Abstract
Aims
To understand the epidemiology and transmission patterns of hepatitis C virus (HCV), the predominant blood borne-pathogen infecting drug injectors (IDUs), in a part of the former Soviet Union.
Design
Cross-sectional respondent driven sample of IDUs.
Setting
St. Petersburg, RF
Participants
387 IDUs were recruited in late 2005 and throughout 2006.
Measurements
Participants were surveyed to collect demographic, medical, and both general and dyad specific drug injection and sexual behaviors. A blood sample was collected to detect antibodies to hepatitis C and to amplify viral RNA for molecular analysis. The molecular data, including genotypes, were analyzed spatially and linkage patterns were compared to the social linkages obtained by respondent driven sampling (RDS) for chains of respondents and among the injection dyads.
Findings
HCV infection was all but ubiquitous: 94.6% of IDUs were HCV-seropositive. Among the 208 viral sequences amplified, genotype 3a predominated (n=119, 56.9%) followed by 1b (n=61, 29.2%), and 1a (n=25, 11.9%). There was no significant clustering of genotypes spatially. Neither genotypes nor closely related sequences were clustered within RDS chains. Analysis of HCV sequences from dyads failed to find associations of genotype or sequence homology within pairs.
Conclusions
Genotyping reveals that there have been at least five unique introductions of HCV genotypes into the IDU community in St. Petersburg. Analysis of prevalent infections does not appear to correlate with the social networks of IDUs, suggesting that simple approaches to link these networks to prevalent infections, rather than incident transmission, will not prove meaningful. On a more positive note, the majority of IDUs are infected with 3a genotype that is associated with sustained virologic response to antiviral therapy.
Keywords: HCV, genotyping, Injection drug users, Respondent Driven Sampling, Russia
INTRODUCTION
It is estimated that over 170 million people are infected worldwide with the hepatitis C virus (HCV) (1). Between 70 and 80% of those infected are likely to have chronic HCV infections, which put them at a high risk for development of cirrhosis and hepatocellular carcinoma with resultant morbidity and mortality. The transmission of HCV occurs predominantly through parenteral exposure, which in many parts of the world means unsafe injection drug use (2, 3). The prevalence of HCV among injection drug users (IDUs), in the range of 50 to 90%, exceeds that of HIV in surveys of IDUs throughout the developed world (4). The transmission of HIV and HCV among IDUs has been associated with the sharing of syringes and other items used by injectors to prepare and distribute their drugs and with shared injections that lead to contaminated injection equipment (5-7).
Following the Afghan war, which gave young Russian soldiers fighting a disruptive and confusing guerrilla war easy access to locally produced opium gum that could be easily converted into heroin, and the collapse of the Soviet Union, Russia has experienced a substantial upsurge in injection drug use, especially among urban young adults, and concomitant well-documented increases in the prevalence and incidence of HIV among IDUs (8-13). Similar concomitant increases in HCV have been reported, but only in few locales (14-16). Furthermore, HCV transmission patterns within social networks of IDUs have not been well characterized.
St. Petersburg, situated at the center of Northwestern Russia, is the artistic, educational, scientific and cultural center of modern Russia. Among its population of 4.5 million, an estimated 30,000 to 80,000 inject drugs and more than 35,000 diagnosed HIV cases have been documented (17, 18). However, since less is known about the extent of HCV infection and the scant published data are more than half a decade old (19, 20), better data are needed on the extent of the HCV epidemic among injectors in the city and its spread through networks of injectors. Toward this end, we assembled a sample of active IDUs in St. Petersburg using a chain referral method known as respondent driven sampling (RDS) that is widely considered best suited for obtaining reliable sampling of hidden populations such as IDUs (21, 22). This sample was interviewed and serum specimens were analyzed to determine the HCV prevalence, genotypes, and viral sequences.
In this report, we describe the spatial and molecular epidemiology of HCV infection in the sample. We take advantage of the extensive molecular information about the HCV genome that has defined six major HCV genotypes that show nucleotide divergences of more than 30% (23, 24). The genotypes have been used to track the source of infection, mode of transmission, the geographic origin of the virus, and as surrogate markers for disease severity, and likelihood of sustained virologic response to available antiviral treatment (25-32).
Several studies have published data exploring the transmission of HIV in social networks (33-36). To our knowledge, no similar studies have been published exploring the role of social networks in HCV transmission and nothing is published that combines social network data and HCV molecular epidemiology. The combination of social network data from RDS, dyad specific questions from interviews, and molecular epidemiology data provide a unique opportunity to triangulate these three sources of information, i.e., comparing recruitment patterns to the molecular epidemiology of HCV, to test the hypothesis that closely related viral sequences will cluster within RDS chain and dyads, reflecting the transmission dynamics of HCV.
MATERIALS AND METHODS
Study subjects
The Sexual Acquisition and Transmission of HIV- Cooperative Agreement Program (SATH-CAP) is a NIDA-funded, multi-site, cross-sectional study of groups at high risk for HIV infection (IDUs, and men who have sex with men [MSM]) recruited by RDS. Four multidisciplinary teams are conducting studies using parallel tools in St. Petersburg, Los Angeles, North Carolina, and Chicago. The teams used RDS to recruit as seeds individuals who belong to the core populations of drug users and MSM; subsequent chains of recruitment sought to extend the core populations beyond the seed’s own social network. In addition, the seeds and cores were encouraged to recruit sexual partners who are not members of the core populations into the study. Details about the application of RDS across the sites and in St. Petersburg have already been published (37, 38). Although we used a dual core design, IDUs rarely recruited MSM and vice versa and there was less than 5% cross-over between the sample of IDUs and the sample of MSM recruited by RDS in St. Petersburg. Only one of 35 seeds was an IDU/MSM, but he recruited only other IDUs. Therefore, we considered IDUs and MSMs as independent samples and for analysis we able to focus this report on IDUs recruited in St. Petersburg.
