Abstract
Background
Fatigue is highly prevalent and causes serious disruption in quality of life. Although the underlying biological mechanism is unknown, increases in inflammation have been implicated. This prospective study examined the association between C-reactive protein (CRP), a biomarker of systemic inflammation, and fatigue five years later.
Methods
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a population-based longitudinal study conducted in four US cities. Highly sensitive CRP concentration and fatigue were measured in 2983 African-American and white adults at both Year 15 (2000-2001, ages 33-45 years) and Year 20 examinations (2005-2006). Fatigue was assessed as a loss of subjective vitality using the Vitality Subscale of the 12-item Short Form Health Survey.
Results
Plasma CRP concentration at baseline (i.e. CARDIA Year 15) was a significant. predictor of fatigue level five years later (unadjusted β=0.126, P<0.001). After adjustment for potential confounders, this prospective association remained significant (adjusted β=0.044, P=0.033). Additionally, baseline CRP independently predicted fatigue in the subgroup of participants without medical comorbidity (adjusted β=0.051, P=0.039). Fatigue was associated with a persistent elevation of CRP at both examinations but not with a transient elevation of CRP at only one of the examinations.
Conclusions
This is the first study to demonstrate a prospective association between an inflammatory marker and fatigue in a general population. Furthermore, the association between low-grade systemic inflammation and fatigue seems primarily driven by a persistent immune activation and not explained by the presence or development of medical comorbidity.
Keywords: inflammation, C-reactive protein, fatigue, population-based prospective study
INTRODUCTION
Fatigue is a highly prevalent symptom with up to 38% of community-dwelling individuals suffering from this subjective sense of weariness, tiredness, lack of energy, and low vitality (1, 2). Fatigue is a comorbid symptom found across many major medical and psychiatric disorders - e.g. HIV/AIDS, cancer, multiple sclerosis, chronic fatigue syndrome, major depression, and schizophrenia - and causes serious disruption in quality of life (3-5). However, it also occurs independently, in otherwise healthy individuals, and can lead to disability and cost for society. US workers with fatigue cost employers $136.4 billion annually in lost productivity (6) - far higher compared to $61.2 billion for pain (7) and $44.0 billion for depression (8).
The underlying biological mechanisms that contribute to fatigue are largely unknown although recent basic research on neuroimmune interactions has suggested that inflammatory processes may play a role in fatigue through cytokine effects on the central nervous system. Indeed, animal studies have shown that peripheral immune activation and increases in proinflammatory cytokines - e.g. interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α - induce fatigue-like behaviors such as reduction in voluntary daily running distance (9, 10). Intracerebrally administered recombinant TNF has also been found to induce such behaviors in mice (9). Similarly, human studies have shown administration of IL-6 and interferon-α, also a proinflammatory cytokine, induces fatigue in healthy men (11) and patients with malignant melanoma (12, 13), respectively. However, these experimental strategies resulting in a highly robust and acute immune activation might not reproduce the effects of low-grade chronic inflammation which is thought to be responsible for many pathological processes (14).
Evidence regarding the role of low-grade systemic inflammation on fatigue is limited to a small number of cross-sectional or case-control studies conducted primarily in medical populations (15-28). This prior work has examined the association between fatigue severity and circulating levels of IL-1, IL-6, TNF-α, and C-reactive protein (CRP) but has yielded conflicting results represented by positive, null, and even negative correlations. Furthermore, the design of these studies does not address the direction of causality, and the generalizability of these data is constrained by sampling of patients with conditions such as cancer, multiple sclerosis, and chronic fatigue syndrome. Presence of severe medical comorbidity may either compound or obscure the associations between inflammation and fatigue, and small sample sizes have further contributed to conflicting results. No study, to our knowledge, has examined the association between markers of inflammation and fatigue in a large-scale community sample. Moreover, no data are available that have examined the prospective association between low-grade systemic inflammation and fatigue.
