SUMMARY
Type I diabetes is a T cell-mediated autoimmune disease, characterized by lymphocytic infiltration of the pancreatic islets. It is currently thought that islet antigen-specificity is not a requirement for islet entry and that diabetogenic T cells can recruit a heterogeneous bystander T cell population. We tested this assumption directly by generating TCR retrogenic mice expressing two different T cell populations. By combining diabetogenic and non-diabetogenic and/or non-autoantigen specific T cells, we demonstrate that bystander T cells cannot accumulate in the pancreatic islets. Autoantigen specific T cells which accumulate in islets, but do not cause diabetes, were also unaffected by the presence of diabetogenic T cells. Additionally, 67% of TCRs cloned from NOD islet-infiltrating CD4+ T cells were able to mediate cell-autonomous islet infiltration and/or diabetes when expressed in retrogenic mice. Therefore islet entry/accumulation appears to be a cell-autonomous and tightly-regulated event and is governed by islet antigen specificity.
INTRODUCTION
Juvenile type 1 diabetes is a T cell-mediated autoimmune disease, characterized by progressive lymphocytic infiltration of the pancreatic islets. This insulitis ultimately leads to the destruction of the insulin producing β-cells, resulting in diabetes. As with most autoimmune diseases, patients present once the disease has progressed for some time. This inevitably makes study of early events in the disease, such as T cell islet entry and accumulation difficult. However, the non-obese diabetic (NOD) mouse model closely mimics the human disease, with approximately 80% of female NOD mice developing diabetes spontaneously between 12–22 weeks of age (Delovitch and Singh, 1997). In addition, the disease is T cell mediated with both CD4+ and CD8+ T cells contributing to βcell destruction (Delovitch and Singh, 1997).
A large number of putative autoantigens have been proposed, including insulin, glutamic acid decarboxylase (GAD) 65, IGRP, IA-2, and IA-2β (Phogrin), on the basis of autoantibody detection and the isolation of autoantigen-specific T cell clones (Lieberman and DiLorenzo, 2003). In addition, a number of T cell clones of unknown antigen-specificity have been identified that proliferate in response to whole islets and can cause disease when adoptively transferred into mice (Haskins, 2005). Of these, TCRs from BDC2.5, BDC6.9 and NY4.1 have been used to generate NOD transgenic mice which exhibit accelerated diabetes (Gonzalez et al., 1997; Pauza et al., 2004; Schmidt et al., 1997).
There is a widely-held, but largely unsubstantiated theory that the insulitis seen in NOD mice and T1D patients is initiated by diabetogenic T cells that then recruit a heterogeneous mixture of cells that includes bystander T cells which are not autoantigen-specific and/or do not contribute to disease. Thus it is perceived that such diseased islets are ‘leaky’ and that only a proportion of accumulating T cells is actually responsible for β cell destruction. However, direct support for this notion is very limited, and in part conflicting. While a heterogeneous population of T cells is recruited to the islets of two week-old NOD mice following adoptive transfer of BDC2.5 or BDC6.9 T cell clones (Peterson et al., 1998), the recruited cells may have been insulitogenic and/or diabetogenic anyway. Two GAD-specific CD4+ T cell clones, generated from human HLA transgenic mice, were shown to induce insulitis in recipient mice but only after treatment with low dose streptozotocin (Wen et al., 1998). It was suggested that this caused damage to the islets, providing an inflammatory environment that attracted T cells to the islet. In a virus-induced model of diabetes, expression of the chemokine CXCL10 alone in the islets was sufficient to cause peri-insulitis but not β cell destruction (Rhode et al., 2005). Conversely, NOD mice treated with a neutralizing anti-CCR5 antibody, which blocks the action of CCL5 expressed by pancreatic islets, did not alter peri-infiltration but it did inhibit β-cell destruction and diabetes (Carvalho-Pinto et al., 2004). Several studies have suggested that Th1 T cells are responsible for aggressive disease, while Th2 T cells infiltrate more slowly and do not induce diabetes (Hill et al., 2003). It was inferred that the inflammation generated by Th1 cells might attract T cells that would not normally accumulate in the islets. Thus it is still not clear whether the ability of T cells to home to and/or be retained in the islets is cell autonomous, due to their antigen specificity and/or expression of chemokine receptors, or whether this is induced directly or indirectly by autoantigen-specific diabetogenic T cells (Sercarz, 2000).
Our laboratory has previously used 2A-linked peptide-based, multi-cistronic retroviral vectors containing both chains of the T cell receptor to efficiently reconstitute NOD/SCID mice with T cells bearing a defined receptor (referred to as “retrogenic” mice; see Figure 1A) (Arnold al., 2004; Holst et al., 2006b; Holst et al., 2006a; Burton et al., 2008). We have used this system to investigate the expression, infiltration and diabetogenicity of a variety of T cell populations. Our findings suggested that although some autoantigen-specific T cells can enter and accumulate in the islet, they do not appear to cause destruction. Also, some T cells, which are antigen responsive in vitro do not infiltrate in vivo (Arnold et al., 2004; Burton et al., 2008). This inferred that the ability to enter and accumulate in the islets (insulitogenic potential) and the ability to destroy the β-cells and cause diabetes (diabetogenic potential) may be separate. In this study, we used this system to generate double retrogenic mice, containing two distinct T cell populations, to ask if diabetogenic T cells can indeed mediate the recruitment of bystander T cells, or whether T cell islet entry and accumulation is an antigen-specific, cell autonomous event.
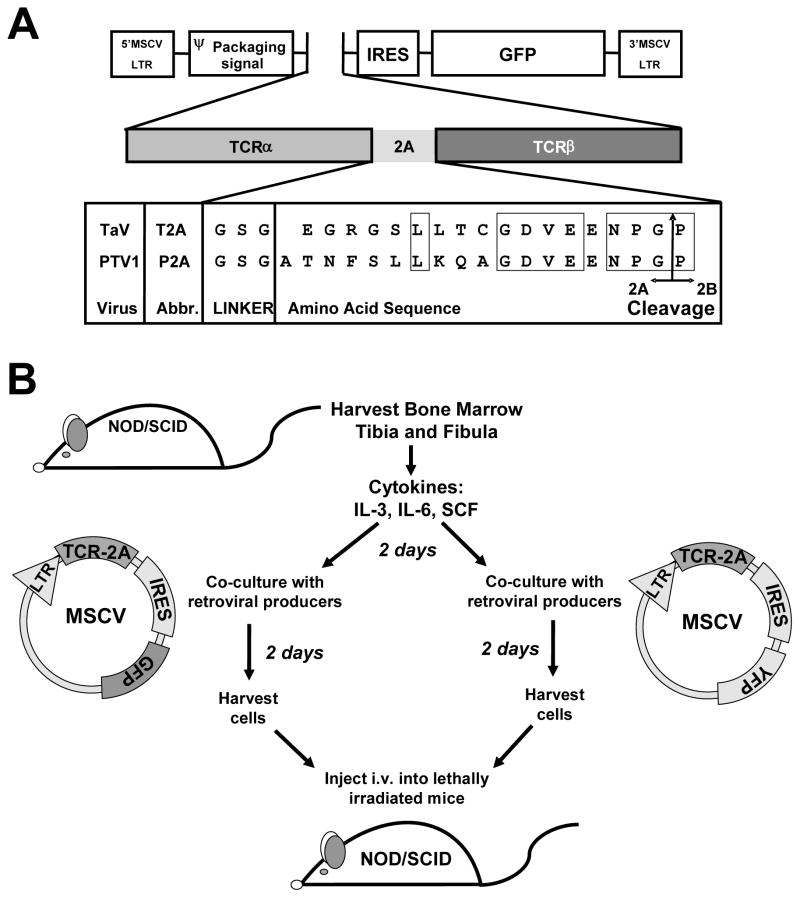
Figure 1. Retroviral-Mediated Stem-Cell Gene Transfer.
(A) Schematic representation of the TCRα-2A-TCRβ constructs, including the amino acid sequence of the 2A regions of Thosea asigna virus (T2A) and porcine teschovirus-1 (P2A), GSG linker, and insertion point in the MSCV-IRES-GFP vector. Conserved residues are boxed. The arrow indicates the ‘cleavage’ point between the 2A and 2B peptides. (B) Schematic representation of protocol to produce dual TCR retrogenic mice in NOD/SCID recipients. Retroviral-mediated stem cell gene transfer was performed with MSCV-IRES-GFP and MSCV-IRES-YFP constructs into separate populations of NOD/SCID bone marrow. These were then combined and injected, to produce mice with two different populations of T cells.