Those eligible to be included in the IDU sample are individuals recruited as seeds or respondents who reported injection drug use during the six months prior to their recruitment, had injection drug use verified by the presence of injection stigmata, were at least 18 years old, were neither intoxicated nor unresponsive during the interview, and were deemed by trained study psychologists of giving informed consent. Individuals were excluded only if they had already participated. The sample was recruited between November 2005 and December 2006, inclusive, and interviewed at a location convenient to public transportation. Participation was anonymous and the coupon system employed by RDS allows only those individuals who wish to participate to contact the study.
Recruited IDUs were asked detailed questions about their sociodemographic and health status, their drug using and sexual behaviors, and their social environment. Data for analyzing RDS recruitment patterns were collected by trained project staff and the main questionnaires were administered through self-administered computerized interviews (CASI) without audio. The RDS sampling questionnaires collected data on network size and composition for post-hoc assignment of RDS weights and to examine possible recruitment bias. The main questionnaire was drawn from a variety of existing instruments, including the NIDA Risk Behavior Questionnaire for HIV Risk Behaviors; the CDC Project Mix Sexual Behavior Assessment; the EXPLORE Study Instrument; the YMS Study Instrument; and the HIV Partnership Survey (39-44). Risk behavior questions were asked at the global participant level, i.e., whether participants had ever or recently engaged in a certain activity, and at the event level, i.e., whether they engaged in certain behaviors with specific recent partners whose initials they provided at the start of the risk sections of the questionnaire. Event-level injection risk behavior was collected for injections with their three most recent injection partners. Event-level sexual risk was collected for the last three sex partners, as well as drug injecting partner or main partner, if these partners were not among the last three. This questionnaire reflected the overall study design by emphasizing the identification of individuals serving a functional role as bridges for HIV transmission from identified high-risk groups to lower-risk groups via sexual transmission. A third questionnaire, an RDS questionnaire designed to assess the characteristics of individuals who declined coupons, was administered to participants returning to obtain payment for successful referrals. In it, each participant was queried about individuals to whom they gave coupons but who did not accept them or redeem them. This questionnaire also was designed to facilitate the examination of recruitment bias.
Serum samples were taken to ascertain their infectious diseases status (HIV-1, and HCV) by enzyme-linked immunoassays (EIA) and PCR. At the completion of study procedures, participants were compensated for their time with gifts valued at approximately US$10 and core participants were given coupons to recruit other study participants. Coupon distribution for the wave of data collection described in this study occurred between November 2005 and December 2006.
The study was approved by institutional review boards at the Yale University School of Medicine and the Biomedical Center, St. Petersburg. Separate consents were obtained for participation in the study, for permission to use serum samples for molecular diagnostics, and for permission to export samples for testing from Russia.
HCV RNA isolation and reverse-transcription polymerase chain reaction (RT-PCR)
HCV sequencing and genotyping were conducted at Yale University. This required transport of serum samples in a solution that preserves the integrity of viral RNA (RNALater; Ambion Inc., Austin, TX). This also limited molecular aspects of the study to those samples for which study participants agreed both to let their serum be used for molecular analysis and to let the sample be exported. Additionally, in the summer of 2007 Russia imposed without warning a ban on the export of human genetic material; this was interpreted to include serum samples for diagnostic purposes. Thus, we were limited in our testing to 216 of the 366 HCV positive study participants. Since these restrictions impose no obvious bias on the samples, we have no reason to believe that the subset of samples transported to Yale were not representative of the entire set of samples.
For molecular analysis of HCV, serum samples were stored at - 80°C at the Biomedical Center until aliquots from the HCV-seropositive samples were transported to Yale University. HCV RNA was isolated from stored serum/RNALater mixture using the Viral RNA Isolation Kit (Qiagen) following the manufacturer’s protocol. Viral RNA sequences were amplified targeting 360 bases of the core gene with primers Sc2 (GGGAGGTCTCGTAGACCGTGCACCATG) and Ac2 (GAG(AC)GG(GT)AT(AG)TACCCCATGAG(AG)TCGGC) using a one-step RT-PCR kit (Qiagen) (45). The amplified products were then purified (PCR Purification Kit,Qiagen) for bidirectional sequencing on an automated fluorescent sequencer (Keck DNA Sequencing Facility, Yale). Nucleotide sequences were optimally aligned using the CLUSTAL W program (46). Mega software (version 3.1; available at http://www.megasoftware.net) was used to construct a phylogenetic tree by the neighbor-joining method based on Kimura’s two-parameter distances. The reliability of the tree topologies was assessed by bootstrapping with 100 replicates, with a bootstrap agreement of ≥70% required to define a phylogenetic cluster. The genotypes and genetic distances obtained were used to analyze the relationship in sequences among and between recruiter-recruit pairs entering the study.
Statistics
The statistical significance of association between possible risk factors and HCV seropositivity or genotype was measured by χ2 or Fisher’s exact test as appropriate. The comparison of the genetic relatedness of HCV within a referral chain in a given social network was made using the Mann-Whitney/Wilcoxon rank sum test or t test as appropriate. P values for comparisons of HCV genotypes with level of social connectedness were derived using paired t test. Differences were considered significant for p <0.05. All statistical analyses were performed using the software Stata (version 9.0; SAS Institute).
