Abstract
Even simple biological signals vary in several measurable dimensions. Understanding their evolution requires, therefore, a multivariate understanding of selection, including how different properties interact to determine the effectiveness of the signal. We combined experimental manipulation with multivariate selection analysis to assess female mate choice on the simple trilled calls of male gray treefrogs. We independently and randomly varied five behaviorally relevant acoustic properties in 154 synthetic calls. We compared response times of each of 154 females to one of these calls with its response to a standard call that had mean values of the five properties. We found directional and quadratic selection on two properties indicative of the amount of signaling, pulse number and call rate. Canonical rotation of the fitness surface showed that these properties, along with pulse rate, contributed heavily to a major axis of stabilizing selection, a result consistent with univariate studies showing diminishing effects of increasing pulse number well beyond the mean. Spectral properties contributed to a second major axis of stabilizing selection. The single major axis of disruptive selection suggested that a combination of two temporal and two spectral properties with values differing from the mean should be especially attractive.
Keywords: nonlinear selection, stabilizing selection, canonical rotation, acoustic signals, gray treefrog
Female mate choice is often influenced by multiple properties of mate-attraction signals. These different properties can influence the success of the signal, and thus the attractiveness of the male, independently or via more complex interactions between properties. Nonetheless, most experimental studies of female choice manipulate only one property of a signal at a time. As a result we seldom have information about how prospective mates deal with simultaneous variation in multiple properties, including the relative importance of different signal properties, and how they interact to influence signal quality. Multivariate analysis can often yield new insights into the sexual selection that female preferences exert (Lande and Arnold 1983; Ryan and Rand 2003; Blows et al. 2003, Hine et al. 2004). Multivariate linear selection analysis (Lande & Arnold 1983), for example, uses multiple linear regression to estimate simultaneously the directional selection operating on a suite of traits. Nonlinear selection analysis (Lande and Arnold 1983; Phillips and Arnold 1989) estimates quadratic selection (including stabilizing and disruptive selection) on each trait as well as the correlational selection that arises due to interactions between traits. Most selection analyses of signal evolution, however, are based on observational data and do not have the inferential power of experimental approaches. Here we use a recently-developed approach (Brooks et al. 2005; Bentsen et al. 2006; Donohoe at al. 2009) that combines experimental power with the multivariate statistical insights of selection analysis to dissect the effects of five components of the call of the gray treefrog (Hyla versicolor).
Female responses to the acoustic signals of many kinds of insects and frogs are especially useful for experimental analysis of multivariate selection because females base their choice of mates mainly, if not exclusively, on such signals (review: Gerhardt and Huber 2002). Moreover, acoustic signals are relatively simple to analyze and synthesize; playbacks can be achieved with a high degree of stimulus control to reproductively active females, which reliably respond in an unequivocal fashion (positive phonotaxis). Not surprisingly, two important recent studies of multivariate selection have involved the acoustic mate-attracting signals of the Túngara frog Physalaemus pustulosus (Ryan and Rand 2005) and the cricket Teleogryllus commodus (Brooks et al. 2005). In the Túngara frog study, a multidimensional scaling approach was used to generate nine different test signals that were representative of the range of multivariate space based on the analysis of 15 call properties. Univariate preference functions were available for some of the acoustic properties but were not formally compared with their contribution to the multivariate preference space. In the cricket study, five of 14 acoustic properties were chosen and experimentally manipulated by simultaneously drawing random values for each trait from that trait’s original univariate distribution, thus breaking up the correlations between traits. These calls were then used in playbacks to females in the laboratory (Brooks et al. 2005) and, later, the field (Bentsen et al. 2006).
In the present study we used the experimental approach developed by Brooks et al. (2005) to study patterns of multivariate selection in the gray treefrog (H. versicolor). We independently manipulated the values of five properties of the advertisement call: pulse rate, dominant frequency; the relative amplitude of the two spectral peaks; pulse number (the main determinant of call duration), and call period (the inverse of call rate). We emphasize that the advertisement call of H. versicolor is a simple trill rather than a two-part signal such as the calling song of T. commodus and the advertisement call of P. pustulosus. Thus, these five properties constitute a large proportion of the acoustic properties of this signal. These five properties are subject to strong correlations with one another for both biophysical and energetic reasons. The approach we take here significantly reduces these correlations up in order to assess the directional and quadratic selection operating on each as well as the interactions between traits in the form of correlational selection. Previous experimental studies have established that variation in each of these properties affects selective phonotaxis in females (Gerhardt 2001, 2005a,b). Moreover, those properties that we held constant in our experimental stimuli (pulse shape, pulse duty cycle, frequency modulation within pulses) show extremely little variation within and between the calls of males in any single population. Our study, therefore, presents an ideal opportunity to assess the insights into sexual selection and signal evolution that an experimental multivariate approach offers over and above what we have learned from univariate experiments.