RESULTS
Presence of diabetogenic CD4+ T cells does not facilitate islet accumulation of bystander CD4+ T cells
We have previously used our 2A-linked retroviral vectors to generate retrogenic mice (Figure 1A) expressing a variety of T cell receptors, specific for various antigens, and with varying abilities to infiltrate the pancreatic islets and cause diabetes (Arnold et al., 2004 ; Burton et al., 2008). Here we created double retrogenic mice by combining two groups of bone marrow, each expressing a different TCR, and then transplanting them into sublethally-irradiated NOD/SCID mice (Figure 1B) (Holst et al., 2006b; Holst et al., 2006a). By incorporating either GFP or YFP in the vectors, we were able to distinguish the two T cell populations after engraftment even if they used the same Vβ-chain.
Initially, we investigated whether a bystander T cell, specific for an antigen not present in the islet, could gain entry to the islet and accumulate in the presence of a diabetogenic T cell (Figure 2 and Figure S1, S2). To do this, we used NY4.1 (4.1) and BDC2.5, two diabetogenic CD4+ T cells of unknown antigenic specificity, with PA21.14H4 (14H4) and PA21.5F2 (5F2), two hen egg lysozyme (HEL)-specific CD4+ T cells, as bystander T cells (Table S1). When 4.1 T cells were combined with either 14H4 or 5F2 T cells, both populations are comparably expressed in the spleen (Figure S1). When BDC2.5 was combined with 14H4, however, the BDC2.5 T cells were expressed in much lower numbers than the 14H4, despite both vectors being comparably expressed in the Lineage−Sca-1+c-Kit+ stem cells of the bone marrow (Figure S2). In mice expressing 4.1 with either 14H4 or 5F2, the pattern in the inguinal lymph nodes mirrored that seen in the spleen (Figure S1). However, there appeared to be a much larger percentage of diabetogenic 4.1 T cells in the pancreatic lymph nodes, compared to the bystander cells (Figure S1). In the pancreatic islets, little or no HEL-responsive 5F2 or 14H4 T cells were observed, regardless of the diabetogenic T cell used (Figures S1 and S2), which was comparable to 5F2 or 14H4 T cells expressed alone (Figure S1).
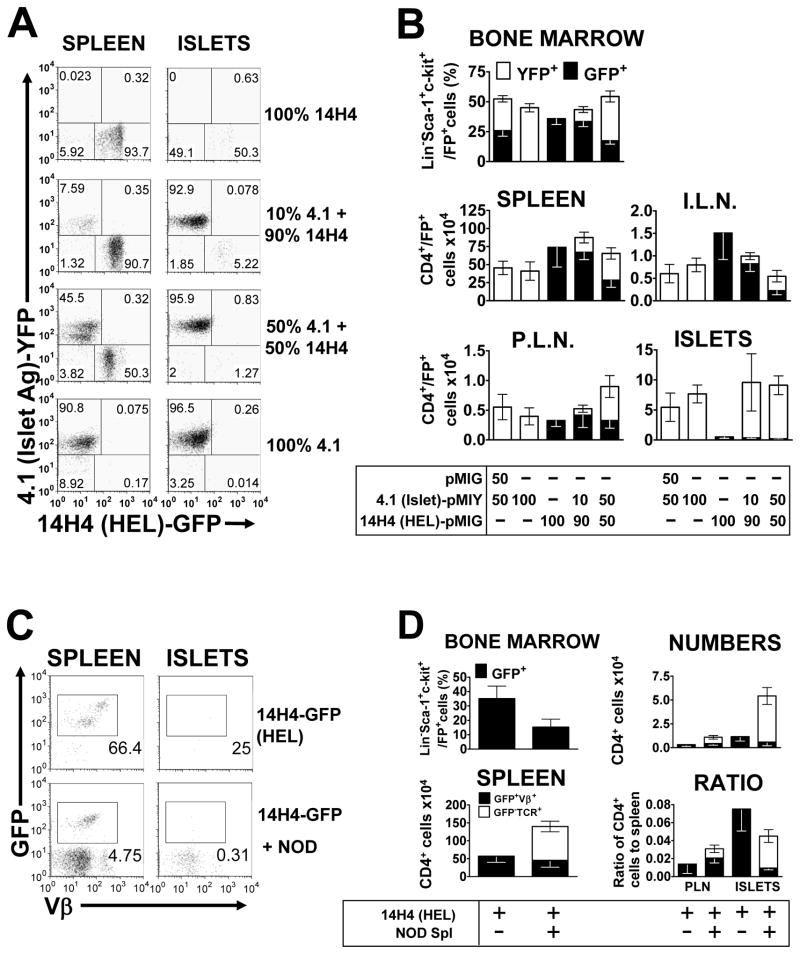
Figure 2. HEL-specific “Bystander” CD4+ T cells do not accumulate in the pancreas.
Double retrogenic mice, with a diabetogenic T cell labeled with YFP and a HEL-specific “bystander” T cell labeled with GFP. Mice were analyzed 4.5–6.5 weeks post transfer, with the bone marrow, spleen, inguinal lymph nodes, pancreatic lymph nodes and pancreatic islets being extracted, processed, cells counted and subjected to flow cytometric analysis. (A) Representative flow cytometric dot plots from B, gated on live and CD4+ T cells. (B) Mice received bone marrow transduced with either 4.1 or 14H4, or both, at a 50:50 ratio, or a 10:90 ratio. One group received 4.1-YFP bone marrow and bone marrow transduced with empty pMIG vector as a control. Top panel; mean (±SEM) percentage of stem cells for each T cell in the bone marrow, as measured by percentage GFP/YFP expression on Lineage−Sca-1+c-kit+ cells (n=10). Middle and bottom panels, mean (±SEM) numbers of each T cell in the spleen (n=9–10), inguinal lymph nodes (I.L.N, n=7–9), pancreatic lymph nodes (P.L.N., n=6–9) and pancreatic islets (ISLETS, n=10), respectively, as calculated from cell counts and the percentage CD4+FP+ live cells. (C) Representative flow cytometric dot plots from D, showing analysis of T cells from 14H4 HEL-specific mice, injected with 10×106 whole splenocytes from 13 week-old NOD mice, two weeks after bone marrow engraftment, and analyzed 4.5–6.5 weeks post-transplant. Plots are gated on live cells and CD4+ cells. (D) Left panels; percentage of stem cells for each T cell in the bone marrow (n=7–10), as measured by percentage GFP expression on Lineage−Sca-1+c-kit+ cells and total numbers of T cells in the spleen. Right panels show the numbers and ratio to the numbers in the spleen for the pancreatic islets (ISLETS) and pancreatic lymph nodes (P.L.N.), respectively (n=5–9). All figures show mean (±SEM) and numbers were calculated from cell counts and the percentage CD4+GFP+Vβ+ live cells for the retrogenic T cells and the percentage CD4+GFP−TCR+ live cells for the NOD T cells.
Given that the double retrogenic mice received twice as much bone marrow as the single retrogenic mice, we also wanted to rule out the possibility that this might affect reconstitution. To address this issue, we combined 4.1-bone marrow with an equal amount of bone marrow which had been transduced with an empty vector to confirm that transplanting twice as much total bone marrow to generate double, compared to the single, retrogenic mice did not affect reconstitution. As can be seen, the levels of 4.1 T cells in all organs were identical between the two groups suggesting there was no adverse consequence of only transplanting half as much bone marrow on T cell development and islet infiltration (Figure 2B).