RESULTS
Twenty-three IDU seeds were identified in the first wave of study recruitment, and through chain referrals, a total of 387 injection drug users were recruited into the study. The age range was 18 to 53 years, with a median age of 29 years. We determined by EIA that HCV infection is nearly ubiquitous among the IDU population in St. Petersburg, with a seroprevalence rate of 94.6% (366 of 387). The demographic characteristics and possible HCV-associated risk factors among the participants are shown in Table 1. The ratio of male to female with HCV infection was 1:3 and six of the men reported having sex with other men at some time in their lives.
Table 1.
HCV Prevalence and Demographics of IDUs in St. Petersburg
| n | HCV-Seropositive | p-value | |
|---|---|---|---|
| Study Participants | 387 | 366 (95%) | -- |
| Sex | |||
| Male | 285 | 273 (96%) | 0.07 |
| Female | 101 | 92 (91%) | |
| Age | |||
| ≤25 years | 127 | 119 (94%) | 0.60 |
| >25 years | 260 | 247 (95%) | |
| Injected in the past 30 days | |||
| Yes | 342 | 331 (97%) | <0.01 |
| No | 36 | 26 (72%) | |
| Age at first injection | |||
| ≤18 years | 184 | 179 (97%) | 0.02 |
| >18 years | 200 | 184 (92%) | |
| Duration of injection drug use | |||
| ≤5 years | 106 | 92 (87%) | <0.01 |
| >5 years | 280 | 273 (98%) | |
| Number of injections, past 30 days | |||
| < 30 / month | 260 | 241 (93%) | 0.03 |
| ≥30 / month | 118 | 116 (98%) | |
| Number injection partners, past 6 months | |||
| ≤2 | 162 | 150 (93%) | 0.20 |
| >2 | 208 | 192 (96%) | |
| New injection partner, past 30 days | |||
| Yes | 112 | 108 (96%) | 0.20 |
| No | 228 | 199 (93%) | |
| Unsafe injection, past 30 days | |||
| Yes | 253 | 245 (97%) | <0.01 |
| No | 112 | 101 (90%) | |
| HIV positive | |||
| Yes | 193 | 188 (97%) | 0.014 |
| No | 194 | 178 (92%) | |
Six factors were significantly associated with HCV infection at p<0.05. These included from among recent behaviors any injection, more frequent injection, and unsafe injection in the past 30 days. For items from life history these included age at first injection and a longer duration of injection. Among medical comorbidities it included being HIV positive. However, it should be kept in mind that none of the lower HCV prevalences are less than 90% with the exception of having injected for not more than five years (87%).
HCV-RNA was amplified from 209 of 216 samples for which serum was available for testing, yielding an HCV-RNA positive rate of 96.8%. Failure to amplify HCV-RNA from the remaining seven samples suggests that they individuals had been infected with HCV, but have cleared the virus. Comparison of the 216 people who provided samples for HCV testing and the 171 who did not revealed no significant differences in relevant demographic characteristics (p = 0.175 for sex and p = 0.245 for age).
Three HCV genotypes (1, 2 and 3) were identified; in descending order of prevalence, 3a was identified in 56.9% (119/209), 1b in 29.2% (61/209), 1a in 11.9% (25/209), and genotypes 2a and 2b in three and one participants, respectively. Our findings are consistent with the predominance of genotype 3a in IDU than the general population.
We examined if any of the factors associated with prevalent HCV infection with a p value < 0.1 (younger age at initiation of drug injection, being male, longer duration of injecting, recent injecting, and daily injecting in the past month) were also associated with a particular genotype (Table 2). The only variable that reached statistical significance was sex (p=0.03); women were more likely to infected with genotype 3a.
Table 2.
Association of Genotype and Factors Associated with Prevalent HCV Infection
| HCV risk | Genotype 1a n = 25 | Genotype 1b n = 61 | Genotype 3a n = 119 | p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age at initiation of IDU | |||||||
| ≤ 18 years | 13 | 13 | 28 | 28 | 60 | 59 | 0.813 |
| >18 years | 12 | 12 | 33 | 32 | 59 | 57 | |
| Sex | |||||||
| Male | 23 | 16 | 44 | 30 | 79 | 54 | 0.034 |
| Female | 2 | 3 | 16 | 26 | 43 | 70 | |
| Duration of IDU | |||||||
| > 5 years | 10 | 17 | 14 | 23 | 36 | 60 | 0.269 |
| ≤ 5 years | 15 | 10 | 47 | 32 | 83 | 57 | |
| Recent injection | |||||||
| Within 30 days | 24 | 13 | 57 | 30 | 110 | 58 | 0.810 |
| Not within 30 days | 1 | 7 | 4 | 29 | 9 | 64 | |
| Injection Frequency | |||||||
| <30 in past 30 days | 14 | 10 | 46 | 34 | 75 | 56 | 0.137 |
| ≥30 in past 30 days | 11 | 16 | 15 | 21 | 44 | 63 | |
Sampling weights were calculated for the five independent variables significantly or marginally associated with HCV seroprevalence as well as the dependent variable, HCV serostatus. Recent injection (within the past 30 days) was the only statistically significant (p<0.01) difference between the weighted and un-weighted.
All the metropolitan districts were well represented in the study population. We investigated if genotypes clustered according to metro-stations or districts. The spatial distribution of HCV genotypes by district is illustrated in Figure 1. Genotype 3a predominated in most districts; some districts with fewer participants had only 3a genotypes present in the sample.
