Abstract
Bladder cancer is the most common malignancy among Egyptian males and previously has been attributed to Schistosoma infection, a major risk factor for squamous cell carcinoma (SCC). Recently, transitional cell carcinoma (TCC) incidence has been increasing while SCC has declined. To investigate this shift, we analyzed the geographical patterns of all bladder cancers cases recorded in Egypt’s Gharbiah Population-Based Cancer Registry from 1999 through 2002. Data on tumor grade, stage, and morphology, as well as smoking, community of residence, age and sex, were collected on 1,209 bladder cancer cases. Age-adjusted incidence rates were calculated for males, females, and the total population for the eight administrative Districts and 316 communities in Gharbiah. Incidence Rate Ratios (IRR) and 95% Confidence Intervals (CI) were computed using Poisson Regression. The male age-adjusted incidence rate (IR) in Gharbiah Province was 13.65/100,000 person years (PY). The District of Kotour had the highest age-adjusted IR 28.96/100,000 among males. The District of Kotour also had the highest IRR among all Districts, IRR=2.15 95% CI (1.72, 2.70). Kotour’s capital city had the highest bladder cancer incidence among the 316 communities (IR=73.11/100,000 PY). Future studies on sources and types of environmental pollution and exposures in relation to the spatial patterns of bladder cancer, particularly in Kotour District, may improve our understating of risk factors for bladder cancer in the region.
Keywords: bladder carcinoma, environmental exposures, developing countries, pollution, schistosomiasis
Introduction
Bladder cancer is the ninth most common cancer throughout the world and is considerably more common in developed than developing countries1. In the United States, bladder cancer is the fourth most common cancer in males with an age-adjusted incidence rate of 37.3/100,000 person years (PY) using the US standard population 2,3. In Europe, these rates are also relatively high, with male world age-adjusted incidence rates (IR) of 17.1 and 29.8/100,000 PY in Italy and the United Kingdom, respectively. Spain’s male age-adjusted IR of 33.0/100,000 is among the highest in Europe4.
In developed countries, over 90% of the bladder cancer cases diagnosed are transitional cell carcinoma (TCC), with squamous cell carcinoma (SCC), adenocarcinomas, and rare types of bladder cancer comprising the remaining 10% of bladder cancer cases5. Exposures to tobacco smoke, occupational toxins, and environmental sources of heavy metals such as arsenic are the major reported risk factors for TCC6–9. Active exposure to tobacco smoke has a strong relationship to TCC bladder cancer10–12. Previous studies have reported a 2.6 fold risk of developing bladder cancer in smokers compared to nonsmokers after adjusting for age, education and marital status13. Other studies report a 5.5 increase in bladder cancer risk comparing male regular-smokers to never-smokers after adjusting for high risk occupations14. Bladder cancer risk is also dependent on the duration of smoking and the amount of cigarettes smoked per day15,16. An additional source of tobacco exposure is from smoking with a water pipe or “shisha,” which is prevalent in Egypt with 15.3% of rural males and 10.9% of urban males reporting regular, current “shisha” use17,18.
The relationship between various occupational exposures and TCC of the bladder has been documented in western countries, most notably to aromatic amines and to a lesser extent polycyclic aromatic hydrocarbon6,19,20. Workers exposed to heavy metals, paints, solvents, emissions, diesel engine, and textiles have higher risk of bladder cancer21. Exposure to aromatic amines, which are present in the dye, rubber and textile industries, accounts for an estimated 25% of bladder cancer cases in developed countries20.
Unlike TCC, the main risk factors for SCC are not environmental exposures, but exposure to infectious agents11,22. The main cause of SCC in developing countries is Schistosoma haematobium, a trematode that induces inflammation and increased bacterial infections that eventually lead to SCC23,24. Bladder cancer is the most prevalent malignancy among Egyptian males (16%), producing >7900 deaths annually, which is strikingly higher than most other parts of the world25. According to IARC, the world age adjusted incidence rate for Egyptian males is an estimated 27.9/100,000 PY26, representing one of the highest bladder cancer incidence rates in North Africa and the Middle East. Countries geographically close to Egypt have much lower rates of bladder cancer: For example, the age-adjusted male IR is for Jordan, Saudi Arabia, Lebanon and Morocco are 7.2, 7.9, 17.5, and 9.7 per 100,000 PY, respectively27. The male IR of bladder cancer in Egypt is almost four times that in Jordan despite a lower prevalence of smoking among Egyptian males (35%) compared to Jordanian males (45%)27.