Next, we investigated whether increasing the number of bystander T cells would allow them to enter. To address this issue, we skewed the ratio of diabetogenic and bystander TCR-transduced bone marrow transplanted to increase the splenic 14H4 bystander population and determine if this would increase infiltration of the bystander T cells or effect the infiltration of the diabetogenic 4.1 T cells (Figures 2A and 2B). Transplanting a 10:90 (4.1:14H4) mix of the two bone marrow populations did indeed result in a marked reduction in the percentage of YFP+ (4.1) and an increase in percentage of GFP+ (14H4) Lineage−Sca-1+c-Kit+ hematopoietic stem cells (HSCs) in the bone marrow, compared to the mice receiving a 50:50 ratio of the two bone marrows (Figure 2B). A similar skewing is observed in the spleen and inguinal lymph nodes, with reduced numbers of 4.1 T cells, compared to the single retrogenic control and the 50:50 mix. 14H4 T cell numbers in the spleen were similar to the single retrogenic control and larger than the 50:50 mix (Figure 2B). Although, the numbers of 14H4 cells in the distal inguinal lymph nodes were reduced compared to a single retrogenic control, the ratio of the two TCRs remained similar to the spleen. In the pancreatic lymph nodes an increase in 14H4 T cells was not seen but there was a marked reduction in the numbers of 4.1 T cells such that the 14H4:4.1 ratio was similar in all lymph nodes examined. This skewing towards 14H4 and away from 4.1 did not have any effect on the populations accumulating in the pancreatic islets. Similar numbers of islet-antigen responsive 4.1 T cells were recovered from the islets of all four groups receiving 4.1-transduced bone marrow and no significant accumulation of HEL-responsive 14H4 T cells was observed in any group, regardless of how many were present in the spleen and lymph nodes (Figures 2A and 2B). Thus, these data suggest that islet entry and/or accumulation is independent of the ratio of diabetogenic to bystander CD4+ T cells and that the former may not be able to induce the islet entry and/or retention of the latter.
A heterogeneous NOD diabetogenic T cell population cannot facilitate bystander CD4+ T cell islet accumulation
We then considered the possibility that our diabetogenic T cells, BDC2.5 and 4.1, were unusual in being unable to mediate bystander accumulation or that this is most efficiently facilitated in the presence of a ‘natural’ cohort of diabetogenic T cells such as those found in NOD mice. To test this, we generated retrogenic mice using the HEL-specific PA21.14H4 TCR, which cannot accumulate in the islet, and adoptively transferred 107 whole NOD splenocytes from 13 week-old mice, two weeks after bone marrow engraftment. While the percentage of GFP+ HSCs was reduced in the bone marrow of the mice with the addition of NOD T cells, the numbers of 14H4 cells in the spleen were comparable to what we see in all other experiments and these did not significantly differ in the presence of NOD T cells. Although, wild type NOD splenocytes were able to infiltrate the islets, the presence of the NOD T cells did not increase the numbers of 14H4 cells in the pancreatic lymph nodes or the pancreatic islets, but instead resulted in a slight decrease (Figure 2C and 2D).
Ectopic antigen expression in the islet enables bystander CD4+ T cell islet entry
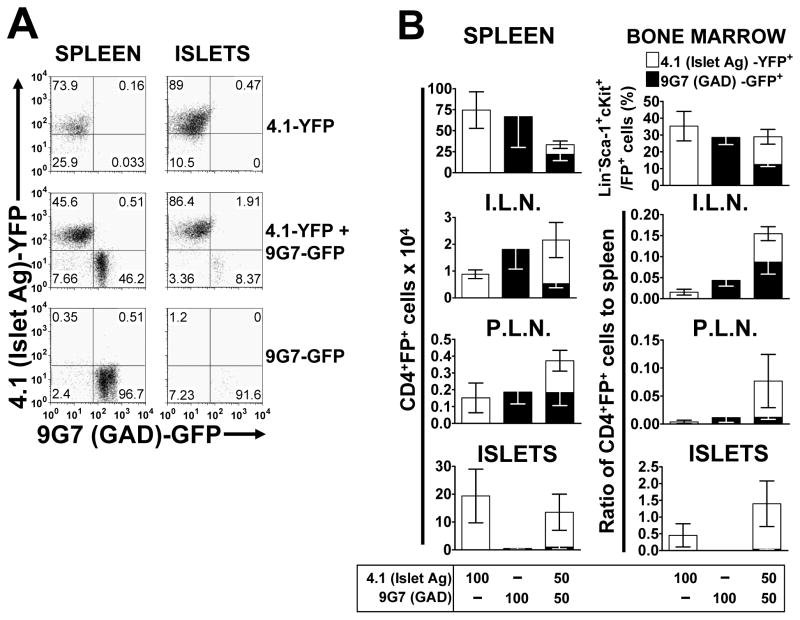
If indeed diabetogenic T cells cannot mediate T cell islet entry and/or retention, then this should also apply to autoantigen-specific T cells that do not accumulate in the islets on their own. To verify this, we determined whether diabetogenic 4.1 T cells could mediate islet accumulation of CD4+ T cells expressing the GAD-specific TCR, PA19.9G7, that does not cause insulitis when expressed in NOD/SCID retrogenic mice (Burton et al., 2008). The percentage of YFP+ (4.1) and GFP+ (9G7) Lineage−Sca-1+c-Kit+ HSCs in the bone marrow was greatly reduced when both populations were transferred together, which led to reduced T cell numbers in the spleen (Figure 3B). However both T cell populations were expressed equally and at the same level as the single retrogenic mice in the PLN (Figure 3B). Importantly, the accumulation of 9G7 T cells in the islets was minimal in both the single and double 4.1+9G7 TCR retrogenic mice (Figures 3A and 3B).
Figure 3. An autoantigen-specific CD4+ T cell which does not accumulate in the islet, is increased, but not to a significant extent by the presence of a diabetogenic CD4+ T cell.
Retrogenic mice, with diabetogenic 4.1 T cells labeled with YFP and PA17.9G7 GAD-specific T cells labeled with GFP, were analyzed 5.5 weeks post-transplant. (A) Representative flow cytometric dot plots from B, showing 4.1 (YFP) and 9G7 (GFP) T cells in the spleen and pancreatic islets. Plots are gated on live cells and CD4+ cells. (B) Top panels; percentage of stem cells for each T cell in the bone marrow, as measured by percentage GFP/YFP expression on Lineage−Sca-1+c-kit+ cells and total numbers of T cells in the spleen (n=3–5). Next three panels, show numbers and ratio to the numbers in the spleen for the pancreatic islets (ISLETS, n=3–5), inguinal lymph nodes (I.L.N., n=3–5) and pancreatic lymph nodes (P.L.N., n=3–5), respectively. All Figures show mean (±SEM) and numbers were calculated from cell counts and the percentage CD4+FP+ live cells.
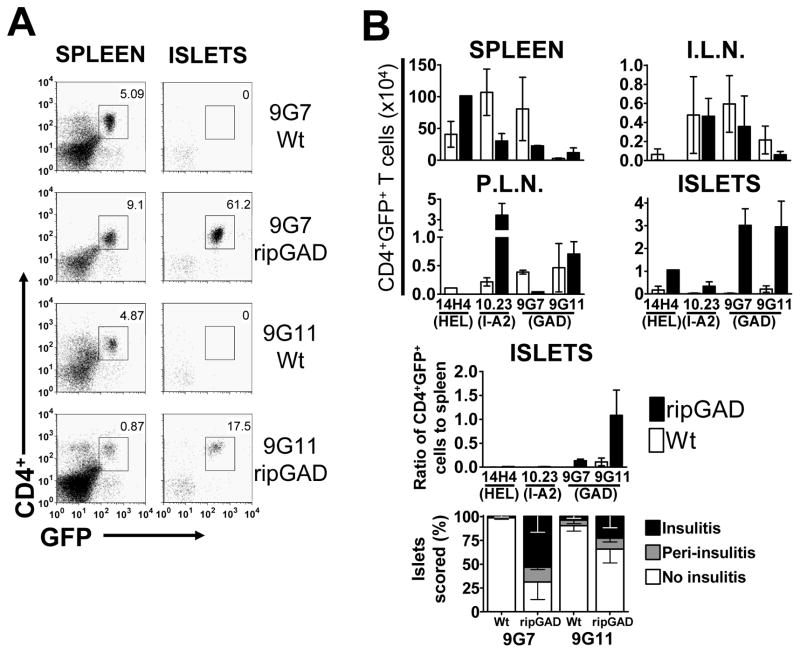
We then used this system to verify that there was no inherent incapacity of these GAD-reactive CD4+ T cells to enter and accumulate in the islets, by assessing whether cognate antigen expression in the islets would result in T cell accumulation. Mouse islets express low levels of GAD65 and GAD67 isoforms compared to rat and human pancreatic islets (Kim et al., 1993). We took advantage of the low level of GAD expression in murine islets, and utilized rip.GAD transgenic NOD/SCID mice, which strongly express GAD in the pancreatic islets (Bridgett et al., 1998) and their littermate controls as recipients for the generation of retrogenic mice. These mice were reconstituted with NOD/SCID bone marrow transduced with PA17.9G7, the GAD-specific CD4+ T cell which reacts with GAD284–300 or PA19.9G11 another GAD-specific CD4+ T cell, which reacts with GAD206–220 (Arnold et al., 2004; Arnold et al., 2002). PA21.14H4, the HEL-responsive T cell, and 10.23, an I-A2-specific T cell which does not infiltrate the islets to any significant degree, were included as controls. Both GAD-specific T cells show strong reactivity to GAD protein, but neither infiltrates the pancreatic islets, or causes diabetes (Arnold et al., 2004). The data clearly show that both 9G7 and 9G11 T cells enter and accumulate in the islets of the rip.GAD transgenic mice without further intervention, but do not do so in the non-transgenic littermate controls (Figure 4). This suggests that antigen expression in the islets is required for CD4+ T cell islet entry and/or retention. Furthermore, in situ activation of auto-antigen specific CD4+ T cells may also be important as HEL-specific 14H4 T cells do not accumulate in the islets following in vivo administration of HEL peptide (data not shown).
