Abstract
Aims
To test the efficacy of two smoking cessation interventions in an HIV+ sample: standard care (SC) treatment plus nicotine replacement therapy (NRT) versus more intensive motivationally-enhanced (ME) treatment plus NRT.
Design
randomized controlled trial.
Setting
HIV+ smoker referrals from eight Immunology clinics in the Northeastern US.
Participants
444 participants enrolled in the study (mean age=42 years; 63% male; 52% European-American; mean cigarettes/day=22.8).
Interventions
SC received two brief sessions with a Health Educator. Those setting a quit date received self-help quitting materials and NRT. ME received four sessions of motivational counseling and a quit-day counseling call. All ME intervention materials were tailored to the needs of HIV+ individuals.
Measurements
Biochemically-verified 7-day abstinence rates at 2-month, 4-month, and 6-month follow-ups.
Findings
Intent-to-Treat (ITT) abstinence rates at 2-month, 4-month, and 6-month follow-ups were 12%, 9%, and 9% respectively in the ME condition, and 13%, 10%, and 10% respectively in the SC condition, indicating no between-group differences. Among 412 participants with treatment utilization data, 6-month ITT abstinence rates were positively associated with low nicotine dependence (p=0.02), high motivation to quit (p=0.04), and Hispanic-American race/ethnicity (p=0.02). Adjusting for these variables, each additional NRT contact improved the odds of smoking abstinence by a third (OR=1.32, 95% CI= 0.99–1.75).
Conclusions
Motivationally-enhanced treatment plus NRT did not improve cessation rates over and above standard care treatment plus NRT in this HIV+ sample of smokers. Providers offering brief support and encouraging use of nicotine replacement may be able to help HIV+ patients quit smoking.
Keywords: HIV, tobacco cessation, nicotine replacement, Transtheoretical Model
An estimated 1.2 million people in the United States are currently living with HIV/AIDS1. Advances in the treatment of HIV in the U.S. have reduced AIDS-related morbidity and mortality2, 3, making HIV a more medically manageable chronic illness and allowing for increased efforts to improve health behaviors and quality of life4–6. Smoking prevalence among HIV+ populations is high, with estimates of 47%-70%4, or more than two to three times the prevalence in the non-HIV-infected general population7. Indeed, HIV+ smokers have been found to smoke at higher rates and to be more nicotine dependent than demographically similar, non-HIV-infected smokers6. Smoking creates a well-known risk for conditions such as cancer, stroke, heart disease, and chronic obstructive pulmonary disease. It also poses additional threats to individuals living with HIV/AIDS, such as pulmonary-related complications (bronchitis, pneumonia, and asthma) 8–12. Despite the vast improvements made with introduction of antiretroviral therapy (ART), recent research suggests HIV+ individuals are at increased risk of developing cardiovascular disease13 and non-AIDS-related malignancies14, 15, in particular lung cancer. Indeed, control of these diseases is critical to management of HIV disease. Given the added risk of smoking in causing these diseases, implementation of effective smoking cessation into the clinic setting is needed.
Not only are racial/ethnic minority groups - in particular African-American and Hispanic populations - disproportionately affected by HIV16, 17, there are inequalities across minority groups in current smoking prevalence rates. American Indians/Alaska Natives having the highest smoking prevalence (33.4%), followed by European-Americans (22.2%), African-Americans (20.2%), Hispanic-Americans (15.0%) and Asian-Americans (11.3%)18. Reducing smoking prevalence among HIV+ patients is critical to increasing survival rates, as well as enhancing quality of life and disease management efforts19. Research suggests this population may face additional obstacles to quitting smoking, including co-occurring behavioral risk factors (e.g., illicit drug use), limited socioeconomic resources and diminished access to healthcare 5, 6, 20, 21.
Few studies have investigated the impact of tailoring smoking cessation programs to an HIV+ population. One pilot study found that HIV+ smokers who received Public Health Service (PHS)-guided intervention were more likely to quit than a self-help control group (63% vs 0%)22. Another pilot study found that HIV+ smokers (N=34) receiving nurse-delivered individual cessation counseling plus nicotine replacement reported greater 12-month abstinence rates than a non-randomized control group of smokers (38% vs 7%)23. A randomized controlled trial found that HIV+ participants (N=95) receiving a cellular telephone-delivered intervention were more likely to quit smoking at 3-month follow-up than those receiving standard care intervention (consisting of advice to quit smoking, tailored handouts for quitting smoking, and nicotine patches), although between-group differences failed to attain significance (7-day point-prevalence abstinence (PPA) 16.7% vs. 6.4%, p=0.28) 24. More recently, Ingersoll and colleagues randomized HIV+ smokers (N=40) to receive either self-guided reading plus nicotine patch or motivational interviewing plus nicotine patch. While no group differences were noted, 22% were abstinent at 3-month follow-up25. Taken together, these results suggest that HIV+ smokers are receptive to smoking intervention and can be successful at quitting, particularly when nicotine replacement is included.
Developing smoking cessation interventions tailored to the characteristics and needs of an HIV+ population is critical. The current study seeks to add to the few small-scale studies of cessation interventions for HIV+ smokers that have reported 3-month abstinence rates. We compare a standard care (SC) intervention to a motivationally-enhanced (ME) intensive treatment and report abstinence rates at 2-month, 4-month, and 6-month follow-up. We also explore racial/ethnic differences in smoking outcomes among this diverse sample of HIV+ patients.