Previous research has reported a significant decrease in SCC in Egypt, although the overall bladder cancer incidence in Egypt has remained steady due to an increase in TCC over the past 30 years28. One study examining 2,778 cases in the Nile Delta Region, Metropolitan Cairo area, and the South of Egypt reported that, in 1980, 22% of Egyptian bladder cancer cases were diagnosed with TCC and 78% were diagnosed with SCC28. By 2005, however, that ratio was nearly the opposite with 73% of bladder cancers diagnosed as TCC and 28% diagnosed as SCC28. Patients diagnosed in 2005 were six times as likely to be diagnosed with TCC compared to patients diagnosed in 198028. The decrease in SCC cases has been explained by declining S. haematobium infection in the past 30 years due to public health interventions and changes in the Nile river system29, 30. There has also been a replacement of S. haematobium with S. mansoni, which is largely attributed to the construction of the Aswan Higher Dam in the 1960’s. The prevalence of S. haematobium infection in residents of the Nile Delta region was estimated to be 74% in 1935, falling to 4% by 198330. With the declining prevalence of S. haematobium infection, the IR of SCC also declined25. While the decrease in SCC has been largely explained by changes in the Nile river water system, there have been few studies investigating the increase of TCC distribution in the Egyptian population. With increasing TCC incidence, additional risk factors for bladder cancer in Egypt need to be identified 31.
In order to explore possible etiologies for bladder cancer in Egypt, this study describes the geographical patterns of bladder cancer in Egypt’s Gharbiah Province. Gharbiah Province was selected for this study because it has the only population-based registry in Egypt. Additionally, the Gharbiah Province is located in Nile Delta region and contains mixed land use (industrial and agriculture), which allowed us to examine eight heterogeneous administrative districts in this region.
Materials and methods
Study Population
The study population included all residents of Gharbiah Province who were diagnosed with primary bladder cancer tumors during 1999 through 2002 and recorded in the Gharbiah Population-based Cancer Registry. Age at diagnosis, race, community of residence, smoking status, tumor grade, stage, morphology, and basis of diagnosis were abstracted from routinely collected registry data. The study was approved by the University of Michigan Institutional Review Board and the Gharbiah Cancer Center Ethics Committee.
Study Region
Gharbiah Province is an administrative region centered ~90 kilometers north of Cairo in the Nile delta region. The Province contains eight administrative Districts, with one main city serving as the capital of each District. Gharbiah Province is home to 3.4 million people, with a population density of 1,752/km2 (51% male; 49% female). Approximately 30% of the population resides in urban areas and 48% of the population is <20 years old 32. The predominant occupation in the Province involves agriculture, but pesticide manufacturing in Kafr El Zayat City (west) and textile production in El Mehalla City (east) are also important occupations and industries in Gharbiah (Figure I). It is important to note that the pesticide and textile factories in Gharbiah are among the few largest and oldest factories of each respective industry in Egypt.
Figure I.
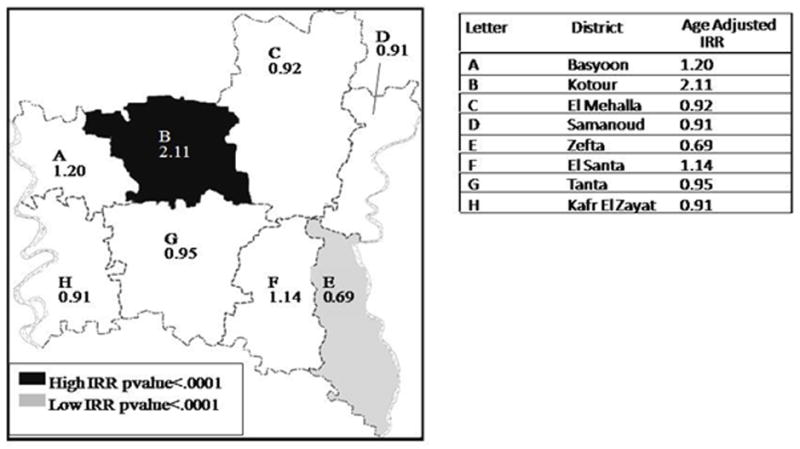
Age-Adjusted Incidence Rate Ratios of Bladder Cancer located by District in Gharbiah Province, 1999–2002.
Gharbiah Cancer Registry
The Gharbiah Cancer Registry was founded in 1998 and is located in Tanta, capital of Gharbiah Province. Case information is actively collected from university hospitals, private clinics and cancer centers located throughout Gharbiah and its surrounding regions, including cancer clinics in Cairo. Staff members of the Gharbiah Cancer Registry have been well trained and activities are periodically monitored by Emory University’s Rollins School of Public Health and the International Agency for Research on Cancer (IARC). Because of the completeness of the data and its accuracy, the Gharbiah registry was included in the NCI’s monograph on cancer in the Middle East 27 and the IARC’s publication of Cancer Incidence in 5 Continents volume IX in 200726. The registry is funded, in part, by the National Cancer Institute, Bethesda, Maryland through the Middle East Cancer Consortium (MECC).