We were especially interested in independently assessing the effects of call duration and call rate on the attractiveness of the call. These traits are strongly negatively correlated because the aerobic metabolic rate of calling individuals is directly correlated with both properties, and calling is energetically constrained (review Wells 2007). Most experimental studies of female choice have explored the effects of varying call duration because males increase duration and reduce call rate (increase call period) in competitive environments (dense choruses)(Wells and Taigen 1986). Indeed, females chose long calls produced at a slow rate even when the call rate of short calls was increased so that the acoustic “on-time” of both alternatives was equalized (Klump and Gerhardt 1987; Gerhardt et al. 2000). Thus, whereas detailed preference functions and assessments of preference-strength are available for call duration in H. versicolor (Gerhardt et al. 1996, 2000; Bush et al. 2002), the effects of small differences in call rate, or of systematic and simultaneous variation in both call duration and call rate are largely unexplored.
The three other acoustic variables that we manipulated show much less variation within and between males than do call duration and call rate, and there is good evidence from univariate studies that pulse rate (Gerhardt 2005a; Bush et al. 2002) and dominant frequency (Gerhardt 2005b) are under stabilizing or weakly directional selection. Furthermore, pulse rate is weakly and negatively correlated with call duration simply because pulses are shorter and have shorter inter-pulse intervals in calls with high pulse rates than in calls with low pulse rates. We were interested to test how these traits might interact with one another and with call duration and call rate.
Previous studies have demonstrated the power of analyses and visualization of nonlinear selection to discover unexpected and counter-intuitive patterns of selection involving particular combinations of acoustic properties (e.g., Phillips and Arnold 1989; Blows et al. 2003; Bentsen et al. 2006). Such insights have opened up previously intractable questions, including how estimates of multivariate selection correspond with multivariate phenotypic and genetic variation in natural populations (e.g. Hine et al 2004; van Homrigh et al. 2007; Hunt et al. 2007, Hohenlohe and Arnold 2008), and how variation in different signal properties affects their interaction and integration in the receiver’s sensory system. The powerful and novel insights that a combination of experimental and multivariate selection analysis can yield provides further rationale for our study.
We will show that measures of signal output -call duration and call rate - are subject to both directional and nonlinear selection by female choice, even when their values are uncorrelated. We use canonical rotation of the nonlinear fitness surface to identify the main trait combinations under stabilizing and disruptive selection (Phillips and Arnold 1989; Blows and Brooks 2003). This approach revealed evidence for stabilizing selection along an axis dominated by pulse rate, pulse number and call rate. These results are generally consistent with studies showing that there are diminishing returns in terms of female preferences of increasing pulse number (call duration) well beyond mean values (Gerhardt et al. 2000; Bush et al. 2001). Thus, pulse rate and call rate are also likely to contribute interactively to limits on the attractive power of increasing the acoustic “on-time” beyond a certain point. Canonical rotation also provided evidence for stabilizing selection on spectral properties of the call and revealed disruptive selection that should make certain combinations of values of spectral and fine-scale temporal properties especially attractive. These results suggest new experiments that could not be conceived in the absence of such an analysis, thus reinforcing the fact that these methods often point to counterintuitive hypotheses about sexual selection.
Methods
ACOUSTIC STIMULI
Synthetic calls were made with custom-designed software (written by J. J. Schwartz) that created 16-bit digital files with an output sampling rate of 20 kHz. The standard call had the same properties as those used in previous studies that generated univariate preference functions for pulse rate, pulse number (≈ call duration) and dominant frequencies (Gerhardt 2005a,b; Bush et al. 2002). The temporal properties of the standard call had values that were close to or matched the temperature-corrected (20° C) mean values in the advertisement calls of a sample of 168 males recorded in 1987 in the same population from which most females were collected for testing (Gerhardt et al. 1996; Fig. 1). The pulse duty cycle (ratio of pulse duration to pulse period) was held constant at 50% in the standard call and all alternatives, mirroring the stability of this derived property in natural calls (Gayou 1984).
Figure 1.
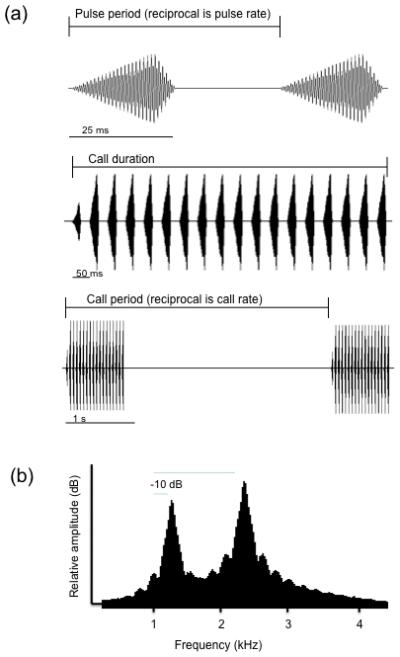
Acoustic properties of the standard synthetic stimulus. (A) Temporal properties as shown by oscillograms. Top trace: two pulses showing the pulse period (the reciprocal is the pulse rate); in alternatives, the pulse duty cycle (ratio of pulse duration to pulse period) was also 50%; Middle trace: a complete call of 18 pulses; call duration of alternatives was mainly determined by pulse number, and influenced by pulse rate such that call duration of alternatives was shorter in calls with higher pulse rates and longer in calls with lower pulse rates. Bottom trace: two complete calls showing the call period (the reciprocal is the call rate); call period in alternatives was not affected by variation in any other temporal property. (B) Spectral properties as shown by the power spectrum of one call. The frequency of both components was varied in alternatives, but the harmonic relationship (2:1) was maintained. The relative amplitude (RA) of the two peaks was also varied in alternatives; high values of RA indicate that the amplitude favoring the high-frequency peak was greater than the usual 10 dB difference; low values indicate the amplitudes of the two peaks were more nearly equal.