Methods
Participants
Patients were eligible if they: (1) were HIV+, (2) were 18+ years of age, (3) were current, regular smokers (five cigarettes/day for the past three months), (4) spoke English or Spanish, and (5) agreed to be available over the next six months. Participants were not required to quit smoking or to use the nicotine patch. Participants were excluded if they: (1) suffered from any unstable medical condition precluding use of the nicotine patch (e.g., uncontrolled hypertension) or an active skin condition (e.g., psoriasis, eczema), (2) were currently using smokeless tobacco, nicotine replacement therapy (NRT) or other smoking cessation treatment, (3) were pregnant or nursing. Participants who met criteria were offered monetary compensation for intervention and follow-up visits. All materials and interventions were available in English and Spanish. The study protocol was approved by the institutional review boards of participating hospitals/clinics. Participants provided written informed consent.
Procedure
The study was performed at six outpatient HIV clinics and two primary care medical offices in Southeastern New England. Study physicians were trained to ask all patients about their smoking status and to provide brief cessation advice to smokers. Those patients who smoked, were deemed eligible to participate by their physician, and were willing to speak with a Health Educator (HE) were referred to the study.
After providing informed consent, patients were administered the baseline assessment via laptop computer. For those with reading difficulties, assessments were administered by study staff. Patients were then randomized (using block randomization to ensure stratification by gender and level of motivation to quit smoking, see Assessments below) into one of two treatment conditions: NRT + brief standard care intervention (SC) or NRT + more intensive motivationally-enhanced counseling intervention (ME). Participants in both intervention arms who were willing to set a quit date were provided 8-weeks of NRT (delivered via bi-weekly “patch pick-ups” scheduled between the participant and the HE). Follow-up assessments were administered at two, four, and six months following enrollment. Intervention sessions were administered by HEs trained in smoking cessation counseling. Sessions were tape recorded to evaluate treatment fidelity. Follow-up assessments were administered by research staff blinded to participant intervention assignment.
Standard Care Intervention
SC participants received two brief sessions, consisting largely of baseline assessments, randomization, and brief assessment of quitting plans. Those willing to set a target quit date (TQD) received NRT (i.e., nicotine patches) and self-help quitting materials26. Participants returned to the clinic bi-weekly for distribution of additional patches, allowing the HE to briefly (five minutes) reinforce quit efforts, check on patch side effects, and answer questions. In order to keep HEs supportive role to a minimum standard, HEs were instructed to provide praise of participant’s efforts and answer any questions asked, but not to initiate additional discussion of the quit effort. A typical participant receiving SC treatment who opted to use the full 8-week course of NRT participated in two brief intervention contacts and four NRT contacts with their HE. Those participants not willing to set a quit date were instructed to contact the HE when they were ready (anytime within the next six months). This brief treatment reflects the minimum standard of care recommended by the Agency for Health Research and Quality (AHRQ) panel convened to address smoking cessation treatment27.
Motivational Enhancement Intervention
In the ME intervention, smoking materials were tailored to the needs of HIV+ individuals (e.g., emphasis on improved immune functioning, prevention of infections based upon feedback provided in qualitative interviews (N=25) with HIV+ patients)28. Participants randomized to ME received four 30-minute intervention sessions, as well as a quit-day counseling call. Quit dates were determined by the individual participant with consultation with the HE, usually taking place between sessions 2 and 3. Motivational Interviewing (MI) elements were delivered throughout all intervention contacts, derived from the work of Miller and Rollnick29 and based on the acronym “FRAMES”: Feedback (HEs provided each participant with feedback following initial baseline assessment as well as carbon monoxide (CO) measurements at each session); Responsibility (HEs emphasized the participant’s personal responsibility for change, as well as discussions of the pros and cons of quitting smoking and the barriers to cessation); Advice (HEs offered clear advice on how to reduce and eliminate their smoking habit, as well as goal-setting to enhance motivation and preparedness to quit); Menu (HEs discussed a menu of strategies for aiding in quitting smoking, including ways to structure the social and physical environments in order to maximize success); Empathy (HEs provided feedback in an empathic manner); and Self-efficacy (HEs were trained to reinforce participants beliefs in the ability to make positive changes). Nicotine patches were distributed to interested participants on a bi-weekly schedule for the duration of 8-week use (patch pick-ups). Participants not willing to set a quit date were engaged in discussion of “quitting as a process” and the barriers to quitting.
Assessments
Assessment measures have been previously described6 and are briefly summarized herein. Socio-demographic information was obtained from participants on age, sex, sexual orientation, marital status, race/ethnicity, language, employment, years of education, and living situation. All participants that self-identified as Hispanic were treated as such, irrespective of race. Remaining participants were classified according to their racial origin, with participants of Asian, Native American or Mixed descent coded as “Other” due to small cell sizes.
Smoking status and characteristics
Self-reports of smoking status were obtained at baseline and follow-up appointments. Outcome analyses were based on 7-day PPA. Those participants reporting any amount of smoking in the past seven days were considered smokers. Participants reporting 7-day abstinence were verified with a 24-hour biochemical measure of abstinence via CO < 10 ppm, using CMD/CO Carbon Monoxide Monitors (Spirometrics, Inc., Auburn, ME). In those cases where participant self-report was discrepant with CO measure (misreporting rates ranged from 19.6% to 23.2% across the three follow-up points), participants were considered smokers. The Fagerstrom Test for Nicotine Dependence (FTND)30, a reliable and well-validated six-item measure of nicotine dependence, was used to classify dependence rated on a 10-point scale, with higher scores indicating greater levels of nicotine dependence. The FTND was used in analyses as the primary outcome of interest. The Readiness to Quit Ladder was used to assess motivation to quit smoking along ten “rungs”31, with higher scores suggesting a greater willingness to quit smoking.