Census Data
Census data were obtained from the Central Agency for Public Mobilization and Statistics (CAPMAS) for the 1996 census, the most recent data available. Gender-specific CAPMAS population projections for the years 2001 and 2005 for the eight Districts were also obtained. We applied these projections to estimate annual population using linear regression of the 1996 and 2001 data to predict the study population from 1999 to 2001, and separately using the 2001 and 2005 to estimate the 2002 population. This provided District-specific annual gender specific population estimates throughout the study period. The gender-specific projections of the eight Districts were applied to communities within that District to determine community populations.
Data Reduction
Regions were defined by community identification numbers and then grouped into eight administrative Districts. Small communities with <1,000 residents are extensions of other larger communities and are termed “ezbets”. These ezbets had their own code in the cancer registry, but not in the CAPMAS census. For the 0.17% of cases where this occurred, the community of residence was coded as the nearest community. Residents of each District’s capital city were considered urban, while those living in any other community were considered rural (CAPMAS coding of urban/rural). Sixteen age categories (0–4, 5–9, 10–14, …, 65–69, 70–74, and ≥75 years) were created to correspond with the CAPMAS census categories for calculating age-standardized incidence rates. Tumor morphologies were divided into four categories, squamous cell carcinoma, transitional cell carcinoma, adenocarcinoma, and other carcinomas (eg: leiomyosarcoma). The International Classification of Diseases version 9 (ICD9) coding system of the World Health Organization (WHO) for cancer types was used to determine types of cancer (WHO, 1994) 33. Cases with missing histology information were coded as unknown33. Diagnoses were divided into three categories: microscopic, non- microscopic, and death certificate diagnosis. Microscopic diagnosis included histology-based, cytology-based diagnoses, while non-microscopic diagnoses were made through surgical or autopsy procedures. Smoking status was only available for ~22% of cases and was not evenly distributed throughout the study sample so was not included in the analyses.
Statistical Analysis
To address possible differences in age structure among the Districts and communities, age-adjusted, gender-specific incidence rates were calculated and assessed for deviations. Chi square tests were carried out to assess if missing information varied by district and by gender. Additionally, Chi-square tests were conducted to determine if bladder cancer rates varied throughout the study period.
Descriptive statistics and annual crude and age-adjusted incidence rates were calculated for each of the eight Districts and the 316 communities within those Districts (SAS Ver. 9; SAS Institute, Cary, NC). Crude incidence rates were calculated using the number of cases per year (1999 through 2002) divided by the person-year estimates for 1999, 2000, 2001 and 2002. Age-adjusted IRs were calculated by direct age-standardization for each District and for each community by gender for 1999 through 2002. Gender specific and total IRRs were calculated through Poisson regression analysis using the GENMOD procedure in SAS for total bladder cancer cases and by specific bladder cancer tumor type (TCC, SCC, Adenocarcinoma and other). District-specific age-adjusted IRRs were calculated using the IR of that District relative to the seven other District IRs.
Results
The Districts of Tanta and El Mehalla contributed the most cases, representing ~25% each (Table I). Approximately 80% of cases were male (male: female ratio of 4.2). The average age and standard deviation of males (60.9 ±10.1 years) was similar to that of females 60.0 (±11.8 years). Over 97% of cases were Muslim and all were identified as Arabic, thus these two factors were not considered further.
TABLE I.
Number and percent of bladder cancer cases in Gharbiah Province, Egypt, 1999–2002 by sex, age, urban/rural, District of residence, smoking status, tumor type, year of diagnosis and basis of diagnosis.