Figure 4. Expression of an antigen under the control of a rat-insulin promoter (RIP) allows an antigen-specific CD4+ T cell which does not normally accumulate in the islet, to accumulate.
NOD.Cg-Prkdcscid Tg(Ins2-GAD2)2Lt/LtJ transgenic mice and NOD/SCID littermate controls were used as recipients, to generate single retrogenic mice, with either PA21.14H4 “bystander” T cells, 10.23 I-A2-specific T cells, PA17.9G7 GAD-specific T cells or PA19.9G11 GAD-specific T cells. Mice were analyzed at 4.5–6.5 weeks post-transplant. (A) Representative flow cytometric dot plots from B, showing T cells in the spleen and pancreatic islets. Plots are gated on live cells. (B) Top four panels; mean (±SEM) numbers of each T cell in the spleen, inguinal lymph nodes (I.L.N.) pancreatic lymph nodes (P.L.N.), and pancreatic islets (ISLETS), respectively, as calculated from cell counts and the percentage CD4+GFP+ live cells (all n=1–5). Third panel, mean (±SEM) ratio of CD4+ T cells in the islets, relative to the numbers in the spleen. Bottom panel: Percentage insulitis, at 6.5 weeks post-transplant (n=3–6).
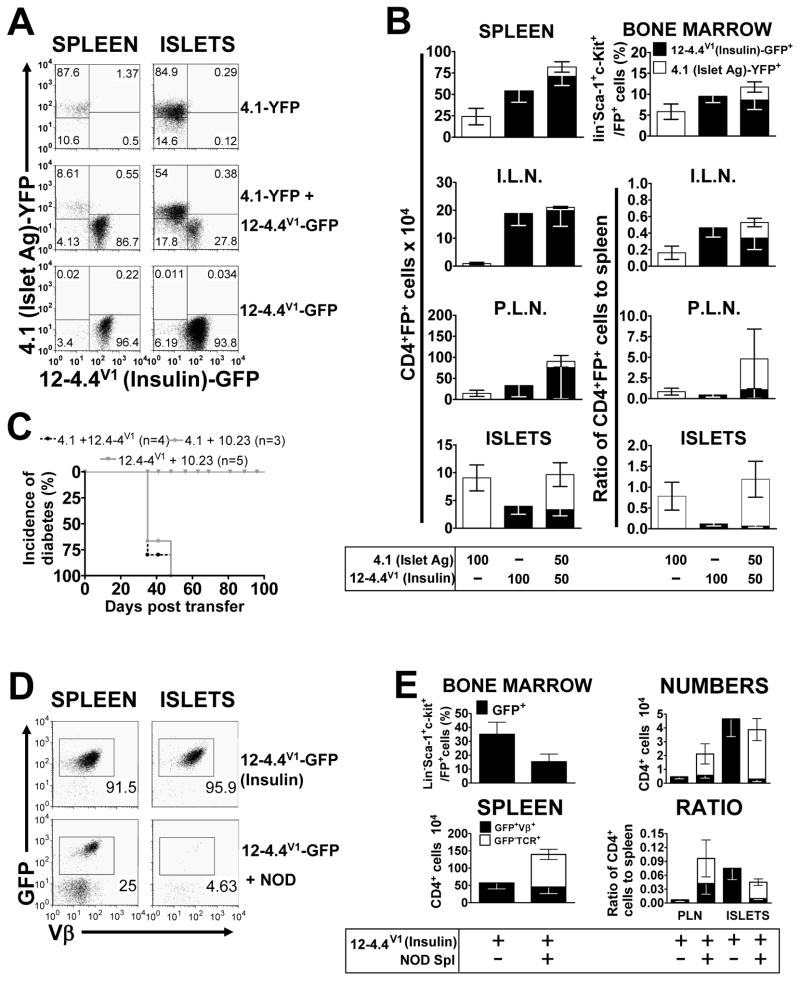
Diabetogenic CD4+ T cells have a minimal effect on the islet accumulation of insulitogenic, auto-antigen specific CD4+ T cells
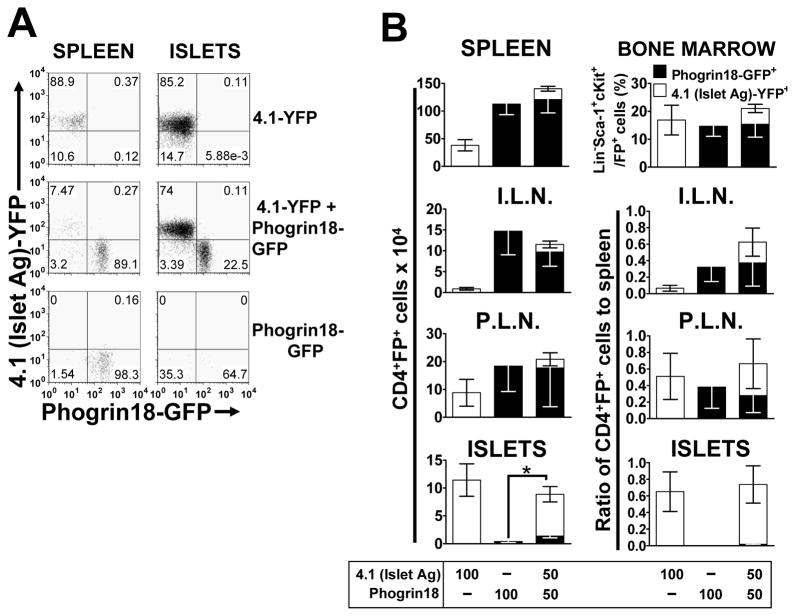
It is possible that for diabetogenic T cells to mediate islet accumulation of other T cell populations the latter must have some inherent cell-autonomous capacity for islet entry, in which case the diabetogenic T cells may serve to boost/augment this process. To test this we used Phogrin18, a phogrin-specific CD4+ T cell that enters and accumulates in islets poorly but reproducibly on its own, and 12-4.4V1, an insulin-specific T cell that accumulates in the islets freely, is insulitogenic, but does not cause any diabetes (Burton et al., 2008).
The percentages of GFP+ (Phogrin18) Lineage−Sca-1+c-Kit+ HSCs in the bone marrow of the single and double retrogenic mice were equal (Figure 5B). However, there was a reduced percentage of YFP+ (4.1) HSCs in the double versus the single retrogenic mice. Development of Phogrin18 T cells was clearly superior as there were significantly more of these T cells than the 4.1 T cells in the spleen, ILN and PLN (Figure 5B). These characteristics were duplicated in the 12-4.4V1+4.1 double TCR retrogenic mice (Figure 6B). If the lymph node data are expressed as a ratio to the number of T cells in the spleen, then the diabetogenic (4.1) and non-diabetogenic (Phogrin 18 or 12-4.4V1) T cells are present in similar proportions, suggesting that none of these T cells have an enhanced capacity to traffic to a particular lymph node (Figure 5B, 6B). While the presence of the 4.1 diabetogenic T cells did increase the number of Phogrin18 T cells accumulating in the islets [from 0.397±0.186 to 1.380±0.333 in the double retrogenic mice (mean x 104 ± SEM x 104); t-test, p=0.018, t=2.526, 27 d.f.] (Figure 5B), this is still very small compared to the number of islet-antigen specific 4.1 T cells in the islets. In contrast, a significant number of 12-4.4V1 T cells accumulate in the islets, albeit to a lesser extent than 4.1 (Figure 6B). However, they were completely unaffected by the presence of 4.1 diabetogenic T cells. We also tested whether a pathogenic CD8+ T cell can increase recruitment of CD4+ T cells. Islet antigen specific, diabetogenic CD8+ AI4 T cells (DiLorenzo et al., 1998; Chaparro et al., 2008) did not increase infiltration of 10.23 CD4+ T cells, which suggests that the presence of pathogenic CD8+ T cells does not affect insulitogenic potential of CD4+ T cells (Figure S3). Interestingly, it appeared that while AI4 CD8+ T cells did not affect CD4+ T cell accumulation, insulitogenic Phogrin18 CD4+ T cells did affect AI4 CD8+ T cell islet accumulation.