Other substance use
Past 30-day alcohol and other substance use was assessed using a 30-day Timeline Follow Back (TLFB) 38, a calendar-based form in which participants provide retrospective estimates of the number of days out of the past 30 in which alcohol or other drugs were used.
NRT-related and counseling treatment contacts
HEs were trained to document the duration and nature of each patient care activity, including preparation of session materials, counseling intervention contact with patient, and NRT-related contact (e.g., patch pick-ups). Importantly, NRT-related contacts were tracked separately from counseling intervention contacts. Treatment utilization measures included: number of counseling intervention contacts, number of NRT-related contacts, minutes of counseling contacts, and minutes of NRT-related contacts.
Process Evaluation and Treatment Fidelity
We monitored the delivery of both intervention conditions by: (1) audio-taped supervision on a random sub-sample of counseling sessions; (2) patient exit interviews (conducted by the intervention-blinded research assistant); and (3) documentation of time spent in each intervention. To examine fidelity to each intervention protocol, two independent raters reviewed audiotapes of 20% of all sessions and rated a) the degree to which intervention providers of the ME intervention adhered faithfully to the spirit of motivational interviewing (i.e. establish rapport, express empathy, reflective listening, explore ambivalence)29, and b) the degree to which there was contamination across conditions. Results revealed that ME content delivered to those in the ME condition was appropriate, and that it significantly exceeded that delivered to SC participants (p<0.001). Reliability between raters was high, with intra-class correlations of 0.78 and 0.85 for sessions 1 and 2 (sessions with the lowest attrition rates).
Data Analysis Plan
Results presented in this manuscript consist of analysis of 2-month, 4-month, and 6-month assessments, presented separately by follow-up. Frequencies and descriptive statistics in Table 1 summarize baseline socio-demographic and smoking-related characteristics of study participants. These are supplemented by univariate analyses examining differences by treatment condition that might have confounded the relationship between motivational enhancement and smoking outcome; significance levels are calculated using Chi-square tests for categorical variables and 2-sample t-tests for continuous variables. Table 2 examines cross-sectional treatment effects on 7-day PPA rates defined using both Available Case (Quit/Interviewed) and Intent-to-Treat (ITT; Quit/Eligible) analyses. Table 3 examines the cross-sectional effects of race/ethnicity on smoking outcome. Longitudinal effects of treatment, race/ethnicity and their interaction on ITT quit rates are depicted graphically in Figure 1.
Table 1.
Demographic, smoking, and other substance use characteristics of study participants at baseline (N=444).
| Variable | N | Overall | ME | SC | p-val | |
|---|---|---|---|---|---|---|
| Age | 440 | 42.07 (7.68) | 41.21 (7.28) | 42.87 (7.96) | 0.02 | |
| Gender | 0.05 | |||||
| Male | 281 | 63.28% | 67.67% | 58.49% | ||
| Female | 163 | 36.71% | 32.32% | 41.50% | ||
| Race/Ethnicity | 0.98 | |||||
| European-American | 230 | 51.80% | 51.72% | 51.88% | ||
| Hispanic-American | 72 | 16.21% | 15.08% | 17.45% | ||
| African-American | 82 | 18.46% | 18.96% | 17.92% | ||
| Native-American | 20 | 4.50% | 4.74% | 4.24% | ||
| Cape Verdean | 22 | 4.95% | 5.17% | 4.71% | ||
| Other | 18 | 4.05% | 4.30% | 3.76% | ||
| Marital Status | 0.47 | |||||
| Single | 206 | 46.39% | 43.10% | 50.00% | ||
| Living with Significant Other | 52 | 11.71% | 12.93% | 10.37% | ||
| Married | 49 | 11.03% | 10.34% | 11.79% | ||
| Divorced | 113 | 25.45% | 27.15% | 23.58% | ||
| Widowed | 24 | 5.40% | 6.46% | 4.24% | ||
| Employment | 0.71 | |||||
| Yes - Full | 60 | 13.51% | 13.79% | 13.27% | ||
| Yes - Part | 34 | 7.66% | 8.62% | 6.64% | ||
| No | 349 | 78.60% | 77.59% | 80.09% | ||
| Education | 0.08 | |||||
| Grades 1–8 | 43 | 9.68% | 6.46% | 13.20% | ||
| Grades 9 – 11 | 118 | 26.57% | 25.43% | 27.83% | ||
| High School or GED | 147 | 33.10% | 35.34% | 30.66% | ||
| Tech/Business School | 18 | 4.05% | 5.17% | 2.83% | ||
| Some College | 76 | 17.11% | 19.39% | 14.62% | ||
| College Graduate | 42 | 9.45% | 8.18% | 10.84% | ||
| Lifetime Patch Use | 0.90 | |||||
| Yes | 300 | 67.87% | 67.39% | 68.39% | ||
| No | 142 | 32.13% | 32.61% | 31.60% | ||
| Ever Quit > 1 Year | 0.58 | |||||
| Yes | 89 | 20.14% | 19.13% | 21.23% | ||
| No | 354 | 79.86% | 80.87% | 78.77% | ||
| Motivation to Quit | 0.99 | |||||
| Low (Ladder 1–4) | 88 | 19.81% | 19.82% | 19.81% | ||
| High (Ladder 5–8) | 356 | 80.18% | 80.17% | 80.18% | ||
| Cigarettes per Day | 390 | 18.27 (9.81) | 18.34 (9.84) | 18.20 (9.79) | 0.89 | |
| Fagerstrom (FTND) | 442 | 5.91 (2.33) | 5.91 (2.34) | 5.92 (2.33) | 0.97 | |
| Alcohol Use in Past 30 Days | 390 | 3.16 (7.04) | 3.16 (6.99) | 3.16 (7.11) | 0.99 | |
| Marijuana Use in Past 30 Days | 390 | 2.60 (7.39) | 2.52 (7.34) | 2.68 (7.46) | 0.83 | |
| Other Substance Use in Past 30 Days | 390 | 0.85 (3.69) | 0.86 (3.69) | 0.84 (3.69) | 0.94 | |
Notes: Mean (SD) for continuous variable; percentages for categorical variables. Sample size column (N) refers to available subjects for continuous variables, and individual cell counts for categorical variables.