| Factors | Number | % |
|---|---|---|
| Sex | ||
| Male | 977 | 80.81 |
| Female | 232 | 19.19 |
| Age | ||
| 0–49 | 179 | 14.81 |
| 50–59 | 341 | 28.21 |
| 60–69 | 432 | 35.73 |
| 70+ | 257 | 21.26 |
| Urbanization | ||
| Urban | 607 | 50.21 |
| Rural | 602 | 49.79 |
| District of Residence | ||
| Tanta | 325 | 26.88 |
| El Mehalla | 282 | 23.33 |
| Kafr El Zayat | 107 | 8.85 |
| Zefta | 92 | 7.61 |
| Samanoud | 77 | 6.37 |
| El Santa | 118 | 9.76 |
| Kotour | 121 | 10.01 |
| Basyoon | 87 | 7.20 |
| Smoking Status1 | ||
| Ever Smoker | 97 | 29.85 |
| Never Smoker | 228 | 70.15 |
| Tumor Type2 | ||
| Squamous Cell Carcinoma | 256 | 24.71 |
| Transitional Cell Carcinoma | 716 | 69.11 |
| Adenocarcinoma | 47 | 4.54 |
| Other | 17 | 1.64 |
| Year of Diagnosis | ||
| 1999 | 291 | 24.07 |
| 2000 | 335 | 27.71 |
| 2001 | 287 | 23.74 |
| 2002 | 296 | 24.48 |
| Basis of Diagnosis | ||
| Microscopic | 1098 | 90.82 |
| Non-microscopic | 50 | 4.14 |
| Death Certificate Only | 61 | 5.05 |
Missing smoking information: 884 cases
Missing specific tumor morphology: 173 cases
Approximately 5% of cases in the study were identified through a death certificate, and most (~87%) were diagnosed microscopically through histology of the primary tumor. The annual incidence of bladder cancer was relatively stable throughout the four-year study period, with yearly differences not statically significant (χ2=4.87, p =0.18). Furthermore, the male:female ratio did not vary greatly among years (χ2= 1.94, p =0.59) nor did the type of bladder cancer (χ2= 11.11, p= 0.27). Specific tumor diagnosis information was missing from 173 (14%) of cases but did not vary across gender or district. The number of TCC and SCC cases among females was similar (TCC:SCC ratio of 1.13:1. However, there were relatively more TCC male cases compared to females cases (TCC:SCC ratio of 3.61:1).
Geographical Patterns
Table II presents gender-specific incidence rates (IR) for SCC, TCC and both by District. The total bladder cancer IR was the highest in Kotour District, which had more than twice the IR of bladder cancer compared to the rest of Gharbiah (Table II). Elevated IRs in Kotour District were evident for both males and females and for SCC and TCC tumor types. After adjusting for urban/rural status and age, the IRR among males and females of Kotour was significantly higher than the remaining Districts, with an IRR of 2.10 (95% CI 1.32, 3.29) and 2.78 (95% CI 2.20, 3.51), respectively (Table III). Kotour city (capital of Kotour District) also had the highest age-adjusted incidence rate among the 316 communities located in the Gharbiah Province (male age-adjusted IR = 134.30/100,000 PY and the total age-adjusted IR = 73.11/100,000 PY).
TABLE II.
Comparison of mean age-adjusted incidence rates (IR) of bladder cancer by gender and cancer type among male and female cases residing in the eight Districts of Gharbiah Province, Egypt 1999–2002
| Age-Adjusted Incidence Rates per 100,000 Person Years | ||||||
|---|---|---|---|---|---|---|
| TCC | SCC | Total1 | ||||
| Males | Females | Males | Females | Males | Females | |
| Tanta | 8.58 | 1.54 | 1.73 | 1.41 | 13.01 | 3.50 |
| El Mehalla | 7.91 | 1.30 | 2.13 | 0.77 | 12.88 | 3.27 |
| Kafr El Zayat | 8.59 | 1.09 | 2.39 | 0.72 | 13.30 | 2.35 |
| Zefta | 6.84 | 1.18 | 1.56 | 0.65 | 10.20 | 2.24 |
| Samound | 8.94 | 0.81 | 1.90 | 1.71 | 12.36 | 3.75 |
| El Santa | 9.26 | 0.93 | 3.41 | 1.38 | 16.27 | 2.81 |
| Kotour | 17.26 | 1.94 | 8.22 | 2.79 | 28.96 | 5.73 |
| Basyoon | 10.95 | 1.71 | 3.60 | 0.89 | 17.67 | 2.61 |
| TOTAL | 8.68 | 1.29 | 2.40 | 1.14 | 13.65 | 3.15 |
Total incidence rates include cases with a nonspecific bladder cancer diagnosis
TABLE III.