FIG. 1. Spatial Distribution of HCV Genotypes among IDUs in St Petersburg.
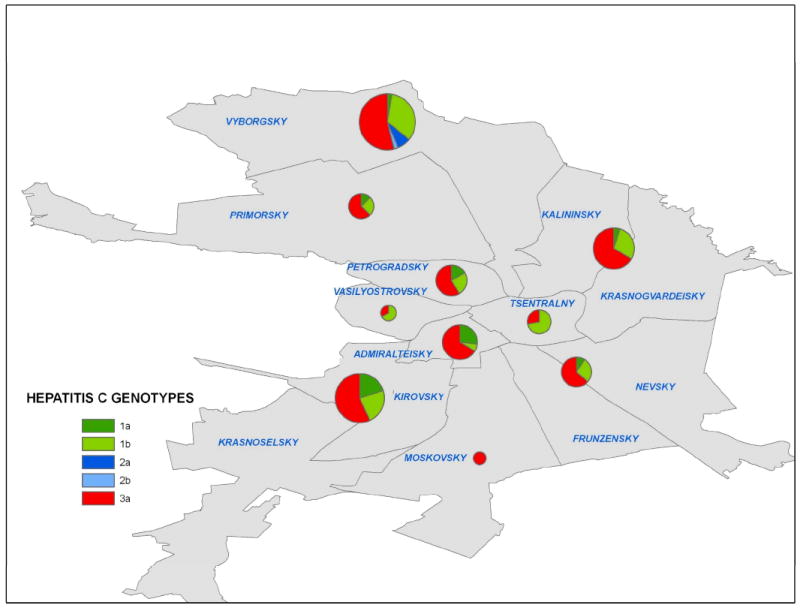
The prevalence and distribution of the five HCV genotypes found among IUDs are shown in the map in relation to the Metro-districts in St. Petersburg. The size of the pie-chart is proportion to the number of participants recruited from a Metro-district.
Figure 2, a phylogenetic tree of 217 HCV core sequences (comprising of 209 IDUs, and 8 others), shows monophyletic clusters for each of the three genotypes (3a, 1a, and 1b). The largest cluster is that of genotype 3a. The colors of the labels identify participants in the same recruitment chain. The colors are dispersed throughout the tree with no obvious clustering vis-a-vis recruitment chain. All seven chains of twelve or more participants have at least two genotypes of HCV and three of the seven have four genotypes represented.
FIG.2. Phylogenetic Analysis of Partial Core Sequences of HCV Isolates from St. Petersburg.
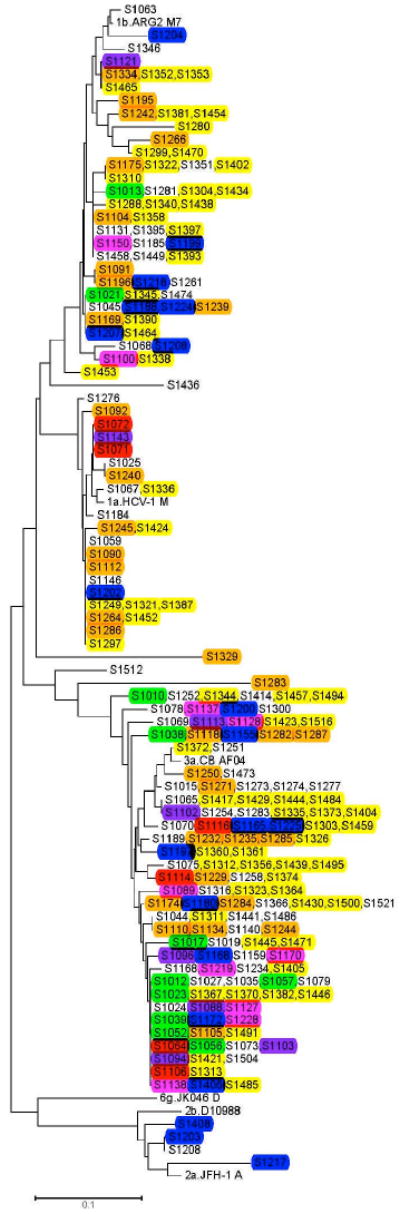
Sequences from 217 study participants (109 IDUs, and 8 others) and references from GenBank. The strains recovered from participants are numbered with an “S” prefix. The colors of the labels identify participants in the same recruitment chain (social network). Reference sequences from GenBank are defined by the name of the relevant strain or clone.
We next investigated the association of HCV within dyads of injectors. We identified 105 recruit-recruiter dyads for which HCV sequences were available for both members of the dyad. We selected five variables based on the research hypothesis that these variables might predict closer association and hence greater likelihood of cases in which we could detect person-to-person transmission of HCV. The five variables we assessed were duration of relationship (>5 versus ≤ 5 years), type of relationship (friend versus acquaintance or stranger), sex partner (sexual intercourse between recruit and recruiter in the past six months), drug injecting partner (shared injection between recruit and recruiter in the past 30 days), and overlapping network (recruiter knows more or less than half of the injectors that the recruit knows) (Table 3). Dyads with closer relationships were no more likely than those with more distant relationships to share the same genotype. In addition, genetic distance between the two members of more closely related dyads were not statistically different from genetic distances between more distantly related dyads (Table 4). Furthermore, the genetic distance of 108 pairs generated at random from the 216 individual sequences did not differ from sequences found in known dyads (data not shown).
Table 3.
