseline plasma viscosity was associated with late-life general cognitive ability, processing speed and mental flexibility, with estimated lifetime cognitive decline, and with 5-year decline in mental flexibility. These results are consistent with findings in men participating in the Caerphilly study [25]. Since plasma fibrinogen is a major determinant of plasma viscosity it is not surprising that our findings for both biomarkers were similar (the correlation between the marker levels was 0.454, P < 0.001). After adjusting for fibrinogen, plasma viscosity remained significantly associated with scores on the TMT. This could mean that altered rheology, over and above any effect of fibrinogen molecules themselves, accounts for the association between plasma viscosity and cognitive ability. Contrary to the Caerphilly study [25], we observed no associations between haematocrit and any test of cognitive ability. While further large prospective studies of haematocrit and cognitive function and decline are required, it is possible that our findings reflect a more important role for plasma viscosity than for haematocrit or whole blood viscosity in determining blood flow in the cerebral microcirculation [10].
The strengths of this study were the application of a comprehensive cognitive battery; cognitive change measurements being taken over a 5 year period (which is at least as long as the follow-up period from previous studies); and the assessment of four inter-related measures of inflammation and rheology in a large population-based sample of over 2,000 persons. Also, by selecting participants with no history of cardiovascular disease, we were able to assess the association between biomarker levels and cognition in a section of the population with minimal evidence of vascular disease at baseline. Limitations of the study included reduced power to detect associations in the cognitive change subset, which may help to explain differences between the subset analysis and the estimated cognitive decline analysis. Selection bias may also have occurred in the subset due to the non-random nature of the selection. Furthermore, despite the reliability and consistency of vocabulary based cognitive test scores, the study is limited by the use of estimated cognitive decline instead of an actual measure of cognitive change. In addition, the use of single, peripheral measurements of biomarkers, potentially diluting the associations found [7, 26]. CRP levels in particular are prone to large fluctuations, especially during acute inflammatory processes and it has been recommended that multiple measures be taken for greater accuracy [27]. Peripheral measures of the biomarkers may also not reflect levels within the cerebral circulation. Although the AAA participants were all healthy and living independently in the community at baseline, bias may have resulted from selective non-response and withdrawal from the trial. We hypothesised that persons with poorer cognitive function were less likely to participate and found that those with missing values at follow-up tended to have worse scores at baseline testing (data not shown). In totality, the limitations of the study suggest that, if anything, the observed associations are likely to be underestimating the true relationship between the markers and cognitive ability or decline.
Although effect sizes for associations between inflammatory biomarkers and cognitive ability were small, they were consistent with results from previous studies [6-9]. It is possible, however, that the associations are not causal but the result of other conditions that underlie both cognition and inflammation. One potential confounding, or mediating factor is cardiovascular disease, which has been shown to be associated with both inflammation [28] and cognitive ability [29]. However, when the modelling was repeated with the cardiovascular risk factors as covariates most of the significant associations remained. This, together with the asymptomatic study population, suggests that the relationship between inflammation and cognition is at least partially independent of baseline cardiovascular disease. In addition, as the fibrinogen and CRP levels correlated both negatively and strongly with the MHVT scores there is also a possibility of reverse causation affecting the interpretation of the results. It may be that poorer cognitive ability in earlier life is linked to conditions affecting inflammation in later life, whether directly or through associations with lifestyle and socio-economic status. Furthermore, lower socioeconomic status (SES) in adult life is also a strong predictor of raised inflammatory marker levels, including CRP and fibrinogen [30, 31]. These associations were weakened when comparing the biomarker levels with childhood socioeconomic status with adjustment for adult cardiovascular disease risk factors strongly attenuating the associations between CRP and early life SES.
To help explain the physiological relationship between inflammation, rheology, and cognitive decline and to determine whether they are causal, future research could usefully consider associations between cognitive ability and genetic polymorphisms affecting biomarker levels. A causal role for inflammatory markers in cognition is certainly plausible, either with or without vascular pathology as an intermediary. It is known that microglia produce pro-inflammatory factors including Tumor Necrosis Factor (TNF)-α and Interleukin-1β, excessive quantities of which can be deleterious to neurons [32]. TNF-α also stimulates the production of fibrinogen [33] and (indirectly via synthesis of interleukin-6) CRP [34], which along with other cytokines and products of the complement cascade activate the microglia [35]. Increased plasma viscosity may also have a direct effect on cognitive function by decreasing cerebral blood flow [10, 25]. If blood viscosity within the low-normal range and maintained brain performance by rehydration is beneficial to the acutely challenged, hypo-perfused brain in older patients, then it may be possible to extend the potential link between blood viscosity and brain function to chronic cognitive decline in the general population [10].
In conclusion, we have shown that raised circulating inflammatory marker levels and raised plasma viscosity are associated with poorer late-life cognitive ability, estimated lifetime cognitive decline, and in some cases with late-life cognitive decline. Future studies are required to confirm or refute the causality of these associations and so guide the development of preventive interventions.
Acknowledgments and funding
This research was supported by the Wellcome Trust, Chest Heart and Stroke Scotland, the British Heart Foundation, the Chief Scientist Office, Scotland, and the Medical Research Council (funding for Riccardo Marioni and the MRC Centre for Cognitive Ageing and Cognitive Epidemiology).