Age-adjusted incidence rate ratios (IRR) and 95% Confidence Intervals (95% CI) by District and urbanization among bladder cancer cases, Gharbiah Governate, Egypt, 1999–2002
| Age Adjusted IRR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| TCC | SCC | TOTAL1 | ||||
| Males | Females | Males | Females | Males | Females | |
| District | ||||||
| Tanta | 0.91 (0.75, 1.10) | 1.11 (0.69, 1.79) | 0.56 (0.38, 0.84) | 1.02 (0.61, 1.67) | 0.87 (0.74, 0.99) | 0.99 (0.73, 1.35) |
| El Mehalla | 0.85 (0.69, 1.04) | 0.88 (0.53, 1.48) | 0.95 (0.66, 1.36) | 0.65 (0.36, 1.16) | 0.89 (0.76, 1.05) | 0.95 (0.69, 1.30) |
| Kafr El Zayat | 1.04 (0.78, 1.38) | 1.03 (0.50, 2.13) | 0.86 (0.49, 1.52) | 0.67 (0.27, 1.65) | 0.99 (0.79, 1.25) | 0.85 (0.52, 1.40) |
| Zefta | 0.76 (0.59, 0.96) | 0.92 (0.44, 1.89) | 0.59 (0.31, 1.12) | 0.47 (0.17, 1.27) | 0.76 (0.59, 0.98) | 0.66 (0.39, 1.12) |
| Samanoud | 0.99 (0.70, 1.39) | 0.67 (0.24, 1.83) | 0.82 (0.42, 1.61) | 1.72 (0.86, 3.45) | 0.88 (0.66, 1.16) | 1.25 (0.78, 2.03) |
| El Santa | 1.02 (0.75, 1.38) | 0.67 (0.27, 1.66) | 1.47 (0.91, 2.38) | 1.36 (0.68, 2.73) | 1.16 (0.93, 1.46) | 0.93 (0.57, 1.53) |
| Kotour | 1.98 (1.47, 2.65) | 1.71 (0.79, 3.71) | 3.71 (2.44, 5.66) | 2.99 (1.58, 5.68) | 2.15 (1.72, 2.70) | 2.21 (1.42, 3.46) |
| Basyoon | 1.51 (0.95, 1.80) | 1.42 (0.65, 3.08) | 1.53 (0.88, 2.64) | 0.83 (0.30, 2.28) | 1.31 (1.02, 1.69) | 0.83 (0.30, 2.28) |
| Urbanization | ||||||
| Rural | Referent | --- | --- | --- | --- | --- |
| Urban | 2.08 (1.77, 2.44) | 1.57 (1.05, 2.36) | 1.34 (0.99, 1.81) | 0.93 (0.59, 1.46) | 1.89 (1.67, 2.15) | 1.44 (1.14, 1.86) |
Total incidence rates include cases with a nonspecific bladder cancer diagnosis
Table III presents total, SCC, and TCC gender specific incidence rate ratios (IRR) by District. The highest total IRR was among the Kotour District (IRR=2.15,95% CI 1.72, 2.70), followed by Basyoon (IRR=1.31 95% CI 1.02, 1.69). Zefta and Tanta Districts had the lowest total male IRR. The male IRR for SCC was highest in Kotour followed by Basyoon, while Tanta had the lowest IRR. Kotour also had the highest IRR of TCC and Zefta had the lowest IRR. Females also had a statistically significant IRR in Kotour compared to the other Districts. The tumor type-specific rates were higher among females in Kotour, but this was not statistically significant. The Districts of El Mehalla, Kafr El Zayat, Samanoud, El Santa and Basyoon had unremarkable IRRs when compared with overall TCC and SCC.
In Gharbiah Province, ~30% of the population was considered urban, yet about half of all cases resided in urban areas. The age-adjusted IRRs for urban versus reference rural dwellers for males and for females were 1.89 (95% CI 1.67, 2.15) and 1.44 (95% CI 1.14, 186), respectively (Table III). The IRR for TCC was higher in urban males and was also elevated in females, but was not statistically significant.
Discussion
The incidence of bladder cancers varied among the Districts of Gharbiah Province. In particular, Kotour District had much higher incidence of SCC and TCC compared with the other seven Districts. The elevated incidence of SCC in Kotour is largely unexplained. While there has been an overall decrease of S. haematobium infection in Gharbiah Province during the past few decades, it is unknown whether this declining prevalence was uniform across all Districts. The higher rate of TCC in Kotour is also enigmatic, but could be related to environmental and/or occupational exposures. Environmental exposures may vary within Gharbiah and could be important risk factors and/or promoters among people in Kotour. Elevated pesticide and heavy metal pollution in the Nile river are well recognized34, however, measurements of such environmental exposures have not yet been reported from Gharbiah. Kotour residents may also experience environmental exposures as it borders El Mehalla District where the largest textile factory in Egypt is located. Residents in Kotour may come into contact with these environmental exposures by working in textile factories and through contaminated soil and water from these factories. While exposure to tobacco smoke may explain an overall increase in TCC among Egypt13,16, this probably can not explain geographical differences in bladder cancer observed in our study, as tobacco use is believed to be similarly widespread among all Districts. These findings suggest the need for more detailed study of past exposures that might explain this unusually high incidence in this District.