Association of social connectedness and HCV genotype among 105 dyads
| Degree of social connection | Same genotype n=49 (45%) | Different genotype n=59 (55%) | p-value | |
|---|---|---|---|---|
| Duration of relationship | ||||
| >5 years | 11 (38%) | 17 (61%) | 0.30 | |
| ≤5 years | 37 (51%) | 36 (49%) | ||
| Type of relationship | ||||
| Friend | 18 (41%) | 26 (59%) | 0.52 | |
| Acquaintance/Stranger | 27 (47%) | 30 (53%) | ||
| Sex partner | ||||
| Yes | 12 (44%) | 15 (56%) | 0.93 | |
| No | 35 (45%) | 42 (55%) | ||
| Drug partner | ||||
| Yes | 35 (44%) | 44 (56%) | 0.78 | |
| No | 9 (41%) | 13 (60%) | ||
| Proportion overlapping drug network | ||||
| ≥50% in common | 19 (46%) | 21 (53%) | 0.98 | |
| <50% in common | 22 (47%) | 24 (52%) | ||
Table 4.
Association of social connection and HCV genetic distance among 105 dyads
| Degree of social connection | Median genetic distance | p-value Mann-Whitney |
|---|---|---|
| Duration of relationship | ||
| >5 years | 0.060 | 0.34 |
| ≤5 years | 0.051 | |
| Type of relationship | ||
| Friend | 0.051 | 0.95 |
| Acquaintance/Stranger | 0.051 | |
| Sex partner | ||
| Yes | 0.034 | 0.35 |
| No | 0.051 | |
| Drug partner | ||
| Yes | 0.051 | 0.98 |
| No | 0.051 | |
| Proportion overlapping drug network | ||
| ≥50% in common | 0.051 | 0.64 |
| <50% in common | 0.051 | |
DISCUSSION
This study has produced significant findings about the epidemiology of HCV among IDUs in St. Petersburg in three domains – disease prevalence, genotype distribution, and linkage between the virus and potential transmission pathways.
First, in terms of prevalence, 19 of 20 active IDUs were already infected. This prevalence is higher than any of the previous estimates for the regions in the former Soviet Union and ranks at the top for rates of HCV prevalence found among IDUs worldwide (4, 16, 47, 48). Behaviors common to IDU populations such as the sharing of needles, syringes and other injecting paraphernalia, injecting with others, injecting in public places and ‘shooting galleries’ place them at increased risk of HCV infection (5-7). Consistent with previous studies (49-52), we found that individuals with a shorter duration of injection were less likely to be HCV seropositive and that HCV was the first infection transmitted within our IDU sample.
Our findings underscore the serious and advanced nature of the HCV epidemic among IDUs in St. Petersburg. Generally, 15-20% of infected individual clear virus and do not become chronically infected; such individuals test positive for antibodies to HCV but negative for viral RNA. However, we found that only 3% of IDUs we tested in St. Petersburg were antibody positive and RNA negative. This suggests that repeated exposure and reinfection may be common, but we found little direct evidence for superinfection with multiple genotypes. However, the lack of similarity of genotype within injection dyads or in chains of individuals recruited by RDS suggests that the repeated exposure is not sufficiently strong to produce genotypic convergence within these social structures. Because this study is cross-sectional, however, we cannot draw any conclusions about the rates of viral clearance and reinfection.
Although sexual transmission of HCV is rare, there are reports of HCV acquisition based on sexual behavior (53, 54). Since nearly half of the IDUs are also HIV positive (38), and HIV co-infection is felt to enhance the probability of sexual transmission of HCV, such transmission may be occurring, and not only amongst IDUs (55-57). Our findings reveal a substantial bridge population (57% of the injectors reported recent non-IDU sex partners) that might contribute to HCV transmission via unsafe sex. Furthermore, most of the HCV seropositive participants appear to harbor chronic HCV infection. More than 95% of the serum samples transported to the U.S. for testing yielded viral nucleic acid. Such a high level of chronic infection is another ominous feature portending the continuous spread of HCV.
The second significant finding was the predominance of 3a (56.9%) genotype. The relative proportions of infections by the different genotypes vary from country to country, and regions of the same country partly because of distribution of different at risk groups (58). The 1a genotype is associated with injection drug use in Western Europe (59). In Eastern Europe and Russia, genotype 1a was more prevalent in the general population prior to the epidemic of HCV among drug injectors, but 3a appears to have outstripped 1a in younger people and IDUs (60-63). In a study of hundred and two patients who attended infectious disease clinics in St. Petersburg, subtype 3a was predominant in the patients reporting injecting drug use (56%) and in those with unknown source of infection (46%) (20). The authors concluded that the previously dominant subtype 1b was being replaced by subtype 3a in St. Petersburg mediated through an increased in injection drug use. Our finding of 56.9% of 3a among IDUs in St. Petersburg is consistent with these observations.
The third finding is a negative one concerning the ability of our study to identify transmission pathways by using a chain referral approach to accruing our study population. Contrary to our working hypothesis, we found no significant associations for HCV genotype or genetic distance within a recruitment chain or within the 105 identified dyads. Failure to find such associations may be due to the advanced epidemic of HCV among the IDU population; many participants may have been infected for a long enough period of time that recent behaviors and current social connections have become irrelevant to HCV transmission. Unfortunately, this may have far reaching public health consequences limiting the design of rational infection control and HCV prevention programs (64).