Table 2.
Abstinence rates (percent) using Available-Case (AC) and Intent-to-Treat (ITT) Analyses: 2-month, 4-month, and 6-month differences by treatment arm.
| Time | |||||
|---|---|---|---|---|---|
| 2m | 4m | 6m | |||
| AC Abstinence Rates | |||||
| All | 24 | 15 | 13 | ||
| ME | 24 | 15 | 12 | ||
| SE | 24 | 16 | 14 | ||
| p-val | 0.91 | 0.96 | 0.78 | ||
| ITT Abstinence Rates | |||||
| All | 12 | 10 | 9 | ||
| ME | 12 | 9 | 9 | ||
| SE | 13 | 10 | 10 | ||
| p-val | 0.72 | 0.76 | 0.76 | ||
Note: ME = Motivationally-Enhanced Treatment; SC = Standard Care Treatment. Abstinence based on 24-hour biochemical verification of self-reported 7-day quit status. Column p-values test between-condition differences at each time point.
Table 3.
Smoking abstinence rates (percent) by race/ethnicity using Available-Case (AC) and Intent-to-Treat (ITT) Analyses: 2-month, 4-month, and 6-month differences by race/ethnicity.
| Time | ||||
|---|---|---|---|---|
| 2m | 4m | 6m | ||
| Subjects Retained | ||||
| All | 229 | 274 | 318 | |
| European-American | 48 | 56 | 69 | |
| Hispanic -American | 56 | 62 | 76 | |
| African-American | 46 | 68 | 72 | |
| Other | 67 | 73 | 77 | |
| p-val | 0.05 | 0.05 | 0.47 | |
| AC Abstinence Rates | ||||
| All | 24 | 15 | 13 | |
| European-American | 25 | 16 | 11 | |
| Hispanic -American | 25 | 24 | 25 | |
| African-American | 24 | 12 | 7 | |
| Other | 20 | 11 | 11 | |
| p-val | 0.89 | 0.27 | 0.02 | |
| ITT Abstinence Rates | ||||
| All | 12 | 10 | 9 | |
| European-American | 12 | 9 | 8 | |
| Hispanic -American | 14 | 15 | 19 | |
| African-American | 11 | 9 | 5 | |
| Other | 13 | 8 | 8 | |
| p-val | 0.95 | 0.38 | 0.01 | |
Note: ME = Motivationally-Enhanced Treatment; SC = Standard Care Treatment. Abstinence based on 24-hour biochemical verification of self-reported 7-day quit status. Column p-values test race/ethnicity differences at each time point.
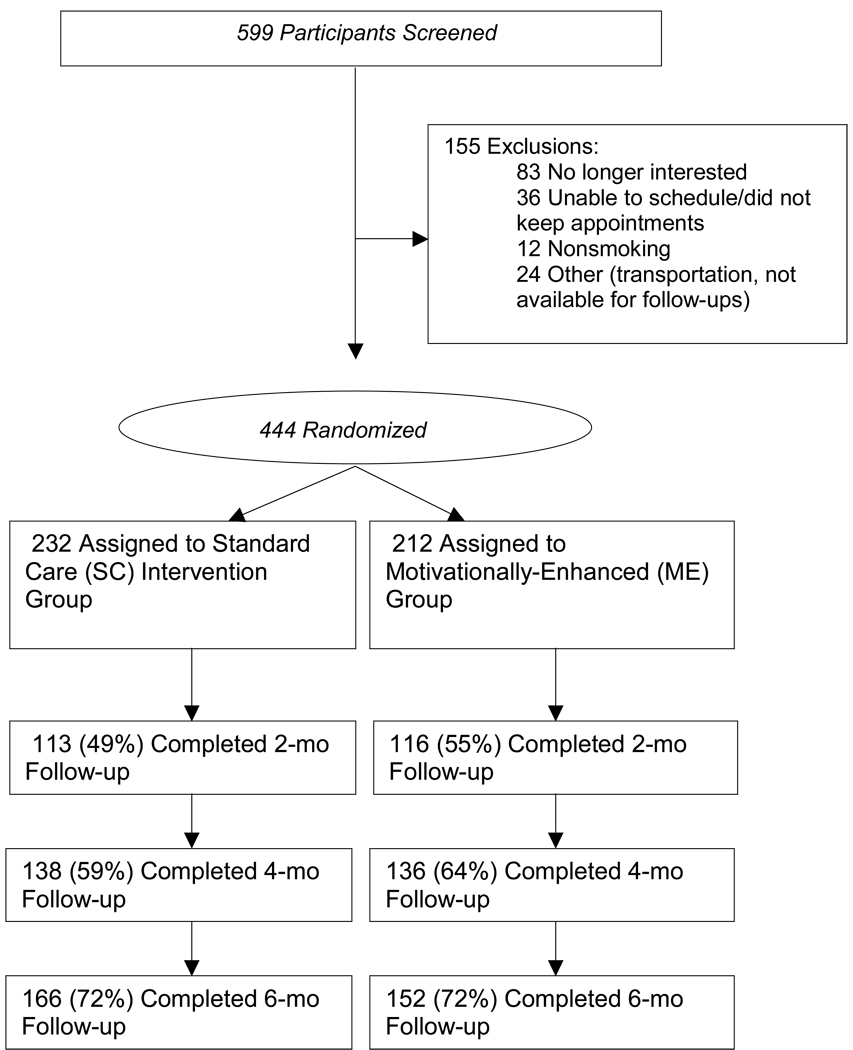
Figure 1.