Acronyms
- CRP
C-reactive protein
- AAA
Aspirin for Asymptomatic Atherosclerosis
- ABI
ankle-brachial index
- AVLT
Auditory Verbal Learning Test
- RAVENS
Raven’s Standard Progressive Matrices
- DST
Digit Symbol Test
- VFT
Verbal Fluency Test
- TMT
Trail Making Test, Part B
- MHVT
Mill Hill Vocabulary Test
- CVD
cardiovascular disease
- TNF
Tumor Necrosis Factor
- BP
blood pressure
- fib
fibrinogen
Footnotes
Conflicts of interest
None
References
- [1].Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, McArdle JJ, Willis RJ, Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cheung RTF, Hachinski V. Vascular Factors in Cognitive Decline. In: Fillit HM, Butler RN, editors. Cognitive Decline: Strategies for Prevention. Cambridge University Press; 1997. pp. 53–69. [Google Scholar]
- [3].Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52(2):168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- [4].Jones RW. Inflammation and Alzheimer’s disease. Lancet. 2001;358(9280):436–7. doi: 10.1016/S0140-6736(01)05667-7. [DOI] [PubMed] [Google Scholar]
- [5].Ravaglia G, Forti P, Maioli F, Brunetti N, Martelli M, Servadei L, Bastagli L, Bianchin M, Mariani E. Serum C-reactive protein and cognitive function in healthy elderly Italian community dwellers. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60(8):1017–21. doi: 10.1093/gerona/60.8.1017. [DOI] [PubMed] [Google Scholar]
- [6].Weuve J, Ridker PM, Cook NR, Buring JE, Grodstein F. High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology. 2006;17(2):183–9. doi: 10.1097/01.ede.0000198183.60572.c9. [DOI] [PubMed] [Google Scholar]
- [7].Schram MT, Euser SM, de Craen AJM, Witteman JC, Frolich M, Hofman A, Jolles J, Breteler MM, Westendorp RG. Systemic Markers of Inflammation and Cognitive Decline in Old Age. J. Am. Geriatr. Soc. 2007;55(5):708–16. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- [8].Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–7. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- [9].Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, Lowe GD, Fowkes FG. Cognitive Decline and Markers of Inflammation and Hemostasis: The Edinburgh Artery Study. J. Am. Geriatr. Soc. 2007;55(5):700–7. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- [10].Lowe GDO. Is sticky blood a treatable determinant of cognitive decline and of dementia? Age Ageing. 2001;30:101–3. doi: 10.1093/ageing/30.2.101. [DOI] [PubMed] [Google Scholar]
- [11].Price JF, Stewart MC, Douglas AF, Murray GD, Fowkes GF. Frequency of a low ankle brachial index in the general population by age, sex and deprivation: cross-sectional survey of 28,980 men and women. Eur J Cardiovasc Prev Rehabil. 2008;15(3):370–5. doi: 10.1097/HJR.0b013e3282f8b36a. [DOI] [PubMed] [Google Scholar]
- [12].Stewart MC, Deary IJ, Fowkes FG, Price JF. Relationship between lifetime smoking, smoking status at older age and human cognitive function. Neuroepidemiology. 2006;26(2):83–92. doi: 10.1159/000090253. [DOI] [PubMed] [Google Scholar]
- [13].Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heald CL, Fowkes FGR, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189:61–9. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [15].Price JF, Stewart MC, Deary IJ, Murray GD, Sandercock P, Butcher I, Fowkes FGR. Low dose aspirin and cognitive function in middle aged to elderly adults: randomised controlled trial. BMJ. 2008;337:a1198. doi: 10.1136/bmj.a1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lezak MD. Neuropsychological Assessment. 3rd ed. Oxford University Press; Oxford: 1995. [Google Scholar]
- [17].Raven J, Raven JC, Court JH. Raven manual: standard progressive matrices. Oxford Psychologists Press; Oxford, UK: 1998. [Google Scholar]
- [18].Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; New York, NY: 1981. [Google Scholar]
- [19].Salthouse T. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–61. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deary IJ, Whalley LJ, Crawford JR. An ‘instantaneous’ estimate of a lifetime’s cognitive change. Intelligence. 2004;32(2):113–9. [Google Scholar]
- [21].Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115(16):2119–27. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- [22].SPSS for Windows, Rel. 14.0.1. SPSS Inc.; Chicago: 2005. [Google Scholar]
- [23].Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin. Chem. 2001;47(3):444–50. [PubMed] [Google Scholar]
- [24].Salthouse T. Aging and measures of processing speed. Biol. Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- [25].Elwood PC, Pickering J, Gallacher JE. Cognitive function and blood rheology: results from the Caerphilly cohort of older men. Age Ageing. 2001;30(2):135–9. doi: 10.1093/ageing/30.2.135. [DOI] [PubMed] [Google Scholar]
- [26].Emberson J, Whincup P, Morris R, Walker M, Lowe GD, Rumley A. Extent of regression dilution for established and novel coronary risk factors: results from the British Regional Heart Study. Eur. J. Cardiovasc. Prev. Rehabil. 2004;11:125–34. doi: 10.1097/01.hjr.0000114967.39211.e5. [DOI] [PubMed] [Google Scholar]
- [27].Koenig W, Sund M, Frohlich M, Lowel H, Hutchinson WL, Pepys MB. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA Augsburg studies, 1984 and 1987. Am. J. Epidemiol. 2003;158(4):357–64. doi: 10.1093/aje/kwg135. [DOI] [PubMed] [Google Scholar]
- [28].Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- [29].Breteler MMB, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam study. BMJ. 1994;308:1604–8. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu B, Hong J. Role of Microglia in Inflammation-Mediated Neurodegenerative Diseases: Mechanisms and Strategies for Therapeutic Intervention. J. Pharmacol. Exp. Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- [33].Pulanić D, Rudan I. The past decade: fibrinogen. Coll Anthropol. 2005;29(1):341–9. [PubMed] [Google Scholar]
- [34].Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- [35].McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat. Disord. 2003;10(1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]