The standard signal had a dominant frequency of 2.2 kHz and secondary peak of 1.1 kHz that had a relative amplitude of -6 dB; these values are slightly lower than the mean values in the advertisement calls of a sample of 59 males from central Missouri (Gerhardt 2005b; Table 1). The standard and other synthetic calls did not incorporate the within-pulse frequency modulation typical of this species; consequently, the bandwidths of these peaks were narrower than in natural calls. Nevertheless, synthetic calls without frequency-modulated pulses were as attractive to females as synthetic calls with such modulation as well as typical, pre-recorded calls (Gerhardt 1978).
Table 1. Summary statistics from call analyses used to generate distributions of values of variables in the experimental signals. See Figure 1.
| Property | Mean | Standard deviation |
|---|---|---|
| A. Temporal properties | ||
| Pulse rate (PR) | 20 pulses/s | 1.7 pulse/s |
| Pulse number (PN) | 17.8 | 4.3 |
| Call period (CPER) | 4.2 s (14.4 calls/ min) | 0.74 s |
| B. Spectral properties | ||
| High-frequency peak1 (Freq) | 2250 Hz | 150 Hz |
| Relative amplitude2 (RA) | 10 dB | 1 dB |
Low-frequency peak frequency was always one-half of that of the high-frequency peak.
High-frequency peak had more energy than low-frequency peak (see Fig. 1).
We generated one standard call and 154 unique alternative calls in which the values of each of the five manipulated properties were determined independently using the methods of Brooks et al. (2005). Briefly, we first generated a standard call with the mean value for each of the five call properties that we wished to manipulate (these mean values and their standard deviations are from Gerhardt 2005a,b; Bush et al. 2002, and are given in Table 1). To assign a value to each property in each call, we drew a random number between zero and one, and converted this from a probability to a z-score (i.e. in standard deviations) using the normsinv function in Microsoft Excel. We then converted this standardized value to the equavalent trait value in the univariate normal distribution for the relevant trait. Values of pulse number and call period (converted from call rate in calls per minute to facilitate call synthesis) were log-transformed to achieve a normal distribution for sampling and then re-converted to linear measures before the call was synthesized.
PLAYBACK SYSTEM, TEST PROCEDURE AND CALCULATION OF PHONOTAXIS SCORES
Females of H. versicolor were tested in the semi-anechoic chamber described in Gerhardt (1994). Sounds were amplified and played back from an Analog-Digital-Systems 200 speaker, which was 1 m from the point at which the female was released. Digital files were output from an IBM-compatible personal computer using Adobe Audition software. Signal amplitude was adjusted at the release point to 85 dB SPL (sound pressure level in dB re 20 μPa) with a Larsen-Davis 720 sound level meter.
Female treefrogs were collected in amplexus at several localities in central Boone County, Missouri (USA)(see Gerhardt 2005a,b). They were refrigerated (about 4°C) to delay oviposition. Before testing (usually within 3 days of capture), each female was acclimated to the target test temperature (20°C) for at least 30 min. Each female was placed in a circular, acoustically transparent (hardware or plastic cloth) cage, which was located 1 m from the speaker. After an acoustic stimulus had been played back three times, the top of the cage was removed remotely. Female movements were observed under infra-red illumination with a closed-circuit video system. A response was tabulated when the female showed phonotactic orientation behavior (Rheinlaender et al., 1979) and moved to within 10 cm of one of the speakers within 5 min. If a female did not begin phonotaxis within 5 min or hopped away from the testing area within the chamber, the trial was recorded as a “no response.”
We timed each response of a female in six tests: three tests were with standard call and three tests with an alternative stimulus from the set of 154 calls. We computed a phonotaxis score following the procedures of Bush et al. (2002) by dividing the mean value of the time required to respond to the standard call by the mean value of the time required to respond to the alternative call. The phonotaxis score was then one if the mean times were the same, >1 if the female responded more rapidly to the alternative call, and < 1 if the female responded more rapidly to the standard call. One half of the females were first tested with the standard call, followed by the alternative, and a retest of the standard call. After a time-out of at least 5 min, the female was tested with the alternative call, followed by the standard call, and a retest of the alternative call. In tests of the other half of the females, the order of these two, 3-test series was reversed, i.e., the first three tests were: alternative, standard, alternative, and the second three tests, standard, alternative, standard. We used only the results from females that responded within 5 min in all six tests because these are untrained animals, whose probability of responding to any signal is strongly influenced by their reproductive status, effects of handling, and other factors unrelated to the attractiveness of the acoustic stimulus.