Participant flow through the screening, randomization, and follow-up processes.
We sought to refine these analyses further in Table 4 by replacing the binary treatment indicator with continuous measures of treatment utilization, whose effects we estimated within a multivariate logistic regression setting. For these analyses, an inclusive approach to model building was implemented: all variables listed in Table 1 were initially entered as individual predictors in univariate logistic regression of between-condition differences in 7-day PPA, regardless of whether there was evidence of randomization imbalances at baseline or not. All variables that showed a significant relationship with outcome at a fairly liberal significance level of alpha=0.10, were subsequently entered in a multivariate logistic regression model, which was simplified using a backward elimination procedure.
Table 4.
Predictors of 6-month smoking abstinence among participants with treatment utilization data (N=412).
| Adjusted Odds Ratios | Value | LCL | UCL | p-val |
|---|---|---|---|---|
| Intercept | 0.10 | 0.06 | 0.17 | <0.001 |
| Motivation to Quit (Ladder < 5) | 0.12 | 0.02 | 0.87 | 0.04 |
| Fagerstrom Score (FTND) | 0.84 | 0.73 | 0.97 | 0.02 |
| Hispanic-American versus European-American | 2.53 | 1.13 | 5.67 | 0.02 |
| African-American versus European-American | 0.14 | 0.02 | 1.05 | 0.06 |
| Other versus European-American | 1.04 | 0.36 | 3.00 | 0.95 |
| NRT-related Contacts | 1.32 | 0.99 | 1.75 | 0.06 |
Note: LCL = 95% Lower Confidence Limit; UCL = 95% Upper Confidence Limit. Intercept denotes odds of smoking abstinence for European-Americans with baseline Ladder score <5, baseline FTND score of 5.91, and 2.31 NRT-related contacts over the 6-month study period.
Results
Summary of the screening, recruitment, and retention process is shown in Figure 1. In sum, 444 participants were enrolled and randomized: 232 to ME and 212 to SC. Of the baseline variables, the only one showing randomization imbalances was age (p<0.02), with ME participants slightly younger than SC participants (average age: 41.21 versus 42.87 years). As expected, significant differences emerged in their mean duration of intervention counseling contact (31 minutes in ME vs. 4 minutes in SC; p<0.001). The mean number of NRT-related contacts per patient did not differ significantly between the SC and ME conditions (2.20 for ME vs. 2.42 for SC; p=0.11).
Although 2-month follow-up data were collected from only 52% of participants (49% for ME, 55% for SC), participation rates rose to 62% at the four-month follow-up (59% for ME, 64% for SC), and to 72% at the six-month follow-up (72% for ME, 72% for SC). Study retention rates did not show statistically significant differences between treatment arms at any of the follow-ups.
Over the entire six-month follow-up period, 72% of initial study participants chose to use the patch at some point, with 80%-85% of those patch-users who were interviewed claiming use at each interview. At the 6-month follow-up, at which we were able to re-contact the largest number of initial study participants, patch use was documented for 60% of the sample. Patch use rates did not show between-group differences at any of the three follow-ups (p’s>0.60). Methods incorporated in this study assumed that those who reported use of the patch during the follow-up window had set a quit date during the study.
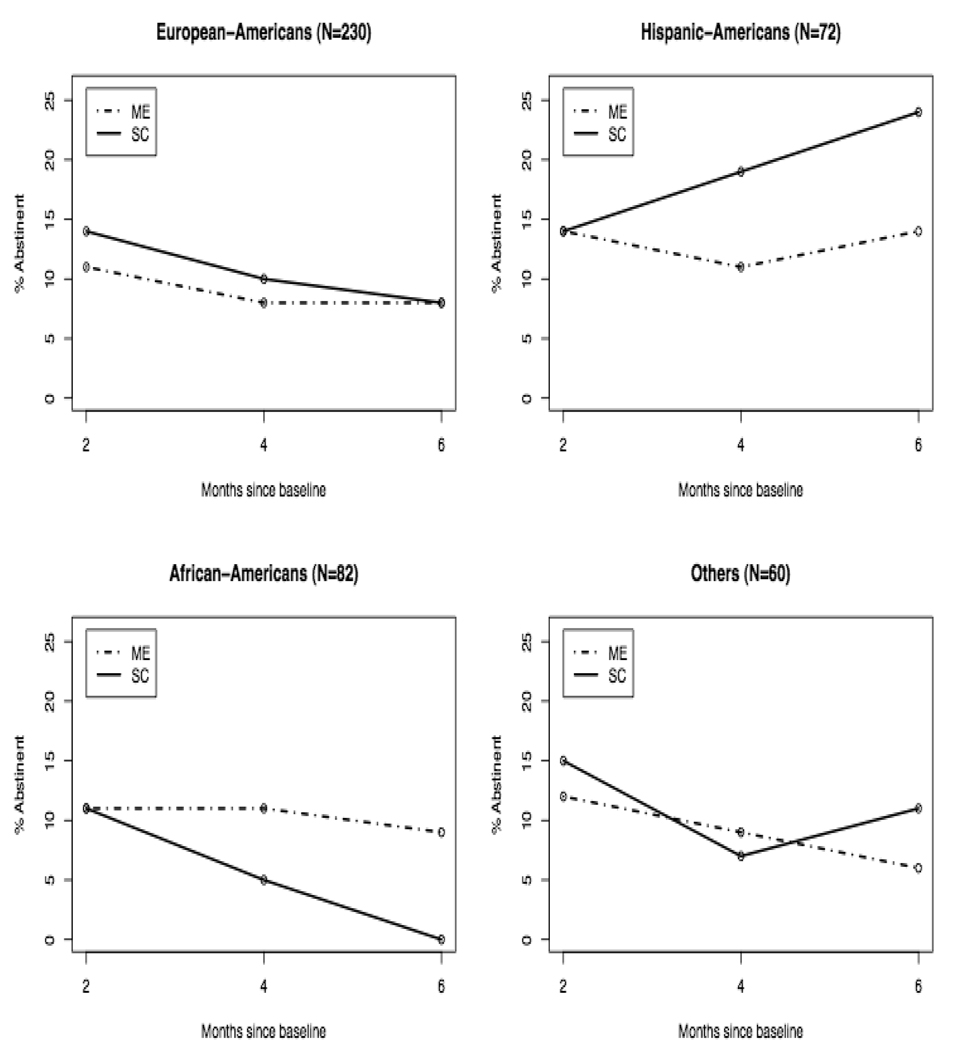
Self-reported 7-day PPA rates were verified with a 24-hour biochemical measure of abstinence. The biserial correlation between self-reported abstinence and CO levels was 0.46 overall (N=412), although the accuracy of self-report appeared to vary by racial/ethnic group (European-Americans: 0.41, African-Americans: 0.39, Hispanic-Americans: 0.79, Other: 0.75). When calculated under an Intent-To-Treat (ITT) criterion that treats participants not attending a particular follow-up visit as smokers, 7-day PPA rates at two-, four- and 6-month follow-ups were 12%, 9%, and 9% respectively, in the ME condition, and 13%, 10%, and 10% respectively, in the SC condition, indicating no between-group differences (all p-values > 0.70). Analyses based on available subjects alone led to the same conclusion. However, Table 3 reveals significant differences by race/ethnicity in the abstinence rates at the 6-month follow-up, whether calculated under an ITT criterion (p=0.01) or among available subjects alone (p=0.02): few African-Americans were able to quit smoking, whereas Hispanic-Americans quit at substantially higher rates than European-American and Other participants.