Figure 5. An autoantigen-specific CD4+ T cell which accumulates in the islet poorly, is increased, but not to a significant extent by the presence of a diabetogenic CD4+ T cell.
Retrogenic mice, with diabetogenic 4.1 T cells labeled with YFP and Phogrin18 phogrin-specific T cells labeled with GFP, were analyzed 4.5–6.5 weeks post-transplant. (A) Representative flow cytometric dot plots from B, showing 4.1 (YFP) and Phogrin18 (GFP) T cells in the spleen and pancreatic islets. Plots are gated on live cells and CD4+ cells. (B) Top panels; percentage of stem cells for each T cell in the bone marrow, as measured by percentage GFP/YFP expression on Lineage−Sca-1+c-kit+ cells and total numbers of T cells in the spleen (n=12–15). Next three panels, show numbers and ratio to the numbers in the spleen for the pancreatic islets (ISLETS, n=12–15), inguinal lymph nodes (I.L.N., n=10–15) and pancreatic lymph nodes (P.L.N., n=10–14), respectively. All Figures show mean (±SEM) and numbers were calculated from cell counts and the percentage CD4+FP+ live cells. * p=0.016, t=2.526, 27 d.f., t-test.
Figure 6. An autoantigen-specific CD4+ T cell which accumulates in the islet but does not cause destruction, does not accumulate in higher numbers in the presence of a diabetogenic CD4+ T cell or T cells from whole NOD splenocytes.
Retrogenic mice, with diabetogenic 4.1 T cells labeled with YFP and 12-4.4V1 insulin-specific T cells labeled with GFP, were analyzed 4.5–6.5 weeks post-transplant. (A) Representative flow cytometric dot plots from B, showing 4.1 (YFP) and 12-4.4V1 (GFP) T cells in the spleen and pancreatic islets. Plots are gated on live cells and CD4+ cells. (B) Top panels; percentage of stem cells for each T cell in the bone marrow (n=7–8), as measured by percentage GFP/YFP expression on Lineage−Sca-1+c-kit+ cells and total numbers of T cells in the spleen. Next three panels, show numbers and ratio to the numbers in the spleen for the pancreatic islets (ISLETS), inguinal lymph nodes (I.L.N.) and pancreatic lymph nodes (P.L.N.), respectively (n=6–9). All Figures show mean (±SEM) and numbers were calculated from cell counts and the percentage CD4+FP+ live cells. (C) Diabetes incidence. (D) Representative flow cytometric dot plots from E, showing analysis of T cells from 12-4.4V1 Insulin-specific mice, injected with 10×106 whole splenocytes from 13 week-old NOD mice, two weeks after bone marrow engraftment, and analyzed 4.5–6.5 weeks post-transplant. Plots are gated on live cells and CD4+ cells. (E) Left panels; percentage of stem cells for each T cell in the bone marrow (n=7–10), as measured by percentage GFP expression on Lineage−Sca-1+c-kit+ cells and total numbers of T cells in the spleen. Right panels show the numbers and ratio to the numbers in the spleen for the pancreatic islets (ISLETS) and pancreatic lymph nodes (P.L.N.), respectively (n=5–9). All figures show mean (±SEM) and numbers were calculated from cell counts and the percentage CD4+GFP+Vβ+ live cells for the retrogenic T cells and the percentage CD4+GFP−TCR+ live cells for the NOD T cells.
We also assessed whether the presence of additional islet-antigen specific T cell population in double retrogenic mice would increase the rate of diabetes incidence compared to 4.1 TCR single retrogenic mice due to alteration of the inflammatory milieu. The addition of insulitogenic 12-4.4V1 T cells or non-insulitogenic 10.23 T cells had no effect on the onset of diabetes induced by 4.1 T cells (Figure 6C).
A heterogeneous NOD diabetogenic T cell population decreases 12-4.4V1 T cell islet accumulation
Finally, we investigated whether a mixed population of whole NOD splenocytes, would allow greater accumulation of the 12-4.4V1 T cells that can accumulate in the islets on their own. Two weeks after bone marrow transplant, we adoptively transferred 107 whole NOD splenocytes into some of the 12-4.4V1 retrogenic mice, to act as a diabetogenic population, and analyzed the mice at 4.5–6.5 weeks post-bone marrow reconstitution. The numbers of both T cell populations (retrogenic and NOD wild type) were comparable to those seen in previous experiments (Figures 2D and 6B). The presence of NOD T cells did not seem to have a major effect on the number of retrogenic T cells in the spleen and PLN (Figure 6E). Surprisingly, the presence of NOD cells actually reduced the number of retrogenic 12-4.4V1 T cells accumulating in the islets, suggesting that regulatory T cells in the NOD population may be influencing the infiltration of these auto-antigen specific T cells or that the NOD T cells are competing for nutrients, space, etc. and thus block T cell islet entry (Figure 6E). Either way our data clearly show that diabetogenic T cells cannot facilitate/enhance the entry/retention of non-diabetogenic T cells.
Insulitogenic potential is restricted to TCRs isolated from islet-infiltrating CD4+ T cells
Our data suggest that the ability of T cells to accumulate in the islets is cell intrinsic and that T cells which are not specific for an antigen expressed in the islets, and processed and presented in the PLN, fail to accumulate. It would therefore follow that the majority of T cells in the islets have accumulated there based on these criteria. One caveat of our approach is that we are assessing the insulitogenic potential of specific T cell populations when present at high precursor frequency, in contrast to what would be found in unmanipulated NOD mice. To mitigate this issue, we reasoned that if T cell accumulation/entry is cell autonomous then most TCRs isolated from CD4+ T cells purified from islets and expressed in naïve T cells should be able to mediate islet infiltration, while none (or very few) of the TCRs isolated from splenic CD4+ T cells should be able to do so.
To test this hypothesis, we cloned paired TCRα and TCRβ chains from multiple single CD4+ T cells, isolated from either the islets or spleen of a 13 week-old NOD mouse. To facilitate cloning, we performed a single cell sort based on Vα2 and Vβ6 or Vβ10 expression, and used corresponding primers to clone Vβ and Vα chains. The Vα2 gene family and the Vβ6 and Vβ10 gene segments were chosen because of their relatively high percentage expression in the NOD pancreas and spleen, the high mean fluorescence intensity of the antibody staining and the presence of unique restriction sites in the coding segments that could be used to facilitate cloning (Figure S4). Since our analysis was limited to four Vα2 and two Vβ chains, the TCRs cloned are only partially representative of the infiltrating CD4+ T cell population. The percentage of Vα2+/Vβ6+ and Vα2+/Vβ10+ T cells in the islets and spleen appeared comparable (data not shown). We successfully cloned a total of 17 unique in-frame sequences (12 islet and 5 splenic) (Figure S4, Tables S2 and S3). Two islet-derived clones: GL24.6I1 and GL25.10I1 were isolated from multiple cells (4 and 14 times, respectively), which likely reflects the in situ proliferation of these clones. We did not obtain any repeated sequences in the spleen sample, nor were any common TCR sequences found between the spleen and islet samples. Given this relatively small sample size, compared with the number of infiltrating T cells, it is not possible to draw definitive conclusions from TCR Jα, Jβ and CDR3 usage (Table S3). However, usage did not appear obviously skewed.
We then generated retrogenic NOD/SCID mice expressing the 17 unique TCRs. Splenic and islet-infiltrating T cells were analyzed 8–10 weeks after bone marrow reconstitution. As expected, the capacity of T cell reconstitution varied amongst the TCRs, which is likely due to a variety of factors such as the efficiency of TCR expression and TCRαβ pairing, the influence of positive and negative selection, and peripheral tolerance. However, it is noteworthy that T cell reconstitution efficiency is clearly an inherent characteristic of each TCR, rather than due to the technique and/or random retroviral integration, as the error was relatively low within each group.