Although female frogs probably assess the calls of more than one male at a time in choruses (Murphy and Gerhardt 2002), our single-speaker design had two advantages over choice-tests. First, we obtained a score from each female that could be compared across females. Second, there was no possibility of acoustic overlap and hence masking of parts of two alternative signals during a playback. Males tend to avoid such overlap with near neighbors, and univariate studies have typically controlled the phase relationship of alternatives to maintain equal periods of silence before and after each stimulus (e.g., Gerhardt 2005a,b). In the present study, however, the high values of pulse number and low values of call period in many stimuli would have made overlap with a standard call inevitable.
ANALYSIS
We standardized all traits to have a mean of zero and a standard deviation of one before selection analysis. We used multiple regression based linear and nonlinear selection analysis (Lande and Arnold 1983), implemented in SPSS (v15.0) to estimate standardized gradients of linear and quadratic selection on the five traits and correlational selection on pairs of traits. Standardized linear selection gradients (βi) were estimated from a multiple regression model that included only the linear terms for each trait. Quadratic and correlational gradients were estimated from a multiple regression that included the linear terms, their squares (i.e., quadratic gradients, γii), and the cross products (i.e. correlation gradients, γij) of each pair of linear terms. Quadratic selection gradients estimated in this way using statistical software packages (including SPSS, ver. 15.0) must be doubled (Stinchcombe et al. 2008) in order to give appropriate Lande-Arnold (1983) gradients and we have done so here.
The multivariate form of nonlinear selection may be misinterpreted by simple inspection of the terms in the gamma matrix. In particular, the strength and significance of nonlinear selection may be underestimated (Phillips and Arnold 1989; Blows and Brooks 2003). We therefore performed a canonical rotation of the gamma matrix to find the major axes of the nonlinear response surface (Phillips and Arnold 1989; see method in app. 1 of Blows and Brooks 2003). This canonical rotation results in a matrix, M, comprising i eigenvectors, mi, each describing a major axis of the response surface (where i is the original number of traits). The strength of nonlinear selection along each eigenvector is given by its eigenvalue, λi. The strength of directional selection (θi) along each eigenvector and the significance of both directional and nonlinear selection along each eigenvector were obtained by including linear and quadratic forms of all eigenvectors in a new multiple-regression model (i.e., the double-linear regression [DLR] method described by Bisgaard and Ankenman [1996]). These results were visualized using thin-plate splines in the fields package in R following the methods of Brooks et al. (2005).
RESULTS
In Table 2 we show the standardized directional selection gradients and the matrix of standardized quadratic and correlational selection gradients. There was significant positive directional selection for calls with higher pulse number and negative directional selection and negative quadratic selection on call period. Because call period is the inverse of call rate, this pattern means that there was positive directional selection for high call rates. We did not detect significant directional or quadratic selection on dominant frequency, pulse rate or relative amplitude.
Table 2. The vector of standardized directional selection gradients (β) and the matrix (γ) of standardized quadratic (on diagonal) and correlational (below diagonal) selection gradients.
| γ | ||||||
|---|---|---|---|---|---|---|
| β | Freq | PR | PN | CPER | RA | |
| Dominant frequency (Freq) | 0.022 | 0.018 | ||||
| Pulse rate (PR) | 0.005 | 0.001 | 0.014 | |||
| Pulse number (PN) | 0.065** | -0.021 | 0.036 | -0.012 | ||
| Call period (CPER) | -0.047* | 0.017 | -0.032 | 0.021 | -0.094** | |
| Relative amplitude (RA) | -0.015 | -0.035 | 0.018 | 0.013 | -0.001 | -0.040 |
P < 0.05
P < 0.01
The small number of significant nonlinear selection gradients (one out of fifteen) may be due to a lack of nonlinear selection, or low power to detect such selection due to the number of terms in the regression model (twenty one terms including linear terms and the intercept). It is often only possible to detect the true extent of nonlinear selection by performing a canonical rotation of the nonlinear fitness surface (Phillips and Arnold 1989; Blows and Brooks 2003). The M matrix of eigenvectors (i.e. major axes of nonlinear selection) and their eigenvalues (equivalent to nonlinear selection gradients, describing the strength and form of nonlinear selection) from the canonical rotation of γ are shown in Table 3 and visualized in Figures 2 and 3. The major features are two axes of stabilizing (negative quadratic) selection (m4 and m5), and one axis (m1) that indicates significant disruptive (positive quadratic) selection. The surface described by m4 and m5 constitutes a peak (Figure 2), indicating stabilizing selection on these two axes.
Table 3. The major dimensions (eigenvectors, (mi)) of nonlinear selection identified by canonical rotation of γ.
The eigenvalues (λi) of each eigenvector are equivalent to the quadratic gradients of selection along each eigenvector
| M | selection | |||||
|---|---|---|---|---|---|---|
| Freq | PR | PN | CPER | RA | λi | |
| m1 | 0.558 | -0.569 | -0.473 | 0.117 | -0.357 | 0.061* |
| m2 | 0.711 | 0.659 | 0.165 | -0.046 | -0.176 | 0.022 |
| m3 | 0.152 | -0.362 | 0.803 | 0.436 | -0.105 | -0.023 |
| m4 | 0.356 | -0.106 | -0.094 | 0.179 | 0.906 | -0.057* |
| m5 | -0.186 | 0.314 | -0.309 | 0.873 | -0.095 | -0.116** |
P < 0.05
P < 0.01
Figure 2.