As demonstrated in Figure 2, all racial/ethnic groups responded similarly to the ME intervention, which was able to arrest the decline in abstinence rates across the three follow-up visits. However, within the SC condition, racial/ethnic differences primarily arose from the opposite manner in which African-American and Hispanic participants responded: African-American subjects receiving SC showed a precipitous decline in abstinence rates over time, in marked contrast to Hispanic participants, whose abstinence rates within the SC condition showed sharp increases from one follow-up visit to the next. However, a logistic regression model that included treatment and race/ethnicity main effects as well as their interaction at 6 months, showed that only the main effect of race/ethnicity achieved statistical significance (p=0.002), whereas the treatment main effect (p=0.54) and the treatment by race/ethnicity interaction (p=0.25) did not.
Figure 2.
Intent-to-Treat (ITT) quit rates over two-month, four-month, and six-month follow-up, by study condition and race/ethnic group.
Note: ME = Motivationally-Enhanced Treatment; SC = Standard Care Treatment.
These findings led us to examine which of the treatment components was of greater importance in determining smoking outcome (NRT-related contacts vs. counseling contacts), as well as the extent to which their addition to the model would help explain racial/ethnic differences in abstinence rates. To this end, we initially fit a multivariate logistic regression model for 6-month ITT abstinence rates that examined simultaneously the effects of baseline motivation to quit (Ladder score), baseline level of nicotine dependence (FTND score), race/ethnicity, NRT-related contacts, and counseling contacts. The effect of counseling contacts was not significantly related to smoking abstinence, whether one used number of contacts as a predictor (p=0.86) or total length of exposure to counseling (p=0.54). Therefore, we fit the reduced model shown in Table 4, in which treatment utilization was measured by NRT contacts alone.
In this model, the reference group is comprised of European-Americans with low baseline motivation to quit smoking (Ladder score < 5) and average baseline nicotine dependence level (FTND = 5.91) that had received 2.31 NRT-related contacts by the time of their 6-month follow-up. For such participants, the odds of abstinence were estimated at 0.10 (95% CI: 0.06–0.17), corresponding to abstinence rates of 9% (95% CI: 6%-15%). Elevated nicotine dependence levels were negatively associated with smoking abstinence (p=0.02), lowering the odds of smoking abstinence by a sixth for every unit increase in the patient’s FTND score above the sample mean of 5.91 (OR= 0.84, 95% CI = 0.73–0.97). A similar association was observed between low baseline motivation to quit smoking and smoking abstinence (p=0.04), whose odds appeared to decrease eightfold among subjects with Ladder scores below 5 (OR=0.12, 95% CI= 0.02–0.87). On the contrary, increased treatment utilization showed a borderline significant positive association with smoking abstinence (p=0.06), whose odds were increased by about a third per additional NRT contact (AOR=1.32, 95% CI= 0.99–1.75). Adjusting for all other variables in the model, overall racial/ethnic differences remained significant (p<0.001), with Hispanic-Americans showing improved odds of abstinence relative to European-Americans (OR=2.53, 95% CI=1.33–5.67), in contrast to the markedly lower odds for African-Americans (OR=0.14, 95% CI=0.02–1.05). However, these latter odds appeared to have been biased downwards by the fact that of only 4 African-Americans to report smoking abstinence at 6 months, 3 did not provide treatment utilization data and were excluded from these analyses (N=412). Such differential missingness was not evident for the remaining racial/ethnic groups.