A key finding of this study is an increased incidence of bladder cancer among urban populations in Gharbiah. This may reflect a growing importance of occupational and environmental exposures located in Gharbiah’s industrial centers. However, some of the increased incidence in urban areas could be due to under-reporting of bladder cancer in rural areas. Yet, there are health care clinics located throughout the Province and access to the health clinics is believed to be adequate, so most rural cases would have been reported to the registry. Despite the increased incidence of bladder cancer in Gharbiah’s urban areas, intense urbanization does not appear to be the driving force behind the geographical patterns in this Province. The two largest urban areas lie in the Districts of Tanta and El Mehalla, neither of which had higher IRRs compared to the rest of the Province. In Kotour, where bladder cancer rates were highest, the IR of bladder cancer was significantly higher than the remaining districts even after adjusting for urban status. Simply living in an urban area does not seem to be the predominant risk in Kotour.
Results from our study appear consistent with those from a previous studies on the histopathologic changes 28 and a systematic review of bladder cancer in Egypt25. Approximately four times as many males as females experienced this disease, a pattern similar to that found in previous studies conducted in Egypt25,28 and in other countries1. Approximately 94% of bladder cancers were typed as either TCC or SCC; with TCC representing roughly four times as many cases as SCC, which is similar to another recent study describing the changing pattern of bladder cancer etiology in Egypt28. Although we only analyzed four years of data, comparisons with previous finding clearly indicate that cases of TCC are rising as those of SCC decline28. It is believed that this transition from TCC and SCC is related to etiologic factors, however, a small portion of this transition may be attributed to changes in pathologic diagnosis of SCC. The diagnosis of squamous cell type of cancer is not unanimous among pathologists and the diagnosis of SCC in recent years has been reserved for pure cases of squamous cell carcinoma, which may influence the actual percentage of SCC in Egypt as well as in other areas. Additionally, there may be variation among pathologist that diagnoses cancers in our study in the diagnosis of bladder cancer of TCC/SCC. Five main laboratories report to the Gharbiah Cancer Registry and tumor slides are regularly taken to another laboratory for a second opinion; the registry compares data across the pathology laboratories for reliability. While we were unable to assess the inter-rater reliability of pathologist in this study, the consistency of our results with previous studies leads us to believe that histological diagnosis was accurate.
This study has manystrengths, one of which is the quality of population based registry from which this study sample was selected. Staff at the Gharbiah Cancer Registry are periodically trained and monitored by IARC and the SEER registries. Despite relatively recent establishment of the registry in 1998, the registry reports covering over 90% of the Gharbiah region27,36. Cases in this study were actively collected in clinics around Gharbiah, as well as cancer centers in neighboring Provinces and from cancer centers in Cairo. The quality of this registry strongly suggests that residents diagnosed with bladder cancer were included in our study. Furthermore, the vast majority of cases were histologically confirmed, providing further confidence in the case data. Additionally, the number of cases was consistent across the study period, which helps ensure overall data integrity.
Several factors could have confounded the patterns we observed. One potential confounder, not evaluated in our study, was exposure to tobacco smoke. Because smoking information was available for only ~20% of cases, we were not able to evaluate this possible risk. Information on smoking frequency and duration is not routinely collected in developing countries, because it is not related to patient care 37. However, it is unlikely that smoking rates would vary greatly across the Districts in Gharbiah due to the wide availability of tobacco products throughout the Province. Occupation and place of birth were not available for this study, which is unfortunate since such data could have helped to nuance possible interactions and confounding of exposure risks. We are unaware of previously reported studies of the relationship between occupational exposures and bladder cancer in Egypt, despite the recognized risk in Western Europe countries and the United States20. In our study, cases may have worked in a different District than their residence, reducing the value of residence in evaluating occupational exposure to carcinogens. Furthermore, information on place of birth could help interpret our findings, although another study utilizing liver cancer data from the Gharbiah Province reported that 89.5% of study participants were born in Gharbiah, indicating limited residential movement38.
Our study is the first to examine the geographic and demographic patterns of bladder cancer in this region. Relatively high incidence of SCC and TCC were found in Kotour and these rates remain largely unexplained. Conversely, other Districts had comparatively low incidence. Follow-up studies investigating the distribution of risk factors, particularly environmental risk factors, are needed to understand and reduce bladder cancer in the Egyptian population.