Using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, an ongoing community-based cohort study, we examined whether high levels of CRP, a biomarker of systemic inflammation, were associated with fatigue five years later, assessed as low vitality, in a general adult population. Furthermore, we examined whether high CRP levels predicted fatigue in the subgroup without medical comorbidity - i.e. no comorbid disorders such as cardiovascular diseases, diabetes, and hypothyroidism. Lastly, we assessed whether a persistent, as opposed to a transient, elevation of CRP was associated with fatigue. We hypothesized that plasma CRP levels would predict fatigue levels five years later in the entire sample as well as in the subgroup without medical comorbidity, and that this association would be most strongly driven by a persistent elevation of CRP.
METHODS AND MATERIALS
Subjects
The CARDIA study is a longitudinal study of cardiovascular risk factors in white and African-American men and women aged 18 to 30 years at study inception. Full details of the study design and methods have been published previously (29). Briefly, 5115 individuals were recruited from four US cities (Birmingham, Ala; Chicago, Ill; Minneapolis, Minn; and Oakland, Calif) to take part in the baseline clinical examination in 1985-1986. Of the 5115 individuals originally enrolled at Year 0, 3178 participated in both Year 15 and Year 20, two CARDIA examinations when both CRP and fatigue were measured. For the purpose of the current paper, Year 15 (2000-2001) was considered as the baseline and Year 20 (2005-2006) as the follow-up. After 195 subjects were excluded due to missing values of CRP or fatigue (n=183) and outlying values of CRP (n=12) at either baseline or follow-up, the remaining sample size was 2983.
Main Outcome Measure
Fatigue was measured as low vitality using the Vitality Subscale of the 12-item Short Form Health Survey (SF-12) (30). The SF-12 is a valid and reliable shorter version of the SF-36 with their subscales being highly correlated in clinical and general populations (30-33). The SF-12 Vitality Subscale consists of a single item (“Did you have a lot of energy?” referring to the past four weeks) on a six-point scale: All of the time, 0; Most of the time, 1; A good bit of the time, 2; Some of the time, 3; A little of the time, 4; and None of the time, 5. Higher scores on this scale reflect more severe fatigue. The SF-12 Vitality Subscale score was treated as a continuous variable given that ordinal variables with five or more categories are referred to as `quasi-interval' and can be treated as if they were continuous (34).
To ensure the suitability of using the SF-12 Vitality Subscale as the main outcome measure, we assessed its convergent validity using two data sets we had access to: the Whitehall II study, a prospective cohort study of British civil servants (35), and the Moving Beyond Cancer (MBC) study, a multisite randomized controlled trial of behavioral interventions (36). Both studies included the SF-36 and the latter also included the Fatigue Symptom Inventory (FSI) (37). Convergent validity was demonstrated by computing correlations between the individual energy item, “Did you have a lot of energy?”, and the full Vitality Subscale of the SF-36. High correlations were observed in 7888 healthy adults from the Whitehall II study (r=0.87; P<0.0001) and in 557 breast cancer patients from the MBC study (r=0.89; P<0.0001). The individual energy item, “Did you have a lot of energy?”, was also highly correlated with the FSI in the latter group (r=0.64; P<0.0001).
C-Reactive Protein
Highly sensitive CRP was measured using a BNII nephelometer (Dade Behring, Deerfield, Ill). Given the skewed distribution of CRP, the values were log transformed. Visual inspection of the distribution of log-transformed CRP identified 12 participants as having outlying values at either baseline or follow-up even after the transformation; consequently, these subjects were excluded from the analyses. Log-transformed CRP was used in all analyses as a continuous variable, except for graphic representation and the analysis of persistent CRP elevation. For the latter purposes, CRP values were categorized according to the Centers for Disease Control/American Heart Association recommendations (<1 mg/L, 1-3 mg/L, and >3 mg/L) (38).