Discussion
Our results demonstrate that HIV+ patients are able to quit smoking, although overall rates of cessation were low. ITT abstinence rates at two-, four- and six-month follow-ups were 12%, 9%, and 9%, respectively, in the ME condition, and 13%, 10%, and 10%, respectively, in the SC condition. This supports the contention that HIV+ patients are a difficult to treat population with regard to smoking cessation. Further, our findings appear consistent with a previous report in which HIV+ smokers randomized to receive MI plus NRT did no better than those receiving self-help materials plus NRT25.
Working with a primarily low-income, African-American HIV+ population, Vidrine et al.24 reported 7-day PPA rates at three-month follow-up of 16.7% in the cellular phone intervention condition and 6.4% in those receiving usual care under an ITT approach. Therefore, short-term cessation rates obtained via a cellular telephone-delivered intervention were comparable with both our SC and ME treatments. Whether Vidrine et al.’s 24 3-month outcomes would demonstrate considerable declines by 6-month follow-up is unknown. These differences aside, Vidrine and colleagues results support the notion that a directive intervention reaching patients in the community with brief and supportive tools may be more effective than a time-intensive office-based MI approach. Although both Vidrine et al.24 and the current study included physician advice, in that study the patch was offered by physicians, as compared to the current study, in which health educators provided patches and brief bi-weekly sessions to respond to questions and ensure patch adherence. These additional contacts may have improved patch compliance, thereby improving long-term abstinence in the SC condition.
We further report in this study that relative to the brief support offered within the SC condition, there was no additional benefit of the considerably more time-intensive ME condition in terms of improving abstinence, even when accounting for between-subject differences in length of exposure to HE counseling. We have also previously reported the cost-effectiveness of the two conditions39 in a sub-sample of participants in which the ME condition incurred higher personnel costs per client compared to SC, $265 vs. $130, respectively. Thus, the current set of analyses provide additional evidence that an approach focused on increasing NRT access and brief contacts supporting continued patch use may be more cost-effective in this population.
Notable among our findings were racial/ethnic differences in abstinence rates. African-Americans faired particularly poorly in cessation outcomes, partly due to the fact that none assigned to SC were able to quit smoking at six months. While our findings suggest that MI may have helped African-Americans with preventing smoking relapse, two recent smoking cessation trials conducted with low-income African-American populations failed to find support for MI40, 41. This inconsistency begs the question of whether there is something about MI as an adjunct to smoking cessation that makes it less or more helpful for certain populations. Perhaps the ME approach, which includes a fair amount of discussion about motivations and weighing the pros and cons of decisions, incurs too heavy of a cognitive processing burden on the participant. It appears plausible that certain population subgroups may respond better to a directive, advice-oriented counseling paradigm41.
Our findings suggest that Hispanic-Americans quit at substantially higher rates than all other racial/ethnic groups, with their overall six-month abstinence rates approaching 25% among study completers and remaining elevated at 19% under an ITT approach. Smoking cessation interventions targeting Latinos are few and have had limited success, with some of the barriers attributed to poor attendance at required multiple sessions42. Perhaps the extra time that these longer, more frequent sessions take leaves patients less enthusiastic to comply with the study protocol.
There are several limitations. First, retention rates at the 2-month and 4-month follow-up points were modest, while 6-month retention was good. This may have been due to the compensation structure of the study, with participants receiving more monetary compensation at the final follow-up. Second, participants were recruited from immunology clinics located in the Northeastern U.S. alone, not allowing for exploration of regional differences in smoking outcomes and the role of psychosocial contributors. Third, findings of racial/ethnic differences relied on small samples of a special population of HIV+ patients recruited in the Northeastern US, and require replication with general population samples at a national level.
Despite these limitations, this study provides evidence that HIV+ patients can quit smoking. It does not indicate conclusively which methods would be best for helping them to do so. However, the association between NRT use and improved smoking outcomes, even when baseline nicotine dependence and motivation to quit have been controlled for, suggests that NRT use should be encouraged. Further, the use of brief supportive counseling may be the most cost-effective approach for this population. Given the important role that smoking cessation plays in the management of infectious diseases, in particular HIV, healthcare providers who can offer counseling in the context of “the whole person” 10 will likely provide more effective cessation advice as well. Innovative culturally-targeted interventions are needed that can more effectively reach and connect to this vulnerable special population of ethnically diverse HIV+ smokers43.
Acknowledgements
This research was supported by grant R01-DA12344-06 from the National Institute of Drug Abuse (R. Niaura, PhD), grant K23-HL069987 from the National Heart, Lung, and Blood Institute (E. Lloyd-Richardson, PhD), grant K07-CA95623 from the National Cancer Institute (C. Stanton, PI), an NIH-funded Transdisciplinary Tobacco Use Research Center (TTURC) Award (P50 CA084719), an NIH-funded Lifespan/Tufts/Brown Center for AIDS Research Award (P30 AI42853), and by the Robert Wood Johnson Foundation.