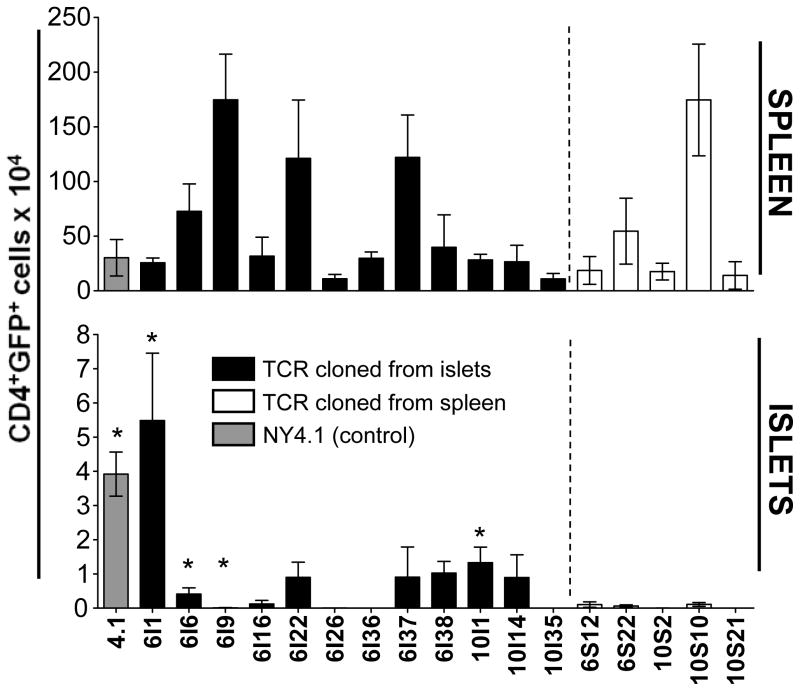
As anticipated, none of the splenic T cell-derived TCRs mediated T cell islet accumulation to any significant degree by 8–10 weeks, consistent with the aforementioned hypothesis (Figure 7). In contrast, eight of the twelve (67%) islet-derived TCRs mediated some degree of T cell accumulation in the islets. Four of these (33%) caused varying degrees of diabetes within the 8–10 week window of analysis [GL24.6I1, GL24.6I6, GL25.6I9, GL25.10I1] (Figure 7; Table S3). 10I1 mice were hyperdiabetogenic as most developed diabetes by 5–6 weeks post-reconstitution, and thus were analyzed when they became diabetic rather than at 8 weeks. Curiously, another TCR, GL25.6I9, caused diabetes at 10 weeks in some mice, but produced no significant accumulation in the remaining mice. The remaining four islet-derived T cells, GL28.6I16, GL28.6I26, GL25.6I36 and GL23.10I35, showed minimal/no accumulation at 8–10 weeks post-engraftment. However it is possible that this may be too early for some TCRs/T cells, as several previously characterized insulitogenic/diabetogenic TCRs mediate minimal insulitis (eg. Phogrin18) and minimal diabetes incidence (eg. 12-4.1 and BDC6.9) at 10 weeks post-transfer (Burton et al., 2008). It is also possible that some of the cellular infiltrate may have resolved by 10 weeks. If one considers the duplicated TCRs that were independently isolated from single islet-infiltrating T cells, these data suggest that an even higher percentage of these T cells (86% – 24 of 28 islet-isolated clones) is able to cell autonomously mediate islet accumulation and/or induce diabetes, while none of the spleen-derived TCRs are able to do so.
Figure 7. TCRs isolated from NOD islets-infiltrating CD4+ T cells can mediate T cell accumulation in the islets of retrogenic mice.
Vα2+Vβ6+ and Vα2+Vβ10+ CD4+ T cells were single-cell sorted from the spleen and islets of a 13 week-old female NOD mouse. The TCR chains were then amplified using a nested RT-PCR, sequenced and cloned into a 2A-linked retroviral vector. Retrogenic mice generated using these vectors, were analyzed 8-10 weeks after engraftment (n=2–11). Shown are numbers of CD4+GFP+ T cells in the spleen (top panel) and pancreatic islets (bottom panel). Plots are gated on live cells. Islet derived clones are indicated by the black bars and the “I” in their name, splenic clones by open bars and the “S” in their name. NY4.1 is included as a control and is indicated by the gray bar. The * indicates that mice in this group were diabetic when analyzed at 8–10 weeks after engraftment. GL25.10I1 T cells induced rapid diabetes, and these mice were analyzed at 5–6 weeks post engraftment.
DISCUSSION
There is a pervasive notion that the majority of infiltrating T cells at the site of auto-immune destruction is not specific for islet antigens and have been recruited via a cell extrinsic mechanism. However, there is little direct support for this with most of the current data being circumstantial (Peterson et al., 1998; Wen et al., 1998; Rhode et al., 2005; Carvalho-Pinto et al., 2004). Our data suggest that this assumption may be incorrect. Despite the presence of significant insulitis mediated by diabetogenic T cells in double TCR retrogenic mice, no additional population was enticed or could be directed to enter and/or be retained in the islets. Importantly, 6 different TCRs were used which display a spectrum of insulitogenic potential, indicating that this is not a deciding factor in whether diabetogenic T cells can mediate islet entry/accumulation of a third party T cell population. Four distinct diabetogenic T cell populations (4.1 and BDC2.5 CD4+ retrogenic T cells, AI-4 CD8+ retrogenic T cells, and heterogeneous NOD T cells) were unable to facilitate third party T cell islet entry and/or retention, reducing the possibility that these observations were due to a limitation in our approach and/or a unique characteristic of certain diabetogenic T cell populations. Finally, we cloned the TCRαβ chain pairs from twelve unique CD4+ T cells isolated by single cell sorting from the islets of a NOD mouse and demonstrated that eight of these (67%) could mediate T cell islet accumulation and/or induce diabetes in mice. Given that two of the cloned TCRs were isolated from eighteen T cells, this may actually represent a higher percentage of in vivo population. A parallel sample of TCRs isolated from five splenic single cell cloned CD4+ T cells of the same mouse failed to mediate T cell islet accumulation to any significant degree. Taken together, our data suggest that inflammation induced by diabetogenic CD4+ T cells alone may not be sufficient to mediate third party/bystander CD4+ T cell islet entry and/or retention and further suggests that islet accumulation is a cell-autonomous event.
Our data also confirm that islet antigen expression is a key factor in determining the ability of a given T cell population to accumulate in the islets, as GAD-specific T cells could only accumulate when GAD was expressed in the islets. This finding correlates well with findings from a previous study, where MHC class I expression was inhibited in the pancreatic β-cells of NOD mice by expression of a RIP-E19 transgene resulting in a reduced accumulation of CD8+ T cells in the islets (Yamanouchi et al., 2003). However, another study found that conditional deletion of MHC class I on β-cells did not affect infiltration but instead reduced the incidence of diabetes and delayed onset (Hamilton-Williams et al., 2003).
A significant benefit of the TCR retrogenic system used here is the fact that one has complete control over the T cell populations present in the mice and thus the ability to track, manipulate and isolate these T cell populations for further analysis. One caveat, however, is that these analyses were not performed at physiological T cell densities in vivo. It could be argued that this may change the ‘rules’ that govern the processes that control T cell islet accumulation, which might be more complex than recapitulated here. Alternatively, it could be argued that the diabetogenic T cell density may in fact be far higher in this setting and thus a ‘sterner test’ of whether islet accumulation is really cell autonomous. Regardless, our data are consistent with the notion that T cell islet accumulation is auto-antigen specific and cell autonomous, and suggest that further analyses of these processes are warranted.