In terms of research methodologies, we had hoped to use RDS to study viral transmission dynamics since RDS is increasingly becoming the method of choice to penetrate difficult to reach networks or populations (21, 65). Our report is the first attempt to link recruitment chains to patterns of viral transmission, and we were therefore disappointed that neither RDS chains nor recruitment dyads within these chains were associated with transmission events as measured by genetic homologies. One of the limitations to this approach may be a consequence of the weak network ties upon which RDS relies. Recruit-recruiter dyads of IDUs need not actually have injected drugs with each other, but even limiting our dyads to those in which injecting together was reported failed to reveal closer genetic linkages at either the genotype or sequence level. Additionally, RDS limits the number of people referred by each respondent, which yields, by definition, only partial network data. Furthermore, study participants may not be willing or able (anonymous injection partners) to recruit those social contacts who represent their actual transmission links. Thus, the social network data obtained in some instances could provide incorrect information that would compromise the utility for analyzing or modeling transmission events. Finally, RDS data pertains to current social networks, and in the case of long-standing infections the current structure of these networks may be quite different from the structure that existed when the virus was transmitted. While the ability of RDS to capture transmission patterns for prevalent infections appears limited, combining the two data set might provide a more robust exploration of incident transmissions (as opposed to prevalent cases) of infectious diseases like HCV and HIV.
This study included some self-reported data, especially regarding behaviors that put the study subjects at risk for becoming infected with and thereafter transmitting HCV. This can lead to recall and socially desirable response biases. However, in keeping with standards for this type of research, we have kept the timeframe for recall to 30 days to minimize recall bias and there are many studies that demonstrate little significant response bias in self-reported data from IDUs (66-68).
In conclusion, the high prevalence and the high infectiousness of HCV among IDU pose challenges for control strategies. Given the scale of HCV prevalence in St. Petersburg, control measures must be increased to significantly reduce the epidemic in the IDU population. Given that the majority of IDUs were infected with HCV of genotype 3a (a genotype that is amenable to currently available therapy) efforts to treat injectors with active HCV infection is an important step not only for the infected individuals but also to reduce the population burden of HCV. More efforts must be made to identify the bridge populations and implement measures to contain the HCV epidemic expansion in the general population.
Acknowledgments
We thank the SATH-CAP participants in St. Petersburg, the SATH-CAP Coordinating Center at RAND, and the research teams at all SATH-CAP sites. This work was supported by a cooperative agreement (5U01-DA017387) with the National Institute on Drug Abuse.
Footnotes
Conflict of Interests: None
References
- 1.Cohen J. The scientific challenge of hepatitis C. Science. 1999;285:26–30. doi: 10.1126/science.285.5424.26. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–S98. doi: 10.1053/jhep.2002.36389. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.Hagan H, Thiede H, Weiss NS, et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaisson RE, Bacchetti P, Osmond D, et al. Cocaine use and HIV infection in intravenous drug users in San Francisco. Jama. 1989;261:561–565. [PubMed] [Google Scholar]
- 6.Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 7.Koester S, Glanz J, Baron A. Drug sharing among heroin networks: implications for HIV and hepatitis B and C prevention. AIDS Behav. 2005;9:27–39. doi: 10.1007/s10461-005-1679-y. [DOI] [PubMed] [Google Scholar]
- 8.Dehne KL, Khodakevich L, Hamers FF, Schwartlander B. The HIV/AIDS epidemic in eastern Europe: recent patterns and trends and their implications for policy-making. Aids. 1999;13:741–749. doi: 10.1097/00002030-199905070-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kalichman SC, Kelly JA, Sikkema KJ, et al. The emerging AIDS crisis in Russia: review of enabling factors and prevention needs. Int J STD AIDS. 2000;11:71–75. doi: 10.1177/095646240001100201. [DOI] [PubMed] [Google Scholar]
- 10.Atlani L, Carael M, Brunet JB, Frasca T, Chaika N. Social change and HIV in the former USSR: the making of a new epidemic. Soc Sci Med. 2000;50:1547–1556. doi: 10.1016/s0277-9536(99)00464-5. [DOI] [PubMed] [Google Scholar]
- 11.Poznyak VB, Pelipas VE, Vievski AN, Miroshnichenko L. Illicit drug use and its health consequences in Belarus, Russian Federation and Ukraine: impact of transition. Eur Addict Res. 2002;8:184–189. doi: 10.1159/000066138. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes T, Sarang A, Bobrik A, Bobkov A, Platt L. HIV transmission and HIV prevention associated with injecting drug use in the Russian Federation. International Journal of Drug Policy. 2004;15:1–16. [Google Scholar]