Potential biobehavioral confounders
Smoking status, originally classified as never, former or current smoker, was recategorized as a binary variable (current smoker or not). A summary score of sleep quality was produced summing up five items of the sleep questionnaire, with higher summary scores reflecting poorer sleep quality: daytime sleepiness (individual score 0 or 1), sleep onset problem (0 or 1), sleep maintenance problem (0 or 1), early awakening (0 or 1) and subjective sleep quality (0 to 4). All the other variables were used in the original format without further reduction or modification. They were binary (sex, ethnicity [White and African-American] and regular use of aspirin) or continuous (age, years of education, body-mass index [BMI], mean systolic blood pressure, self-reported average daily alcohol consumption, physical activity level, depressive symptoms, and pain). Pain was measured using the Bodily Pain Subscale of SF-12 (score range 0-4), with higher scores reflecting more severe pain. Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D), a valid and reliable measure of depressive symptoms in the general population. Physical activity level was assessed with the CARDIA Physical Activity History Questionnaire, eliciting frequency of participation in a range of specific heavy and moderate intensity activities during the previous 12 months. Physical activity score was computed and expressed in `exercise units' (EU). For reference, 300 EU roughly approximates the American College of Sports Medicine recommendations for the amount of exercise needed to support weight loss (39).
Analysis
Baseline characteristics of 2983 participants were described in terms of frequency, mean or median by baseline levels of fatigue. Then the study hypotheses were tested by performing linear regression analyses with standardized regression coefficients (β), which facilitate comparison across models. The prospective associations between CRP and fatigue were examined in both directions: 1) baseline CRP predicting fatigue at follow-up; and 2) baseline fatigue predicting CRP at follow-up. Selection of covariates for multivariable analysis relied on external clinical judgment rather than predetermined P-value criteria; the latter approach, which selects factors for inclusion in a multivariable model only if the factors are `statistically significant' in bivariate screening, is considered less optimal (40). All covariates were assessed at baseline. Model 1 included sociodemographic variables (age, sex, ethnicity, and education). Model 2 further included biological measures and medication (BMI, systolic blood pressure, and regular aspirin use). Model 3 further included fatigue-related symptoms (depressive symptoms, sleep quality, and pain). Finally, Model 4 further included health-related behaviors (smoking, alcohol consumption, and physical activity). Age, sex, ethnicity, and education were tested for potential effect modification. To avoid any artifact due to different sample sizes between the nested models, participants missing the covariates were excluded.
Since depression is a construct closely linked to the presence of fatigue in humans, the prospective associations between CRP and depressive symptoms were also examined. For this purpose, all the analytical procedures described above were repeated simply exchanging fatigue with depressive symptoms.
To examine whether high CRP levels predicted fatigue in those participants without medical comorbidity, a subgroup analysis was conducted. Based on the participants' medical history and laboratory data at follow-up, they were divided into two subgroups: those with or without medical disorders that are usually comorbid with fatigue. Then, the aforementioned multivariable models (Models 1 to 4) were repeated for each subgroup. Given that CRP is an important risk marker for medical disorders comorbid with fatigue such as cardiovascular diseases, this subgroup analysis served to examine whether the prediction of fatigue at follow-up by baseline CRP was due to the presence or development of such medical disorders at follow-up. Presence of comorbid disorders was defined by any one of the following criteria: 1) lifetime history of chronic medical conditions comorbid with fatigue (e.g. coronary heart disease, diabetes, renal failure, hepatitis, cancer, hypothyroidism, inflammatory bowel diseases, and auto-immune diseases); 2) past year history of acute or subacute medical conditions comorbid with fatigue (e.g. asthma, pneumonia, and deep vein thrombosis); 3) fasting plasma glucose level at or above 126 mg/dL (as a criterion for diabetes mellitus); and 4) serum creatinine at or above 2.5 mg/dL (as a criterion for renal dysfunction).