Most of the work that supported bystander recruitment used adoptive transfer studies which may result in an artificially high or robust response. Previous work suggested that adoptive transfer of BDC2.5 T cells into NOD mice induced islet destruction which facilitated the recruitment of a large diverse T cell population, most of which did not appear to be highly activated based on CD25 expression and response to IL-2 (Peterson et al., 1998). It was assumed that these cells were not auto-antigen specific and that the inflammatory environment was responsible for their recruitment and entry into the pancreatic islets. However, this was not directly demonstrated. Another transfer study used BDC2.5 T cells and DO11 T cells, which recognize OVA peptide presented on H-2Ad molecules, injected into NOD/SCID x CB17/SCID F1 mice, which expressed both H-2Ad and H-2Ag7. Injection of mice with OVA peptide prevented diabetes, although it had no effect on infiltration (Suri et al., 2004). As both T cell populations could be detected in the pancreas, it was suggested that the activated DO11 T cells, which do not recognize an islet antigen, could enter the islets when BDC2.5 was present (Suri et al., 2004). However, the timing of the injection was critical and it is possible that soluble OVA peptide could have been presented by APCs in the islets due to damage caused by the presence of the BDC2.5 cells, and/or that the number of diabetogenic T cells was unphysiological. Using transgenic mice that express the OVA peptide at another location might exclude this possibility. Lastly, expression of the chemokine CXCL10 in the β-cells of the pancreas, using a RIP-transgene, led to peri-insulitis and some impairment of β-cell function, but no diabetes (Rhode et al., 2005). The mice were on a C57BL/6 background and do not spontaneously develop diabetes. This would therefore appear to indicate that the expression of molecules involved in inflammatory responses can influence the trafficking of T cells to the islets, regardless of their ability to cause disease. The only known ligand for CXCL10, CXCR3, is upregulated on activated T cells, but only TH1 cells show CXCR3-mediated trafficking in response to inflammation (Xie et al., 2003), which could explain the bias seen in NOD mice. Indeed, neutralization of CXCL10 reduces the incidence of diabetes in NOD mice following cyclophosphamide administration (Morimoto et al., 2004). Also, infection with a virus which expands in the pancreatic lymph nodes abrogates diabetes in both a virus-induced model of diabetes and NOD mice, apparently by attracting T cells from the islets with high CXCL10 concentrations (Christen et al., 2004). It is therefore tempting to speculate that the ability of T cells to enter/accumulate in the islets is due to antigenic activation, leading to expression of CXCR3.
Overall, our data show that the ability of T cells to infiltrate or accumulate in the pancreatic islets is cell-autonomous, antigen-specific and tightly regulated. However, our data also raise the intriguing and potentially important possibility that the majority of the T cells in the pancreatic islets are auto-antigen specific. Indeed, there is evidence that up to forty percent of CD8+ T cell infiltrating the islets of NOD mice are responsive to the islet antigen IGRP (Han et al., 2005) and inhibiting MHC class I expression on β-cells has been shown to impair CD8+ T cell accumulation in the islets (Yamanouchi et al., 2003). When we directly “sampled” the pancreas to determine the cell autonomous insulitogenic potential of islet infiltrating T cells through single cell cloning and re-expression in retrogenic mice, 67% percent of the unique islet-derived TCRs mediated accumulation and/or caused diabetes. However, one should note that only a proportion of the islet-infiltrating CD4+ T cell population (Vα2/Vβ6+; Vα2/Vβ10+) was sampled and thus additional studies will be required to determine if these observations can be extrapolated. Nevertheless, these findings increase the value of direct analysis of islet infiltrating T cells and suggests that the inhibition of islet entry/retention may have a more significant therapeutic benefit than previously appreciated. Furthermore, our data further support the potential therapeutic validity of antigen-specific modalities.
Whether this finding relates in any way to other autoimmune diseases remains to be determined. Indeed, some have suggested that the majority of the T cells in arthritic joints are not autoantigen-specific (Cantagrel et al., 1998). It is possible that the structure, location and chemokine expression of the pancreatic islet in NOD mice makes it uniquely inaccessible to non-autoantigen specific T cells. However, it is possible that the observations made here may apply to other organ-specific autoimmune diseases. Either way, our study raises important questions about the T cell clonality and specificity in autoimmune foci and suggests that additional analysis is warranted.
EXPERIMENTAL PROCEDURES
Mice
NOD/SCID and NOD.Cg-Prkdcscid Tg(Ins2-GAD2)2Lt/LtJ mice were obtained from Jackson Laboratories and were bred in the St Jude Animal Resources Center (Memphis, TN). All animal experiments were preformed in an AAALAC-accredited, SPF facility following national, state and institutional guidelines. Animal protocols were approved by the St. Jude Institutional Animal Care and Use Committee.
TCR reagents and retroviral-mediated stem cell gene transfer
All retroviral TCR constructs used in this paper are listed in Tables S1 and S3 and have been described previously (Arnold et al., 2004 ; Burton et al., 2008), with the exception of AI4 (a gift from T. DiLorenzo, Albert Einstein College of Medicine, and D. Serreze, The Jackson Laboratory) and those cloned from single cells (GL series - see below).
Retroviral transduction of murine bone marrow cells was performed as previously described (Szymczak et al., 2004; Holst et al., 2006a). Briefly, female NOD/SCID bone marrow was cultured for 48 hr in complete DMEM supplemented with 20% FBS, 20 ng/ml IL-3, 50 ng/ml IL-6 and 50 ng/ml stem cell factor (BioSource, Carlsbad, CA). Bone marrow cells were then harvested and co-cultured with irradiated retroviral producer cell lines for an additional 48 hr. The transduced bone marrow cells were then washed-off the retroviral cells and combined, so that mice receiving 50:50 mixes received equal numbers of each type of bone marrow, and that different groups received the same total number of cells, with single retrogenic controls receiving half that number (>4×106 total BM cells). The cells were then washed and resuspended in PBS/2% FBS with 20U/ml heparin, and injected via the tail vein into sublethally irradiated mice (300 rads). Mice were test-bled for TCR reconstitution 4 wk post-transplant, and analyzed 4.5–6.5 wk post-transplant.
Flow cytometric analysis of bone marrow recipient mice
Bone marrow, spleens, inguinal lymph nodes (iln), pancreatic lymph nodes (pln) and pancreatic islets were removed, processed, counted, stained and then analyzed on a LSRII flow cytometer (BD Pharmingen, San Diego, CA). For the bone marrow, cells were stained with anti-Sca-1-biotin, followed by anti-c-kit-APC, anti-CD3e-PE, anti-B220-PE, anti-Gr-1-PE, anti-Mac-1-PE, anti-TER-119-PE and streptavidin-PE-Cy5. Cells were gated on Lineage−Sca-1+c-kit+ (PElo, PECy5hi, APChi) and the percentage of GFP+ and YFP+ stem cells determined from a dot plot. For the lymphocytes from the other organs, cells were stained with anti-CD69-PE, anti-CD25-PE-Cy5 and anti-CD4-AlexaFluor647 or with anti-CD44-PE, anti-CD25-Cy5, anti-CD62L-APC (eBioscience, San Diego, CA) and anti-CD4-APC-Cy7. When staining with Vβ-specific antibodies, cells were stained first with anti-Vβ6-biotin, anti-Vβ12-biotin, or anti-Vβ8.1-biotin, followed by anti-Vβ11-PE, anti-CD25-PE-Cy5, anti-CD4-APC-Cy7 and streptavidin-APC. For staining of T cells from AI-4 Rg mice, anti-CD8-PE was used. Cells were resuspended in DAPI immediately prior to analysis. The unused PE-texas red channel on the LSRII was compensated and used to gate-out auto-fluorescent cells during data analysis. All antibodies were from BD Pharmingen unless stated otherwise.
Recovery of T cells from the pancreatic islets
T cells were isolated from the islets as described previously (Burton, 2008). Briefly, the pancreas was flushed with 3 ml of collagenase type P (Roche diagnostics, Indianapolis, IN), excised, and incubated for 37°C for 15–20 min in 4 ml of collagenase. The pancreata were then disrupted and washed twice with DMEM or RPMI, + 10% FBS. The islets were picked under a dissecting microscope using a P10 pipette, disrupted by the addition of 1 ml of cell dissociation buffer (Gibco, Grand Island, NY) and 15 minute incubation at 37°C and vortexing. After another wash, the cells were resuspended, counted and used.
Cloning of TCRs from T cells isolated by single cell sorting
Cells were prepared from the islets and spleen of a 13 week-old female NOD/Ltj mouse and stained with anti-CD4-PE-Cy7, anti-Vα2-FITC, anti-Vβ10-PE and anti-Vβ6-biotin, then streptavidin-APC (all antibodies BD Pharmingen). Vα2+Vβ6+CD4+ and Vα2+Vβ10+CD4+ T cells were then single-cell sorted into the wells of 96-well PCR plates using the Cyclone attachment on a Mo-Flo cell sorter (Beckman-Coulter, Fullerton, CA). Plates were stored frozen at −80°C until needed. Two-round PCR method modified from (Kedzierska et al., 2004) was used to amplify the Vα and Vβ chains (see Supplementary Methods for more details).