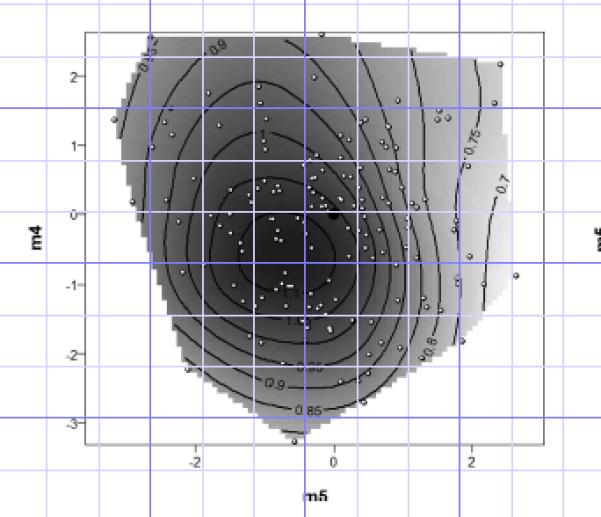
The contour plot of the fitness surface on the two eigenvectors of significant stabilizing selection, m5 and m4. White dots with black outline are the values of the stimulus calls played to females. The black circle is the value of the standard call. Numbers on contours are in units of the phonotaxis score (response time to standard call divided by response time to alternative call). This score equals one if the mean response times are the same (i.e., no preference), >1 if the female responded more rapidly to the alternative call, and < 1 if the female responded more rapidly to the standard call.
Figure 3.
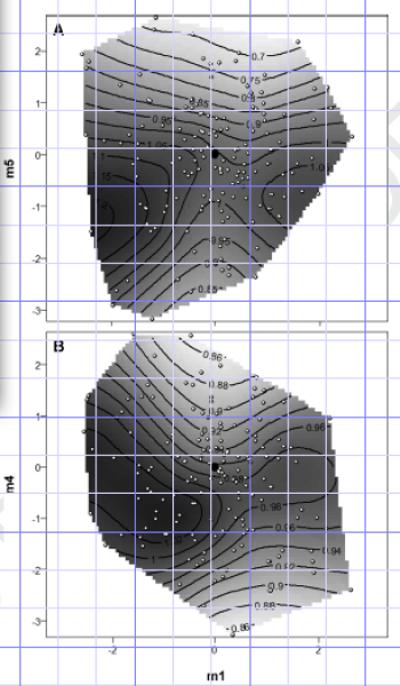
Contour plots of the fitness surface on the single significant axis of disruptive selection m1, against each of the two eigenvectors of significant stabilizing selection, m5 (a) and m4 (b). White dots with black outline are the values of the stimulus calls played to females. The black circle is the value of the standard call.
The axis of strongest stabilizing selection, m5, is heavily influenced by call period, reflecting the significant stabilizing selection on that trait, but it is also heavily loaded by pulse rate and pulse number indicating strong stabilizing selection on the suite of three temporal call traits. The second major axis of stabilizing selection, m4, is weighted heavily to relative amplitude and call frequency, indicating stabilizing selection on the spectral as well as the temporal properties of the call.
The single eigenvector of significant disruptive selection (m1) showed an intermediate minimum (Figure 3), indicating disruptive selection. However, the strong peak at low values of m1 indicates that the combination of high pulse rate, high pulse number, high relative amplitude, and low frequency is particularly attractive to females.
DISCUSSION
DIRECTIONAL AND NONLINEAR SELECTION
We found directional selection for pulse number (≈ call duration) and shorter call period (= higher call rate) in the face of simultaneous and independent variation in other acoustic properties that affect female preferences. Moreover, our methods of constructing the set of synthetic test signals significantly reduced the strong positive correlation (of the order of 0.6 to 0.2) between pulse number and call period (Wells and Taigen 1986; Klump and Gerhardt 1987). Both call properties influence the proportion of “on time” (= ratio of sound to silence), which is an important component of overall calling effort and a reliable indicator of the energetic costs of calling (review: Wells 2007). Call duration and signaling rates have nearly always been shown to be subject to directional preference in univariate studies of acoustically signaling frogs (Klump and Gerhardt 1987; Ryan and Keddy-Hector 1992; Welch et al. 1998); and crickets (Snedden and Sakaluk 1992; Crnokrak and Roff 1995; Bentsen et al. 2006). By contrast, before canonical rotation we detected no significant directional or nonlinear selection on spectral properties (dominant frequency, relative amplitude of the two peaks) nor on pulse rate. This result is consistent with univariate studies have indicated that these properties are under stabilizing and/or weakly directional selection (Gerhardt 2005a,b).