Acknowledgments
The authors would like to thank the Middle East Cancer Consortium (MECC) Registry staff at the Gharbiah Cancer Registry for their assistance in data extraction and Dr. Marie O’Neill and Elizabeth Lehman for her help in reviewing this manuscript. The authors would also like to thank the reviewers of this study. This study was supported by R25 CA112383-01A2-Cancer Epidemiology Education in Special Populations and the Global Health Program at the University of Michigan School of Public Health.
Abbreviations
- CI
Confidence Intervals
- IR
Incidence Rate
- IRR
Incidence Rate Ratios
- ICD9
International Classification of Diseases version 9
- PY
Person Years
- SCC
Squamous Cell Carcinoma
- TCC
Transitional Cell Carcinoma
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, Samuels A, Tiwan R, Ghafoor A, Feuer E, Thun M. Cancer statistics. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance Epidemiology End Results. Cancer Stat Facts Sheet. Available at http://seer.cancer.gov/statfacts/
- 4.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0. IARC Press; Lyon: 2004. [Google Scholar]
- 5.Lynch CF, Cohen MB. Urinary system. Cancer. 1995;75:316–29. doi: 10.1002/1097-0142(19950101)75:1+<316::aid-cncr2820751314>3.0.co;2-t. What journal or book? [DOI] [PubMed] [Google Scholar]
- 6.Golka K, Wiese A, Asseannato G, Bolt H. Occupational exposure and urological cancer. World J Urol. 2004;21:382–398. doi: 10.1007/s00345-003-0377-5. [DOI] [PubMed] [Google Scholar]
- 7.Morales K, Ryan L, Kuo T, Wu M, Chen C. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–662. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith A, Goycolea M, Haque R, Biggs M. Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;7:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 9.Chen GJ, Chuang YC, You SL, et al. A retrospective study on malignant neoplasms of bladder, lung, and liver in Blackfoot disease endemic area in Taiwan. Br J Cancer. 1986;53:399–405. doi: 10.1038/bjc.1986.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Center for Disease Control and Prevention. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 11.Nascimento C, Schnitz-Drager B, Zeegers M, Steineck G, Kogevinas M, Real F, Malats N. Epidemiol of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007;25:285–295. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 12.Pitard A, Brennan AP, Clavel J, Greiser E, Lopez-Abente G, Chang-Claude J, Wahrendorf J, Serra C, Kogeyinas M, Bofetta P. Cigar, pipe and cigarette smoking and bladder cancer risk in European men. Cancer Causes Control. 2001;12:551–556. doi: 10.1023/a:1011291015233. [DOI] [PubMed] [Google Scholar]
- 13.Alberg AJ, Kouzis A, Genkinger JM, Gallicchio L, Burke AE, Hoffman SC, Diener-West M, Helzlsouer KJ, Comstock GW. A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am J Epidemiol. 2007;15:660–6. doi: 10.1093/aje/kwk047. [DOI] [PubMed] [Google Scholar]
- 14.Samanic C, Kogevinas M, Dosemeci M, Malats N, Real F, Garcia-Closas M, Serra C, Carrato A, Garcia-Closas R, Sala M, Lloreta J, Tardon A, Rothman N, Silverman D. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006;15:1348–1354. doi: 10.1158/1055-9965.EPI-06-0021. [DOI] [PubMed] [Google Scholar]
- 15.Castelao J, Yuan J, Skipper P, Tannenbaum S, Gago-Dominguez M, Crowder S, Ross R, Yu M. Gender and smoking related bladder cancer risk. J Natl Cancer Inst. 2001;93:538–45. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- 16.Bedwani R, Khwsky F, Renganathan E, Braga C, Seif H, Azm T, Zaki A, Franceshi S, Boeffeta P, Vecchia C. Epidemiol of bladder cancer in Alexandria, Egypt: tobacco smoking. Int J Cancer. 1997;73:64–67. doi: 10.1002/(sici)1097-0215(19970926)73:1<64::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed M, Loffredo A, Israel E. Tobacco use in shisha: studies on waterpipe smoking in Egypt. Cairo, EG. World Health Organization Regional Office for the Eastern Mediterranean; 2006. [Google Scholar]
- 18.Knishkowy B, Amitai Y. Water-pipe (narghile) smoking: an emerging health risk behavior. Pediatrics. 2005;116:113–119. doi: 10.1542/peds.2004-2173. [DOI] [PubMed] [Google Scholar]
- 19.Talaska G. Aromatic amines and human urinary bladder cancer: exposure sources and epidemiology. J Environ Sci Health. 2003;21:29–43. doi: 10.1081/GNC-120021372. [DOI] [PubMed] [Google Scholar]