- 13.Heimer R, Grau LE, Curtin E, Khoshnood K, Singer M. Assessment of HIV testing of urban injection drug users: implications for expansion of HIV testing and prevention efforts. Am J Public Health. 2007;97:110–116. doi: 10.2105/AJPH.2005.078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes T, Platt L, Judd A, et al. Hepatitis C virus infection, HIV co-infection, and associated risk among injecting drug users in Togliatti, Russia. International Journal of STD & AIDS. 2005;16:749–754. doi: 10.1258/095646205774763180. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes T, Platt L, Maximova S, et al. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi-city study. Addiction. 2006;101:252–266. doi: 10.1111/j.1360-0443.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- 16.Shustov AV, Kochneva GV, Sivolobova GF, et al. Molecular epidemiology of the hepatitis C virus in Western Siberia. J Med Virol. 2005;77:382–389. doi: 10.1002/jmv.20467. [DOI] [PubMed] [Google Scholar]
- 17.Aral SO, St Lawrence JS, Dyatlov R, Kozlov A. Commercial sex work, drug use, and sexually transmitted infections in St. Petersburg, Russia. Soc Sci Med. 2005;60:2181–2190. doi: 10.1016/j.socscimed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.AFEW. AIDS Foundation East-West: Russian Federation. Moscow, RF: AIDS Foundation East-West; 2008. [Google Scholar]
- 19.Abdala N, Carney JM, Durante AJ, et al. Estimating the prevalence of syringe-borne and sexually transmitted diseases among injection drug users in St Petersburg, Russia. Int J STD AIDS. 2003;14:697–703. doi: 10.1258/095646203322387965. [DOI] [PubMed] [Google Scholar]
- 20.Kalinina O, Norder H, Vetrov T, et al. Shift in predominating subtype of HCV from 1b to 3a in St. Petersburg mediated by increase in injecting drug use. J Med Virol. 2001;65:517–524. [PubMed] [Google Scholar]
- 21.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. Aids. 2005;19(Suppl 2):S67–S72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Quader AS, Heckathorn DD, McKnight C, et al. Effectiveness of respondent-driven sampling for recruiting drug users in New York City: findings from a pilot study. J Urban Health. 2006;83:459–476. doi: 10.1007/s11524-006-9052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmonds P, Holmes EC, Cha TA, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 24.Choo QL, Richman KH, Han JH, et al. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991;88:2451–5. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlotsky JM. Hepatitis C virus genetic variability: pathogenic and clinical implications. Clin Liver Dis. 2003;7:45–66. doi: 10.1016/s1089-3261(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Labrador FX, Ampurdanes S, Gimenez-Barcons M, et al. Relationship of the genomic complexity of hepatitis C virus with liver disease severity and response to interferon in patients with chronic HCV genotype 1b infection [correction of interferon] Hepatology. 1999;29:897–903. doi: 10.1002/hep.510290306. [DOI] [PubMed] [Google Scholar]
- 27.Hadziyannis SJ, Koskinas JS. Differences in epidemiology, liver disease and treatment response among HCV genotypes. Hepatol Res. 2004;29:129–135. doi: 10.1016/j.hepres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Hnatyszyn HJ. Chronic hepatitis C and genotyping: the clinical significance of determining HCV genotypes. Antivir Ther. 2005;10:1–11. [PubMed] [Google Scholar]
- 29.Nunez M, Soriano V. Hepatitis C virus (HCV) genotypes and disease progression in HIV/HCV-coinfected patients. J Infect Dis. 2005;191:1–3. doi: 10.1086/426515. [DOI] [PubMed] [Google Scholar]
- 30.Soriano V, Nunez M, Sanchez-Conde M, et al. Response to interferon-based therapies in HIV-infected patients with chronic hepatitis C due to genotype 4. Antivir Ther. 2005;10:167–170. [PubMed] [Google Scholar]
- 31.Hopf U, Berg T, Konig V, et al. Treatment of chronic hepatitis C with interferon alpha: long-term follow-up and prognostic relevance of HCV genotypes. J Hepatol. 1996;24:67–73. [PubMed] [Google Scholar]
- 32.Kobayashi M, Tanaka E, Sodeyama T, et al. The natural course of chronic hepatitis C: a comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996;23:695–699. doi: 10.1053/jhep.1996.v23.pm0008666319. [DOI] [PubMed] [Google Scholar]
- 33.Friedman SR, Neaigus A, Jose B, et al. Sociometric Risk Networks and HIV Risk. Amer J Publ Health. 1997;87:1289–1296. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potterat JJ, Phillips-Plummer L, Muth SQ, et al. Risk network structure in the early epidemic phase of HIV transmission in Colorado Springs. Sexually Transmitted Infect. 2002;78(Suppl 1):159–163. doi: 10.1136/sti.78.suppl_1.i159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothenberg R, Long D, Sterk C, et al. The Atlanta Urban Networks Study: a blueprint for endemic transmission. AIDS. 2000;14:2191–2200. doi: 10.1097/00002030-200009290-00016. [DOI] [PubMed] [Google Scholar]
- 36.Latkin CA, Forman V, Knowlton A, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Social Sci Med. 2003;56:465–476. doi: 10.1016/s0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi M, Ober A, Berry S, et al. Simultaneous Recruitment of Drug Users and Men Who Have Sex with Men in the United States and Russia using Respondent Driven Sampling: Sampling Methods and Implications. Journal of Urban Health. 2009 doi: 10.1007/s11524-009-9365-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niccolai LM, Toussova OV, Verevochkin SV, et al. High HIV Prevalence, Suboptimal HIV Testing, and Low Knowledge of HIV-Positive Serostatus Among Injection Drug Users in St. Petersburg, Russia. [3 April 2009];AIDS & Behavior. 2009 doi: 10.1007/s10461-008-9469-y. on line at www.springerlink.com/content/104828/?k=Niccolai. [DOI] [PMC free article] [PubMed]
- 39.Chesney MA, Koblin BA, Barresi PJ, et al. An individually tailored intervention for HIV prevention: baseline data from the EXPLORE Study. American Journal of Public Health. 2003;93:933–938. doi: 10.2105/ajph.93.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorbach PM, Drumright LN, Daar ES, Little SJ. Transmission behaviors of recently HIV-infected men who have sex with men. Journal of Acquired Immune Deficiency Syndrome. 2006;42:80–85. doi: 10.1097/01.qai.0000196665.78497.f1. [DOI] [PubMed] [Google Scholar]