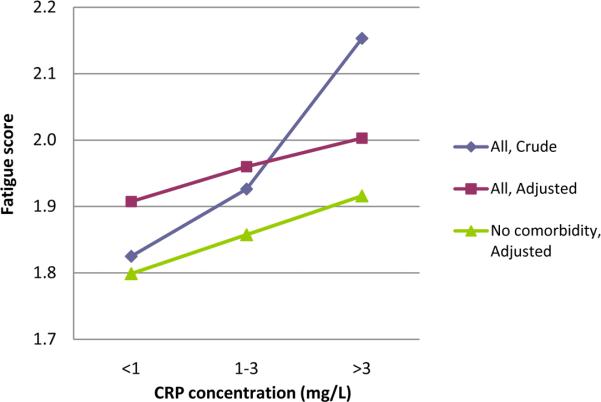
These prospective associations between CRP and fatigue were graphically represented using mean fatigue score at follow-up in each of the three CRP categories at baseline. Crude and fully adjusted means (adjusted using Model 4) in each category were represented as connected lines.
Lastly, to assess whether a persistent, as opposed to a transient, elevation of CRP was associated with fatigue, the participants were divided into four groups according to CRP levels at baseline and follow-up: group 1, the reference category with `low' levels (≤ mg/L) at both examinations; group 2, `high' (>3 mg/L) at baseline and `low' at follow-up; group 3, `low' at baseline and `high' at follow-up; and group 4, `high' at both examinations. Then, multivariable linear regression was performed with this new categorization as the independent dummy variable and fatigue at follow-up as the dependent variable.
RESULTS
Baseline Characteristics
Overall, mean fatigue score and median CRP concentration at baseline were 1.93 (standard deviation 1.15) and 1.36 mg/L (interquartile range 0.53-3.72), respectively (see Figure S1 in Supplement 1). Fatigue score of 1.93 indicates `a lot of energy during a good bit of the time'. Table 1 describes characteristics of the participants by levels of fatigue at baseline. A higher level of fatigue was associated with lower education level, higher BMI, more severe pain, lower physical activity level, higher depressive symptom level, poorer sleep quality, and higher CRP concentration. Those with a higher fatigue level were more likely to be female, currently smoking, and regularly using aspirin. The level of fatigue was not significantly associated with age, systolic blood pressure, or amount of daily alcohol consumption. Although those with low or intermediate fatigue level were more likely to be white and those with a high fatigue level to be African-American, no linear trend was observed. Furthermore, the mean fatigue score was similar in these two ethnic groups (1.92 vs. 1.95, P=0.48 by t-test).
Table 1.
Characteristics of 2983 participants by levels of fatigue at baseline
| Variable (range) | Level of fatigue* |
P for trend | ||
|---|---|---|---|---|
| Low (n = 1334) | Intermediate (n = 1345) | High (n = 304) | ||
| Age (33-45 years), mean (SD) | 40.2 (3.5) | 40.3 (3.6) | 40.4 (3.7) | 0.459 |
| Sex, % | ||||
| Male | 49.9 | 41.8 | 26.0 | <0.001 |
| Female | 50.1 | 58.2 | 74.0 | |
| Ethnicity, % | ||||
| White | 53.7 | 60.0 | 46.1 | 0.920§ |
| African-American | 46.3 | 40.0 | 53.9 | |
| Education (4-20 years), mean (SD) | 15.2 (2.6) | 15.0 (2.5) | 14.4 (2.5) | <0.001 |
| BMI (15.7-65.8 kg/m2), mean (SD) | 27.6 (6.0) | 29.1 (6.9) | 30.7 (7.9) | <0.001 |
| Daily alcohol consumption (0-564.9 mL), mean (SD) | 11.7 (29.7) | 10.2 (20.7) | 9.5 (24.2) | 0.088 |
| Current smoker, % | ||||
| No | 83.0 | 77.8 | 75.0 | <0.001 |
| Yes | 17.0 | 22.2 | 25.0 | |
| Regular use of aspirin, % | ||||
| No | 95.2 | 93.8 | 92.4 | 0.031 |
| Yes | 4.8 | 6.2 | 7.6 | |
| Pain score (0-4), mean (SD) | 0.33 (0.66) | 0.60 (0.88) | 1.24 (1.33) | <0.001 |
| Physical activity score (0-1818), mean (SD) | 423 (313) | 307 (240) | 223 (221) | <0.001 |
| CES-D score (0-54), mean (SD) | 5.9 (5.6) | 9.9 (7.1) | 17.3 (10.6) | <0.001 |
| Sleep quality score (0-8), mean (SD) | 1.7 (1.6) | 2.5 (1.6) | 3.6 (2.0) | <0.001 |
| CRP (0.15-35.6 mg/L), median | 1.15 | 1.47 | 2.16 | <0.001 |
BMI = body mass index; CES-D = Center for Epidemiological Studies Depression Scale; CRP = C-reactive protein; SD = standard deviation
For the purpose of the baseline description, fatigue levels were defined as follows: 1) low - a lot of energy `all of the time' or `most of the time; 2) intermediate - a lot of energy `a good bit of the time' or `some of the time'; 3) high - a lot of energy `a little of the time' or `none of the time'.