Diabetes monitoring
Diabetes onset was monitored by urine analysis using Clinistix® (Bayer Corporation, Elkhart, IN) and confirmed by a blood glucose measurement of over 400 mg/dl, or over 200 mg/dl for two consecutive measurements, using a One Touch Ultra glucometer (LifeScan, Milpitas, CA).
Statistical analysis
The differences in numbers of T cells between groups was calculated by one-tailed t-test, using the statistical analysis function of GraphPad Prism 4.03 (GraphPad Software Inc, San Diego, CA).
Supplementary Material
Acknowledgments
The authors would like to thank T. DiLorenzo and D. Serreze for reagents, K. Forbes and T. Moore for mouse colony management, the Vignali lab for assistance with harvesting bone marrow and helpful discussions, R. Cross and Y. He for single cell sorting and J. Houston in the St Jude Flow Cytometry Facility for help running samples on the LSRII. D.A.A.V. is supported by funds from the Juvenile Diabetes Research Foundation International (1-2004-141 [The Robert and Janice Compton Research Grant, In Honor of Elizabeth S. Compton] and 1-2006-847), the NIH (AI072239), the St Jude Cancer Center Support CORE grant (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC). P.S. is supported by the Canadian Institutes of Health Research and is a Scientist of the Alberta Heritage Foundation for Medical Research.
Abbreviations
- GAD
(Glutamic acid decarboxylase)
- OVA
(Ovalbumin)
- HEL
(Hen egg lysozyme)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold PY, Burton AR, Vignali DA. Diabetes incidence is unaltered in glutamate decarboxylase 65-specific TCR retrogenic nonobese diabetic mice: generation by retroviral-mediated stem cell gene transfer. J Immunol. 2004;173:3103–3111. doi: 10.4049/jimmunol.173.5.3103. [DOI] [PubMed] [Google Scholar]
- Arnold PY, La Gruta NL, Miller T, Vignali KM, Adams PS, Woodland DL, Vignali DA. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J Immunol. 2002;169:739–749. doi: 10.4049/jimmunol.169.2.739. [DOI] [PubMed] [Google Scholar]
- Bridgett M, Cetkovic-Cvrlje M, O'Rourke R, Shi Y, Narayanswami S, Lambert J, Ramiya V, Baekkeskov S, Leiter EH. Differential protection in two transgenic lines of NOD/Lt mice hyperexpressing the autoantigen GAD65 in pancreatic beta-cells. Diabetes. 1998;47:1848–1856. doi: 10.2337/diabetes.47.12.1848. [DOI] [PubMed] [Google Scholar]
- Burton AR, Vincent E, Arnold PY, Lennon GP, Smeltzer M, Li CS, Haskins K, Hutton J, Tisch RM, Sercarz EE, Santamaria P, Workman CJ, Vignali DA. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes. 2008;57:1321–1330. doi: 10.2337/db07-1129. [DOI] [PubMed] [Google Scholar]
- Cantagrel A, Lambert N, Alam A. T cell receptor gene in synovial tissues of rheumatoid arthritis. Int Rev Immunol. 1998;17:323–337. doi: 10.3109/08830189809054409. [DOI] [PubMed] [Google Scholar]
- Carvalho-Pinto C, Garcia MI, Gomez L, Ballesteros A, Zaballos A, Flores JM, Mellado M, Rodriguez-Frade JM, Balomenos D, Martinez A. Leukocyte attraction through the CCR5 receptor controls progress from insulitis to diabetes in non-obese diabetic mice. Eur J Immunol. 2004;34:548–557. doi: 10.1002/eji.200324285. [DOI] [PubMed] [Google Scholar]
- Chaparro RJ, Burton AR, Serreze DV, Vignali DA, DiLorenzo TP. Rapid identification of MHC class I-restricted antigens relevant to autoimmune diabetes using retrogenic T cells. J Immunol Methods. 2008;335:106–115. doi: 10.1016/j.jim.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MB, von Herrath MG. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor alpha chain gene rearrangement. Proc Natl Acad Sci U S A. 1998;95:12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Genetic control of diabetes progression. Immunity. 1997;7:873–883. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- Hamilton-Williams EE, Palmer SE, Charlton B, Slattery RM. Beta cell MHC class I is a late requirement for diabetes. Proc Natl Acad Sci U S A. 2003;100:6688–6693. doi: 10.1073/pnas.1131954100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol. 2005;87:123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- Hill NJ, Van GK, Sarvetnick N. Th1 and Th2 pancreatic inflammation differentially affects homing of islet-reactive CD4 cells in nonobese diabetic mice. J Immunol. 2003;170:1649–1658. doi: 10.4049/jimmunol.170.4.1649. [DOI] [PubMed] [Google Scholar]
- Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006a;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006b;3:191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Turner SJ, Doherty PC. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc Natl Acad Sci U S A. 2004;101:4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Richter W, Aanstoot HJ, Shi Y, Fu Q, Rajotte R, Warnock G, Baekkeskov S. Differential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic islets. Diabetes. 1993;42:1799–1808. doi: 10.2337/diab.42.12.1799. [DOI] [PubMed] [Google Scholar]
- Lieberman SM, DiLorenzo TP. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens. 2003;62:359–377. doi: 10.1034/j.1399-0039.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- Morimoto J, Yoneyama H, Shimada A, Shigihara T, Yamada S, Oikawa Y, Matsushima K, Saruta T, Narumi S. CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J Immunol. 2004;173:7017–7024. doi: 10.4049/jimmunol.173.11.7017. [DOI] [PubMed] [Google Scholar]
- Pauza ME, Dobbs CM, He J, Patterson T, Wagner S, Anobile BS, Bradley BJ, Lo D, Haskins K. T-cell receptor transgenic response to an endogenous polymorphic autoantigen determines susceptibility to diabetes. Diabetes. 2004;53:978–988. doi: 10.2337/diabetes.53.4.978. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Berg R, Piganelli JD, Poulin M, Haskins K. Analysis of leukocytes recruited to the pancreas by diabetogenic T cell clones. Cell Immunol. 1998;189:92–98. doi: 10.1006/cimm.1998.1377. [DOI] [PubMed] [Google Scholar]
- Rhode A, Pauza ME, Barral AM, Rodrigo E, Oldstone MB, von Herrath MG, Christen U. Islet-specific expression of CXCL10 causes spontaneous islet infiltration and accelerates diabetes development. J Immunol. 2005;175:3516–3524. doi: 10.4049/jimmunol.175.6.3516. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Verdaguer J, Averill N, Santamaria P. A mechanism for the major histocompatibility complex-linked resistance to autoimmunity. J Exp Med. 1997;186:1059–1075. doi: 10.1084/jem.186.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercarz EE. Driver clones and determinant spreading. J Autoimmun. 2000;14:275–277. doi: 10.1006/jaut.2000.0380. [DOI] [PubMed] [Google Scholar]
- Suri A, Shimizu J, Katz JD, Sakaguchi S, Unanue ER, Kanagawa O. Regulation of autoimmune diabetes by non-islet-specific T cells - a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004;34:447–454. doi: 10.1002/eji.200324599. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Wegmann DR, Gill RG, Norbury-Glaser M, Schloot N, Daniel D. Analysis of the spontaneous T cell response to insulin in NOD mice. J Autoimmun. 1994;7:833–843. doi: 10.1006/jaut.1994.1066. [DOI] [PubMed] [Google Scholar]
- Wen L, Wong FS, Burkly L, Altieri M, Mamalaki C, Kioussis D, Flavell RA, Sherwin RS. Induction of insulitis by glutamic acid decarboxylase peptide-specific and HLA-DQ8-restricted CD4(+) T cells from human DQ transgenic mice. J Clin Invest. 1998;102:947–957. doi: 10.1172/JCI2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, Rosa R, Di SJ, Mudgett J, Peterson LB, Wicker LS, DeMartino JA. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73:771–780. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- Yamanouchi J, Verdaguer J, Han B, Amrani A, Serra P, Santamaria P. Cross-priming of diabetogenic T cells dissociated from CTL-induced shedding of beta cell autoantigens. J Immunol. 2003;171:6900–6909. doi: 10.4049/jimmunol.171.12.6900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.