- 41.Mansergh G, Flores S, Koblin B, et al. Alcohol and drug use in the context of anal sex and other factors associated with sexually transmitted infections: results from a multi-city study of high-risk men who have sex with men in the USA. Sexually Transmitted Infections. 2008;84:509–511. doi: 10.1136/sti.2008.031807. [DOI] [PubMed] [Google Scholar]
- 42.National Institute on Drug Abuse. Risk Behavior Assessment. Rockville, MD: NIDA/NIH/DHHS; 1991. [Google Scholar]
- 43.Needle R, Weatherby N, Chitwood D, et al. Reliability of self-reported HIV risk behaviors of drug users. Psychology of Addictive Behaviors. 1995;9:242–250. [Google Scholar]
- 44.Shehan DA, LaLota M, Johnson DF, et al. HIV/STD risks in young men who have sex with men who do not disclose their sexual orientation --- six U.S. Cities, 1994--2000. MMWR. 2003;52:81–85. [PubMed] [Google Scholar]
- 45.Ohno O, Mizokami M, Wu RR, et al. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35:201–207. doi: 10.1128/jcm.35.1.201-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins DG, Bleasby AJ, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 47.Onishchenko GG, Shakhgil’dian IV. The current problems in the epidemiology and prevention of viral hepatitis B and C in the Russian Federation. Zh Mikrobiol Epidemiol Immunobiol. 2000:50–54. [PubMed] [Google Scholar]
- 48.Hope VD, Judd A, Hickman M, et al. Prevalence of hepatitis C among injection drug users in England and Wales: is harm reduction working? Am J Public Health. 2001;91:38–42. doi: 10.2105/ajph.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald MA, Wodak AD, Dolan KA, et al. Hepatitis C virus antibody prevalence among injecting drug users at selected needle and syringe programs in Australia, 1995-1997. Collaboration of Australian NSPs. Med J Aust. 2000;172:57–61. doi: 10.5694/j.1326-5377.2000.tb139199.x. [DOI] [PubMed] [Google Scholar]
- 50.Taylor A, Goldberg D, Hutchinson S, et al. Prevalence of hepatitis C virus infection among injecting drug users in Glasgow 1990-1996: are current harm reduction strategies working? J Infect. 2000;40:176–183. doi: 10.1053/jinf.2000.0647. [DOI] [PubMed] [Google Scholar]
- 51.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186:1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 53.Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399–404. doi: 10.1136/sti.74.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van de Laar TJ, Van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–238. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 55.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–991. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 56.Hammer GP, Kellogg TA, McFarland WC, et al. Low incidence and prevalence of hepatitis C virus among sexually active non-intravenous drug-using adults, San Francisco, 1997–2000. Sexually Transmitted Diseases. 2003;30:919–924. doi: 10.1097/01.OLQ.0000091152.31366.E6. [DOI] [PubMed] [Google Scholar]
- 57.Matthews GV, Hellard M, Kaldor J, Lloyd A, Dore GJ. Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: response to Danta et al. AIDS. 2007;21:2112–2113. doi: 10.1097/QAD.0b013e3282ef3873. comment. [DOI] [PubMed] [Google Scholar]
- 58.Nousbaum JB. Genomic subtypes of hepatitis C virus: epidemiology, diagnosis and clinical consequences. Bull Soc Pathol Exot. 1998;91:29–33. [PubMed] [Google Scholar]
- 59.Kleter B, Brouwer JT, Nevens F, et al. Hepatitis C virus genotypes: epidemiological and clinical associations. Benelux Study Group on Treatment of Chronic Hepatitis C. Liver. 1998;18:32–38. [PubMed] [Google Scholar]
- 60.Naoumov NV. Hepatitis C virus infection in Eastern Europe. J Hepatol. 1999;31(Suppl 1):84–87. doi: 10.1016/s0168-8278(99)80380-8. [DOI] [PubMed] [Google Scholar]
- 61.Viazov S, Kuzin S, Paladi N, et al. Hepatitis C virus genotypes in different regions of the former Soviet Union (Russia, Belarus, Moldova, and Uzbekistan) J Med Virol. 1997;53:36–40. [PubMed] [Google Scholar]
- 62.Shustov AV, Kochneva GV, Sivolobova GF, et al. Occurrence of markers, distribution of genotypes and risk factors for viral hepatitis C among some groups of the population in the Novosibirsk region [Russian] Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii. 2004;5:20–25. [PubMed] [Google Scholar]
- 63.Tallo T, Norder H, Tefanova V, et al. Genetic characterization of hepatitis C virus strains in Estonia: fluctuations in the predominating subtype with time. Journal of Medical Virology. 2007;79:374–382. doi: 10.1002/jmv.20828. [DOI] [PubMed] [Google Scholar]
- 64.Singh A, Goering RV, Simjee S, Foley SL, Zervos MJ. Application of molecular techniques to the study of hospital infection. Clin Microbiol Rev. 2006;19:512–530. doi: 10.1128/CMR.00025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdul-Quader AS, Heckathorn DD, Sabin K, Saidel T. Implementation and analysis of respondent driven sampling: lessons learned from the field. J Urban Health. 2006;83:i1–i5. doi: 10.1007/s11524-006-9108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vlahov D, Polk BF. Perspectives on infection with HIV-1 among intravenous drug users. Psychopharmacol Bull. 1988;24:325–329. [PubMed] [Google Scholar]
- 67.Latkin CA, Vlahov D, Anthony JC. Socially desirable responding and self-reported HIV infection risk behaviors among intravenous drug users. Addiction. 1993;88:517–526. doi: 10.1111/j.1360-0443.1993.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 68.Babor TF, Brown J, Del Boca FK. Validity of self-reports in applied research on addictive behaviors: Fact or fiction? Behav Assessment. 1990;12:5–31. [Google Scholar]