Note that there was a significant non-linear association between ethnicity and level of fatigue as assessed by chi-square test (P<0.001).
Cross-sectional Associations between CRP and Fatigue at Baseline
There was a significant positive cross-sectional association between CRP and fatigue at baseline (unadjusted β=0.154, P<0.001). The association remained significant after adjusting for age, sex, ethnicity, education, BMI, systolic blood pressure, regular aspirin use, depressive symptoms, sleep quality, pain, smoking, alcohol consumption, and physical activity (adjusted β=0.046, P=0.013).
Prospective Associations between CRP and Fatigue
Figure 1 and Table 2 describe the prospective association of baseline CRP with fatigue at follow-up. High CRP concentration at baseline predicted fatigue at follow-up five years later (unadjusted β=0.126, P<0.001). This association remained significant in the subsequent multivariable models, including the fully adjusted model (Model 4: adjusted β=0.044, P=0.033). CRP accounted for 0.9% of the variance in fatigue outcome. No effect modification was observed for age, sex, ethnicity, or education. Table 3 describes the prospective association in the opposite direction, baseline fatigue predicting CRP at follow-up. High fatigue level at baseline predicted CRP concentration at follow-up fiveyears later (unadjusted β=0.152, P<0.001). This association remained significant in the subsequent multivariable models, including the fully adjusted model (Model 4: adjusted β=0.053, P=0.006). Fatigue accounted for 0.7% of the variance in CRP outcome. No effect modification was observed for age, sex, ethnicity, or education.
Figure 1.
Crude and adjusted mean fatigue scores at follow-up according to the baseline level of C-reactive protein in the entire sample (N=2921) and in the subgroup with no medical comorbidity (N=2012). The mean score was adjusted for age, sex, ethnicity, education, BMI, systolic blood pressure, regular aspirin use, depressive symptoms, sleep quality, pain, smoking, alcohol consumption, and physical activity level.
Table 2.
Prospective association of baseline CRP with fatigue at follow-up (N=2921)
| Adjustment* | β | P | R2 | ΔR2 due to CRP | % ΔR2 due to CRP |
|---|---|---|---|---|---|
| Unadjusted model | 0.126 | <0.001 | 0.016 | 0.016 | 100% |
| Model 1: Sociodemographic variables | 0.113 | <0.001 | 0.038 | 0.012 | 32.1% |
| Model 2: Model 1 + Biomedical factors | 0.060 | 0.006 | 0.047 | 0.002 | 5.3% |
| Model 3: Model 2 + Fatigue-related symptoms | 0.055 | 0.008 | 0.137 | 0.002 | 1.5% |
| Model 4: Model 3 + Health-related behaviors | 0.044§ | 0.033 | 0.153 | 0.001 | 0.9% |
β = standardized regression coefficient expressing the change in standardized fatigue score per one standard deviation in CRP concentration; CRP = C-reactive protein; R2 = R-square of the model; ΔR2 = change in R-square; % ΔR2= percentage change in R-square
Sociodemographic variables include age, sex, ethnicity, and education. Biomedical factors include body-mass index, systolic blood pressure, and regular aspirin use. Fatigue-related symptoms include depressive symptoms, sleep quality, and pain. Health-related behaviors include smoking, alcohol consumption, and physical activity.