Previous studies have focused heavily on call duration because male gray treefrogs produce longer calls in dense choruses, in which competition to attract females is intense, than they do in sparse choruses (Wells and Taigen 1986). Males producing long calls also produce them at lower rates (i.e., males increase call period), almost certainly because of energetic limitations (Wells and Taigen 1986; Klump and Gerhardt 1987). However, even males that produce long calls at slow rates spend less time calling on a given night (Pough et al. 1992) and during the breeding season (Sullivan and Hinshaw 1992), indicating that such males are incurring additional energetic costs that are not captured in measurements of aerobic metabolic rate. Furthermore, even when call-duty cycle was equalized in a pair of alternative synthetic calls, mimicking signals that would require the same aerobic energetic costs, females preferred long calls repeated at a slow rate to short calls repeated at a high rate (Klump and Gerhardt 1987; Gerhardt et al. 2000). Subsequent studies found that call duration predicted offspring fitness in accordance with the hypothesis that energetically costly signals are honest advertisements of male quality (Welch et al. 1998).
The focus on call duration has drawn attention away from the significance of call rate, for which no detailed preference function has been estimated. In part this is because the results of choice tests would almost certainly be confounded by unavoidable overlap between alternative stimuli in a choice test. Nevertheless, results of a study in which female H. versicolor from central Missouri selected among 4 to 8 simultaneously calling males (98 males in 45 “choruses”) in a semi-natural enclosure (Schwartz et al. 2001) showed that both pulse number (the main determinant of call duration at any single temperature) and total number of pulses produced (a function of duration and call rate = calling effort) explained significant amounts of variation in male mating success. Our results indicate that call rate and call duration are under positive directional selection not only when other call properties are simultaneously and randomly varied but also when the usual negative correlation between these variables is eliminated. Moreover, we also detected significant nonlinear selection on call rate.
NONLINEAR SELECTION: EVIDENCE FOR STABILIZING SELECTION
As in many previous studies (reviewed by Blows and Brooks 2003), the extent and importance of nonlinear selection was not evident from the quadratic and correlational selection gradients (γ), and was only revealed by canonical rotation. All five traits load heavily on one or more canonical axis of significant nonlinear selection.
The idea that the the temporal properties of the call (pulse rate, pulse number and call period) may be tightly integrated by selection is supported by the canonical analysis of the nonlinear fitness surface. The axis of strongest nonlinear selection (m5) indicated very strong stabilizing selection on a combination heavily weighted by all three of these traits. This result seems counter-intuitive in the face of our estimates of positive directional selection on pulse number and call rate (inverse of call period) and the results of univariate playback experiments. However, there is also evidence from univariate studies that the sensory system of the female may well impose limits on the effectiveness of calls that are very long in duration or high in call rate. First, Klump and Gerhardt (1987) reported that all eight responding females chose a super-normal call of 80 pulses to a call with a typical call of 20 pulses; however, 17 other females tested with this combination did not respond to either stimulus. Second, females preferred calls of average duration to calls of very short duration more strongly than they preferred very long calls to calls of average duration as indicated by the distance- (Gerhardt et al. 1996) or intensity-dependence (Gerhardt et al. 2000) of their choices. The diminishing returns of adding pulses were also evident in the single-speaker response functions of Bush et al. (2001). Because call rate was held constant in all of these experiments, increasing pulse number also reduced the length of the silent period between calls. The silent period would obviously also be reduced as call period is reduced (call rate increased) and pulse number is held constant. We speculate therefore that there is probably some minimum period of silence between calls that is necessary to attract females. If so there are probably upper limits on call duration, call rate or a derived combination, the call duty-cycle (ratio of call duration to call period). The lower limit would of course be imposed by some minimum amount of stimulation needed to elicit a response and would be expected to increase to at least the values of these temporal properties in the average call.
The second axis of significant stabilizing selection was weighted heavily by the two frequency-related traits, dominant frequency and relative amplitude. Such selection, again, was not detectable from the quadratic gradients on the two traits or the correlational gradient although the negative quadratic selection on relative amplitude and correlational selection on relative amplitude (RA) and frequency are among the larger gradients in γ. These results are consistent with the weak stabilizing selection on call frequency in univariate manipulative experiments (Gerhardt 2005b). It appears from the strong selection on m4, however, that stabilizing selection on call frequency and the relative amplitude of the two frequency peaks may interact, tightly integrating the spectral components of the call. Frequency-related traits are especially likely to be under stabilizing selection because auditory systems are often most sensitive (i.e. tuned) to the frequencies emphasized in conspecific calls (Ryan et al. 1992; Gerhardt and Schwartz 2001). The phenotypic variation in call frequency and RA were relatively narrow in our experiment, as they are in natural populations. Such narrow variation in the wild may be a consequence of stabilizing selection on the spectral properties of the call as stabilizing selection tends to deplete genetic variation (Johnson and Barton 2005; Hunt et al. 2007). Moreover, the small amount of variation within our experiment may have resulted in an underestimate of the potential for stabilizing selection in a more variable population because the values of very few of the calls we generated fell outside the range of values expected to be as attractive as the standard call. As with the three temporal properties of the call, we predict that studies that independently manipulate the spectral properties of the call may provide new insights into how and why frequency and relative amplitude interact to determine attractiveness.