References
- 1.CDC. HIV/AIDS Surveillance Report, Revised Edition. 2007 [Google Scholar]
- 2.UNAIDS, WHO. Geneva: Switzerland; 2008. North American, Western and Central Europe: AIDS epidemic update regional summary; pp. 1–16. Author. [Google Scholar]
- 3.UNAIDS. Report on the Global AIDS Epidemic (UNAIDS/08.27E/JC1511E) Geneva: Switzerland; 2008. Author. [Google Scholar]
- 4.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 5.Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7:511–522. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Betancourt RM, Stein M, Tashima K, et al. HIV-positive smokers considering quitting: Differences by race/ethnicity. Am J Health Behav. 2008;32:3–15. doi: 10.5555/ajhb.2008.32.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. Cigarette Smoking Among Adults--United States. MMWR Morb Mortal Wkly Rep. 2005;54:1121–1124. [PubMed] [Google Scholar]
- 8.Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006;43:90–98. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Talwar A, Reichert VC, Brady T, Jain M, Kaplan MH. Tobacco and HIV. Clin Occup Environ Med. 2006;5:193–207. doi: 10.1016/j.coem.2005.10.012. xi. [DOI] [PubMed] [Google Scholar]
- 11.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 12.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;20(21):1993–2003. doi: 10.1056/NEJMoa030218. 349. [DOI] [PubMed] [Google Scholar]
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg MJ, Neuhaus J, Bower M, Gey D, Hatzakis A, Henry K, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS. 2007;21:1957–1963. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 16.Dean HD, Steele CB, Satcher AJ, Nakashima AK. HIV/AIDS among minority races and ethnicities in the United States, 1999–2003. J Natl Med Assoc. 2005;97 5S-12S. [PMC free article] [PubMed] [Google Scholar]
- 17.McDavid K, Li J, Lee LM. Racial and ethnic disparities in HIV diagnoses for women in the United States. J Acquir Immune Defic Syndr. 2006;42:101–107. doi: 10.1097/01.qai.0000199353.11479.08. [DOI] [PubMed] [Google Scholar]
- 18.Fagan P, Moolchan ET, Lawrence D, Fernander A, Ponder PK. Identifying health disparities across thhe tobacco continuum. Addiction. 2007;102 Suppl. 2:5–29. doi: 10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 19.Gritz ER, Vidrine DJ, Fingeret MC. Smoking cessation a critical component of medical management in chronic disease populations. Am J Prev Med. 2007;33:S414–S422. doi: 10.1016/j.amepre.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Daza P, Cofta-Woerpel L, Mazas C, Fouladi RT, Cinciripini PM, Gritz ER, et al. Racial and ethnic differences in predictors of smoking cessation. Subst Use Misuse. 2006;41:317–339. doi: 10.1080/10826080500410884. [DOI] [PubMed] [Google Scholar]
- 21.Cummins D, Trotter G, Moussa M, Turham G. Smoking cessation for clients who are HIV-positive. Nurs Stand. 2005;20:41–47. doi: 10.7748/ns2005.11.20.12.41.c4016. [DOI] [PubMed] [Google Scholar]
- 22.Elzi L, Spoerl D, Voggensperger J, Nicca D, Simcock M, Bucher HC, et al. A smoking cessation programme in HIV-infected individuals: a pilot study. Antivir Ther. 2006;11:787–795. [PubMed] [Google Scholar]
- 23.Wewers MEN JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. Journal of the Association of Nurses AIDS Care. 2000;11:37–44. doi: 10.1016/S1055-3290(06)60353-1. [DOI] [PubMed] [Google Scholar]
- 24.Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. Aids. 2006;20:253–260. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]
- 25.Ingersoll KS, Cropsey KL, Heckman CJ. A Test of Motivational Plus Nicotine Replacement Interventions for HIV Positive Smokers. AIDS Behav. 2007 doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.USDHHS. Clearing the Air: Quit Smoking Today. No. 03-1647 vol. Bethesda, Maryland: National Institutes of Health/National Cancer Institute. 2003 [Google Scholar]
- 27.Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care. 2000;45:1196–1199. [PubMed] [Google Scholar]
- 28.Morrow K, Farrell NC, Lloyd EE, Niaura RS. Society for Behavioral Medicine. Nashville, TN: 2000. Barriers and benefits of smoking cessation among HIV-positive individuals: A qualitative investigation. [Google Scholar]
- 29.Miller WR, Rollnick S. Motivational Interviewing: Preparing people to change addictive behaviors. New York: Guilford Press; 1991. [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 32.Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. J Pers Soc Psychol. 1985;48:1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- 33.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15:271–2783. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- 34.Lee C. Perceptions of immunity to disease in adult smokers. J Behav Med. 1989;12:267–277. doi: 10.1007/BF00844871. [DOI] [PubMed] [Google Scholar]
- 35.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D Scale: A self-report depressive scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 37.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 38.Sobell LC, Sobell MB. Alcohol consumption measures. In: Assessing alcohol problems: A guide for clinicians and researchers. Allen JP, Columbs M, editors. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. pp. 55–73. [Google Scholar]
- 39.Shepard DS, Neighbors CJ, Lloyd-Richardson EE, Beaston-Blaakman A, Farrell N, Niaura R. Assessing costs and time of counseling services for HIV+ patients. XIV International AIDS Conference. Barcelona, Spain: Monduzzi Editore, International Proceedings Division; 2002. (paper C708s9823) [Google Scholar]
- 40.Okuyemi KS, James AS, Mayo MS, Nollen N, Catley D, Choi WS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav. 2007;34:43–54. doi: 10.1177/1090198106288046. [DOI] [PubMed] [Google Scholar]
- 41.Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction. 2006;101:883–891. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 42.Nevid JS, Javier RA. Preliminary investigation of a culturally specific smoking cessation intervention for Hispanic smokers. Am J Health Promot. 1997;11(3):198–207. doi: 10.4278/0890-1171-11.3.198. [DOI] [PubMed] [Google Scholar]
- 43.Moolchan ET, Fagan P, Fernander AF, Velicer WF, Hayward MD, King G, et al. Addressing tobacco-related health disparities. Addiction. 2007;102 Suppl 2:30–42. doi: 10.1111/j.1360-0443.2007.01953.x. [DOI] [PubMed] [Google Scholar]