No effect modification was observed for age (P = 0.259), sex (P = 0.542), ethnicity (P = 0.779), or education (P = 0.987).
Table 3.
Prospective association of baseline fatigue with CRP at follow-up (N=2948)
| Adjustment* | β | P | R2 | ΔR2 due to fatigue | % ΔR2 due to fatigue |
|---|---|---|---|---|---|
| Unadjusted model | 0.152 | <0.001 | 0.023 | 0.023 | 100% |
| Model 1: Sociodemographic variables | 0.126 | <0.001 | 0.098 | 0.015 | 15.7% |
| Model 2: Model 1 + Biomedical factors | 0.054 | 0.001 | 0.277 | 0.003 | 1.0% |
| Model 3: Model 2 + Fatigue-related symptoms | 0.057 | 0.002 | 0.277 | 0.002 | 0.8% |
| Model 4: Model 3 + Health-related behaviors | 0.053§ | 0.006 | 0.281 | 0.002 | 0.7% |
β = standardized regression coefficient expressing the change in standardized fatigue score per one standard deviation in CRP concentration; CRP = C-reactive protein; R2 = R-square of the model; ΔR2 = change in R-square; % ΔR2= percentage change in R-square
Sociodemographic variables include age, sex, ethnicity, and education. Biomedical factors include body-mass index, systolic blood pressure, and regular aspirin use. Fatigue-related symptoms include depressive symptoms, sleep quality, and pain. Health-related behaviors include smoking, alcohol consumption, and physical activity.
No effect modification was observed for age (P = 0.401), sex (P = 0.484), ethnicity (P = 0.301), or education (P = 0.553).
Subsequently, we examined whether the following variables at follow-up mediate these independent associations between CRP and fatigue: BMI, depressive symptoms, sleep quality, pain, and physical activity. Sobel-Goodman mediation tests were performed examining each of these variables as a potential mediator of the associations between CRP and fatigue in both directions, maintaining the full set of baseline covariates (41). The only significant mediator was physical activity for the association between baseline fatigue and CRP at follow-up (10.2% of the total effect mediated, P=0.005). As described in the method section, the time frame of physical activity assessment was a period of 12 months prior to the follow-up visit, therefore preceding the measurement of CRP and meeting a necessary condition for mediation.
Finally, we evaluated the prospective associations between CRP and depressive symptoms. High CRP concentration at baseline predicted depressive symptoms at follow-up five years later (unadjusted β=0.086, P<0.001). However, the fully adjusted model indicated that the prospective association of baseline CRP with depressive symptoms at follow-up was entirely explained by the confounding effects of the covariates (Model 4: adjusted β=-0.006, P=0.776). Specifically, the most important confounders were sleep quality and fatigue, respectively accounting for 17.7% and 13.4% of the variance, compared to 0.01% accounted for by CRP. Similarly, although high levels of baseline depressive symptoms predicted CRP at follow-up (unadjusted β=0.106, P<0.001), the association was no longer significant after the full adjustment (Model 4: adjusted β=-0.010, P=0.605).
Prospective Association between CRP and Fatigue in the Subgroup without Medical Comorbidity
Of 2983 participants, 936 had comorbid medical disorders according to our criteria. CRP predicted fatigue only in the subgroup without comorbid disorders, and this prospective association was independent of age, sex, ethnicity, education, BMI, systolic blood pressure, regular aspirin use, depressive symptoms, sleep quality, pain, smoking, alcohol consumption, and physical activity (adjusted β=0.051, P=0.039). CRP accounted for 1.3% of the variance in fatigue outcome.