NONLINEAR SELECTION: EVIDENCE FOR DISRUPTIVE SELECTION
The single eigenvector of significant disruptive selection m1 shows a weakly disruptive pattern. There is, however, a substantial peak indicating that all positive values of m1 are roughly equally unattractive, but that below zero their values then rise rapidly. Low values of m1 are occupied by calls that combine high pulse rate, pulse number and relative amplitude with a low frequency. Of these, only pulse number has been shown to be highly attractive in univariate studies (Gerhardt et al. 1996, 2000). Low frequency is a fairly reliable indicator of large size (Gerhardt 2005b), and high pulse rate is associated with higher-than-average temperatures. Nevertheless, our work indicates that the effects of these variables are multiplicative and that it is the combination of traits and their values and not the traits alone that confer attractiveness. Similar multiplicative effects have already been demonstrated for properties of field cricket calls that combine to shape calling effort (Bentsen et al 2006), and for multiple traits that combine to effect signal intensity in the empidid dance fly Rhamphomyia tarsata, and in the dark-eyed junco Junco hyemalis (McGlothlin et al. 2005). All of these results support the hypothesis that combinations of traits that interact may be especially likely to evolve as signals of individual quality (Kokko et al. 1999; Brooks et al. 2005).
CONCLUSIONS
Our results illustrate once again how important features of selection that are not detectable from the γ matrix of quadratic and correlational selection gradients can be detected after canonical rotation of the fitness surface (Philips and Arnold 1989; Blows and Brooks 2003). In this case the three significant eigenvectors of the fitness surface tell three very important stories. The strong stabilizing selection on m5 indicates strong stabilizing selection on intermediate values of the three temporal properties of the call, each of which loads heavily on this eigenvector. Stabilizing selection on the spectral properties of the call is evident from the selection on m4. The importance of stabilizing selection on each of the call properties is consistent with two studies using the same method to break up the correlations among call traits in gryllid crickets (Brooks et al. 2005; Bentsen et al. 2006) and, interestingly, a recent study using the method to break up correlations among three dimensions in line-drawings of women’s torsos (Donohoe et al, in press). Further, the positive nonlinear selection on m1 (disruptive, but with a higher peak at low values of than at high values) adds to accumulating evidence that traits that interact to influence signal intensity can be under particularly strong directional and nonlinear selection. In this case combinations of high pulse rate, pulse number and relative amplitude with a low frequency are very strongly favored by females. Our results add to a growing body of work that shows that once appropriate multivariate methods are used to find the combinations of traits that are the real targets of selection, stabilizing selection appears to be widespread (Blows 2007, Hunt et al. 2007).
ACKNOWLEDGMENTS
We thank Sarah Humfeld for helping with the synthesis of calls. She and Noah Gordon collected and tested females and supervised the following undergraduates who also collected and tested females: Mitch Tucker, Brice Grunert, Nicole Williams, Alexandra Walter, and Sarah Riley-Land. This research was supported by a grant (R01-DC 5670) from the NIH.
LITERATURE CITED
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Arnold SJ, Pfrender ME, Jones AG. The adaptive landscape as a conceptual bridge between micro- and macroevolution. Genetica. 2001;112-113:9–32. [PubMed] [Google Scholar]
- Bentsen CL, Hunt J, Jennions MD, Brooks R. Complex multivariate sexual selection on male acoustic signaling in a wild population of Teleogryllus commodus. Am. Natur. 2006;167:E102–E116. doi: 10.1086/501376. [DOI] [PubMed] [Google Scholar]
- Bisgaard S, Ankenman B. Standard errors for the eigenvalues in second-order response surface models. Technometrics. 1996;38:238–246. [Google Scholar]
- Blows MW. A tale of two matrices: multivariate approaches in evolutionary biology. J. Evol. Biol. 2007;20:1–8. doi: 10.1111/j.1420-9101.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- Blows MW, Brooks R. Measuring nonlinear selection. Am. Natur. 2003;162:815–820. doi: 10.1086/378905. [DOI] [PubMed] [Google Scholar]
- Blows MW, Brooks R, Kraft PG. Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male guppies. Evolution. 2003;57:1622–1630. doi: 10.1111/j.0014-3820.2003.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Brooks R, Hunt J, Blows MW, Smith MJ, Bussiere LF, Jennions MD. Experimental evidence for multivariate stabilizing sexual selection. Evolution. 2005;59:871–880. [PubMed] [Google Scholar]
- Bush SL, Gerhardt HC, Schul J. Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim. Behav. 2002;63:7–14. [Google Scholar]
- Donohoe ML, von Hippel W, Brooks R. Beyond waist-hip ratio: experimental multivariate evidence that average women’s torsos are most attractive. Behavioral Ecology. in press. [Google Scholar]
- Gayou DC. Effects of temperature on the mating call of Hyla versicolor. Copeia. 1984;1984:733–738. [Google Scholar]