- 20.Vineis P, Pirastu R. Aromatic amines and cancer. Cancer Causes Control. 1997;8:343–355. doi: 10.1023/a:1018453104303. [DOI] [PubMed] [Google Scholar]
- 21.Band P, Le N, MacArthur A, Fang R, Gallagher R. Identification of Occupational Cancer Risks In British Columbia: A population-based case-control study of 1129 cases of bladder cancer. J Occup Environ Med. 2005;47:854–858. doi: 10.1097/01.jom.0000169094.77036.1d. [DOI] [PubMed] [Google Scholar]
- 22.Shokeir A. Squamous cell carcinoma of the bladder: pathology, diagnosis and treatment. BJU Int. 2004;93:216–220. doi: 10.1111/j.1464-410x.2004.04588.x. [DOI] [PubMed] [Google Scholar]
- 23.Mostafa M, Sheweita S, O’Connor P. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12:97–11. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer. Schistosomes, liver flukes, and Helicobacter Pylori. Vol. 61. IARC; Lyon: 1994. IARC monographs on the evaluation of the carcinogenic risk to humans. [PMC free article] [PubMed] [Google Scholar]
- 25.Khaled H. Systematic management of bladder cancer in Egypt: revisited. J Egypt Natl Canc Inst. 2005;17:127–131. [PubMed] [Google Scholar]
- 26.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P, editors. IARC Scientific Publications No. 160. IX. Lyon; IARC: 2007. Cancer Incidence in Five Continents. [Google Scholar]
- 27.Freedman LS, Edwards BK, Ries LAG, Young IL, editors. Cancer incidence in four member countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) compared with US SEER. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 28.Felix AS, Soliman AS, Khaled H, Zaghloul MS, Banerjee M, El-Baradie M, El-Kalawy A, Abd-Elsayed AA, Ismail K, Hablas A, Seifeldin I, Ramadan M, et al. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes Control. 2008;4:421–429. doi: 10.1007/s10552-007-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotb M, Al-Teheawy M, El-Setouhy M, Hussein H. Evaluation of a school-based health education model in schistosomiasis: a randomized community trial. East Mediterr Health. J4:265–275. [Google Scholar]
- 30.El Hawey A, Amr M, Abdel-Rahman A, El-Ibiary S, Agina A, Abdel-Hafez M, Waheeb A, Hussein M, Strickland T. The Epidemiology of schistosomiasis in Egypt: Gharbiah Province. Am J Trop Med Hyg. 2000;62:42–48. doi: 10.4269/ajtmh.2000.62.42. [DOI] [PubMed] [Google Scholar]
- 31.Helal T, Fadel M, El-Sayad N. Human Papilloma Virus and p53 expression in bladder cancer in Egypt: relationship to schistosomiasis and clinicalpathologic factors. Pathol Oncol Res. 2006;12:173–179. doi: 10.1007/BF02893365. [DOI] [PubMed] [Google Scholar]
- 32.Central Agency for Public Mobilization and Statistics. Statistical Year Book. Cairo, Egypt: Central Agency of Public Mobilization and Statistics; 2005. [Google Scholar]
- 33.World Health Organization. International disease classification 1994. Available at http://www.who.int/classifications/icd/en/
- 34.Wahaab R, Badawy M. Water Quality Assessment of the river Nile system: an overview. Biomed Environ Sci. 2004;17:87–100. [PubMed] [Google Scholar]
- 35.Dey S, Soliman AS, Hablas A, Seifeldin IA, Ismail K, Ramadan M, El-Hamzawy H, Wilson ML, Banerjee M, Boffetta P, Harford J, Merajver SD. Urban-rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast Cancer Res Treat. 2009 Jun 23; doi: 10.1007/s10549-009-0427-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim AS, Seif-Eldin IA, Ismail K. Cancer in Egypt, Gharbiah: triennial report of 2000- 2002, Gharbiah Population-based Cancer Registry. Cairo, Egypt: Middle East Cancer Consortium; 2003. [Google Scholar]
- 37.Zhu K, McKnight B, Stergachis A, Daling JR, Levin RS. Comparison of self-reported data and medical record data; results from a case-control study of prostate cancer. Int J Epidemiol. 1999;28:409–417. doi: 10.1093/ije/28.3.409. [DOI] [PubMed] [Google Scholar]
- 38.Lehman EM, Soliman AS, Ismail K, Hablas A, Seifeldin IA, Ramadan M, El-Hamzawy H, Shoushtari CS, Wilson ML. Patterns of hepatocellular carcinoma incidence in Egypt from a population-based cancer registry. Hepatol Res. 2008;5:465–73. doi: 10.1111/j.1872-034X.2007.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