Persistent Elevation of CRP and Fatigue
According to the categorization described in the method section using 3 mg/L as the cutoff for low vs. high CRP, there were 1871 participants in group 1 (low CRP levels at both examinations), 354 in group 2 (high at baseline and low at follow-up), 219 in group 3 (low at baseline and high at follow-up), and 539 in group 4 (high at both examinations). Overall, a significant linear trend was observed with increasingly higher levels of fatigue at follow-up moving from one group to another, and this trend was independent of the aforementioned covariates (adjusted β=0.043, P=0.032) (see Table S1 in Supplement 1). Pair-wise comparisons revealed that group 4 had significantly higher fatigue levels at follow-up as compared to group 1 (adjusted β=0.042, P=0.041). However, neither group 3 nor group 2 had significant difference from group 1 (respectively, adjusted β=0.018, P=0.312; and adjusted β=0.007, P=0.703). In summary, fatigue was significantly associated with a persistent elevation of CRP but not with a transient elevation of CRP.
Additionally, to make the most of the continuous nature of CRP measure in showing the importance of persistent elevation, we performed another analysis using a newly generated variable, the mean of CRP values over baseline and follow-up. This new continuous variable, which reflects both time points with a higher mean corresponding to higher concentration over five years, was significantly associated with fatigue levels at follow-up(adjusted β=0.065,P=0.002).
DISCUSSION
In a community sample, higher plasma CRP concentration predicted higher fatigue level five years later independently of a series of risk factors such as BMI, depressive symptoms, sleep quality, pain, and physical activity. To our knowledge, this is the first study to demonstrate a prospective association between a marker of systemic inflammation and fatigue in a general population. In addition, among participants without comorbid medical disorders, the association between CRP and fatigue was significant, demonstrating that the prospective influence of CRP on fatigue cannot be explained simply by the presence or development of comorbid medical disorders. Moreover, fatigue was predicted by a persistent, as opposed to a transient, elevation of CRP. Lastly, the nature of the association between CRP and fatigue seems bidirectional as higher fatigue level at baseline also independently predicted higher CRP concentration at follow-up five years later. Interestingly, the latter relationship was partly mediated by physical activity level, whereas the prospective effect of CRP on fatigue was neither mediated by physical activity nor any of the following variables including BMI, depressive symptoms, sleep quality, and pain.
Although an association between systemic inflammation and fatigue has been reported in cancer survivors (42, 43), the implications of these data for non-medical community sample is unknown (see Miller et al (44) and Schubert et al (45) for a review) due to the confounding influence of cancer diagnosis and related treatments. Among persons with chronic fatigue syndrome as compared to controls, overproduction (15-20), reduced production (25), and no difference (21-24) of proinflammatory cytokines have been reported, with similar conflicting results in patients with multiple sclerosis (26-28). In a correlational study of 40 healthy young adults, no association of fatigue with TNF-α or CRP was found although this could have been due to limited statistical power (46).
Derived from a community-based prospective study, the current data overcome the limitations of prior studies in humans and translate evidence generated in animals that systemic inflammation induces fatigue-like behaviors. The following features further strengthen the current findings. First, the possibility of selection bias or information bias was less likely than in previous studies, given that the study sample was randomly chosen from the community and the exposure variable was an objective biological measure. Second, as noted above, the association between CRP and fatigue was independent of a series of confounding variables such as obesity, depression, sleep quality, pain, and physical activity. Third, given that the findings were generated in community-dwelling adults including those without medical comorbidity, it does not appear fatigue is simply a byproduct of medical disorders and related inflammation. Fourth, there was two-fold evidence of a dose-response relationship between CRP and fatigue: higher CRP levels were linearly associated with higher fatigue levels; and persistently elevated, but not transiently elevated, CRP concentration predicted fatigue.