- Gerhardt HC. Temperature coupling in the vocal communication system of the gray treefrog Hyla versicolor. Science. 1978;199:992–994. doi: 10.1126/science.199.4332.992. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Reproductive character displacement of female mate choice in the grey treefrog H. chrysoscelis. Anim. Behav. 1994;47:959–969. [Google Scholar]
- Gerhardt HC. Acoustic communication in two groups of closely related treefrogs. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, editors. Advances in the study of behavior. Academic Press; New York: 2001. pp. 99–167. [Google Scholar]
- Gerhardt HC. Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution. 2005a;59:395–408. [PubMed] [Google Scholar]
- Gerhardt HC. Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim. Behav. 2005b;70:39–49. [Google Scholar]
- Gerhardt HC, Schwartz JJ. Auditory tuning and frequency preferences in anurans. In: Ryan MJ, editor. Anuran communication. Smithsonian Institution Press; Washington, D.C.: 2001. pp. 73–85. [Google Scholar]
- Gerhardt HC, Dyson ML, Tanner SD. Dynamic acoustic properties of the advertisement calls of gray treefrogs: patterns of variability and female choice. Behav. Ecol. 1996;7:7–18. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press; Chicago: 2002. [Google Scholar]
- Gerhardt HC, Tanner SD, Corrigan CM, Walton HC. Female preferences based on call duration in the gray treefrog (Hyla versicolor) Behav. Ecol. 2000;11:663–669. [Google Scholar]
- Hine E, Chenoweth SF, Blows MW. Multivariate quantitative genetics and the lek paradox: genetic variance in male sexually selected traits of Drosophila serrata under field conditions. Evolution. 2004;58:2754–2762. doi: 10.1111/j.0014-3820.2004.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Hohenlohe PA, Arnold SJ. MIPoD: A hypothesis-testing framework for microevolutionary inference from patterns of divergence. Am. Nat. 2008;171:366–385. doi: 10.1086/527498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Blows MW, Zajitschek F, Jennions MD, Brooks R. Reconciling strong stabilizing selection with the maintenance of genetic variation in a natural population of black field crickets (Teleogryllus commodus) Genetics. 2007a;177:875–880. doi: 10.1534/genetics.107.077057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Wolf JB, Moore AJ. The biology of multivariate evolution. J. Evol. Biol. 2007b;20:24–27. doi: 10.1111/j.1420-9101.2006.01222.x. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, et al. The strength of phenotypic selection in natural populations. Amer. Natur. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Klump GM, Gerhardt HC. Use of non-arbitrary acoustic criteria in mate choice by female gray treefrogs. Nature. 1987;326:286–288. [Google Scholar]
- Kokko H, Rintamäki PT, Alatalo RV, Höglund J, Karvonen E, Lundberg A. Female choice selects for lifetime lekking performance in black grouse males. Proc. Royal Soc. (London) B. 1999;266:2109–2115. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- LeBas NR, Hockham LR, Ritchie MG. Nonlinear and correlational sexual selection on ‘honest’ female ornamentation. Proc. Royal Soc. (London) B. 2003;270:2159–2165. doi: 10.1098/rspb.2003.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin JW, Parker PG, Nolan VJ, Ketterson ED. Correlational selection leads to genetic integration of body size and an attractive plumage trait in dark-eyed juncos. Evolution. 2005;59:658–671. [PubMed] [Google Scholar]
- Murphy CG, Gerhardt HC. Mate sampling by female barking treefrogs (Hyla gratiosa) Behav. Ecol. 2002;13:472–480. [Google Scholar]
- Phillips PC, Arnold SJ. Visualizing multivariate selection. Evolution. 1989;43:1209–1222. doi: 10.1111/j.1558-5646.1989.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Pough FH, Magnusson WF, Ryan MJ, Wells KD, Taigen TL. Behavioral energetics. In: Feder ME, Burggren WW, editors. Environmental physiology of the amphibians. University of Chicago Press; Chicago: 1992. pp. 395–46. [Google Scholar]
- Rheinlaender J, Gerhardt HC, Yager DD, Capranica RR. Accuracy of phonotaxis by the green treefrog (Hyla cinerea) J. Comp. Physiol. 1979;133:247–255. [Google Scholar]
- Ryan MJ, Perrill SA, Wilczynski W. Auditory tuning and call frequency predict population-based mating preferences in the cricket frog, Acris crepitans. Am. Nat. 1992;139:1370–1383. [Google Scholar]
- Ryan MJ, Rand AS. Sexual selection in female perceptual space: how female túngara frogs perceive and respond to complex population variation in acoustic mating signals. Evolution. 2003;57:2608–2618. doi: 10.1111/j.0014-3820.2003.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Schwartz JJ, Buchanan B, Gerhardt HC. Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol. 2001;49:443–455. [Google Scholar]
- Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution. 2008;62:2435–2440. doi: 10.1111/j.1558-5646.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- Sullivan BK, Hinshaw SH. Female choice and selection on male calling behavior in the gray treefrog Hyla versicolor. Anim. Behav. 1992;44:733–744. [Google Scholar]
- van Homrigh A, Higgie M, McGuigan K, Blows MW. The depletion of genetic variance by sexual selection. Current Biology. 2007;17:528–532. doi: 10.1016/j.cub.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray treefrogs. Science. 1998;280:1928–1930. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- Wells KD. The ecology and behavior of amphibians. University of Chicago Press; Chicago: 2007. [Google Scholar]
- Wells KD, Taigen TL. The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor) Behav. Ecol. Sociobiol. 1986;19:9–18. [Google Scholar]