The mechanisms that drive increases of inflammation and symptoms of fatigue in a healthy community dwelling sample are unknown. Experimental studies suggest that physical and psychological stressors activate the peripheral immune system, mounting an inflammatory response with the release of proinflammatory cytokines and acute phase proteins (`signal generated') (47). These peripheral inflammatory signals are then transduced to the brain through specific pathways across the blood-brain barrier such as vagal nerve afference and IL-1 receptors located on endothelial cells of brain venules (`signal received'), and the brain finally may produce sickness behaviors including fatigue (`response to signal') (48). While extensive research efforts have accumulated mechanistic evidence on the `generation' and `reception' of inflammatory signals (47), the specific mechanisms of how the brain `responds' to these signals producing the symptom of fatigue are still to be elucidated. To date, basal ganglia hypermetabolism - hence altered dopaminergic activities - has been related to physical fatigue and anterior cingulate activation to mental fatigue during interferon-α therapy of patients with malignant melanoma (13, 49). Interestingly, although systemic inflammation has been also linked to depressed mood, the nature and mechanism of this link seem to be distinct from the association between inflammation and fatigue. Among interferon-α treated patients, while fatigue has been related to alterations in dopamine neurotransmission in the basal ganglia, depressed mood has been linked to alterations in corticotropin-releasing hormone pathways and serotonin metabolism (50, 51). Furthermore, fatigue occurs earlier in interferon-α therapy and responds less to antidepressant treatment (12), supporting distinct mechanisms for depression and fatigue.
Persistent inflammation may be a particularly important factor involved with fatigue. Interestingly, data from the CARDIA study reported elsewhere suggest that this persistent inflammation may be driven by genetic predisposition (CRP promoter gene polymorphisms) (52) and early life stress (low childhood socioeconomic status and harsh early family environment) (53). Furthermore, we have previously demonstrated that cytokine gene polymorphisms, which are thought to be associated with persistent elevations of inflammatory markers, correlate with fatigue in breast cancer survivors (54).
As to the association in which higher fatigue level leads to higher CRP concentration, physical activity level was shown to partly mediate it. Fatigued individuals may be less physically active, and low physical activity may lead to increased CRP level. Clinical trials have indeed shown physical exercise decreases CRP level (55, 56).
The following limitations should be considered. First, the assessment of fatigue relied on a single item rather than a composite measure that evaluates the multidimensional nature of this construct. Thus, the current findings should be interpreted taking this limitation into account and future research should employ a more nuanced measure of fatigue such as the Multidimensional Fatigue Symptom Inventory. However, as previously discussed, SF-12 Vitality Subscale is a valid and reliable measure of energy-fatigue. Additionally, supporting the usefulness of this measure, which enquires about energy level, is a previous report that the energy subscale of a composite fatigue measure was the best correlate of the biological substrate for cytokine-induced fatigue (13). Second, CRP was the only marker of systemic inflammation measured in the current study although it is the most extensively researched and the most clinically useful inflammatory marker (57). Third, the magnitude of the association between CRP and fatigue was small, albeit statistically significant. Nevertheless, it was greater than the magnitude of the association between CRP and depressive symptoms in the current sample. The association between CRP and depression, examined by numerous previous studies, is currently considered an established research finding despite its small effect size. A recent meta-analysis has reported Cohen's d of 0.15 overall and 0.11 in community studies (58), both values considered small (59).
These findings suggest that plasma CRP, especially its persistent elevation, is an independent risk factor for fatigue. Despite the study limitations, these prospective observations provide novel information on the role of systemic inflammation on fatigue within the context of a large sample of non-medical, community-dwelling persons. These data should also motivate further investigations to define the effects of proximal factors for persistent inflammation (e.g. CRP gene polymorphism, childhood stress) on fatigue risk. Testing of interventions that target inflammation might identify new strategies to constrain fatigue onset.
Supplementary Material
Acknowledgments
The CARDIA study was supported (or partially supported) by contracts: University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; University of California, Irvine, Echocardiography Reading Center (Year 5 & 10), N01-HC-45134; Harbor-UCLA Research Education Institute, Computed Tomography Reading Center (Year 15 Exam), N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; New England Medical Center (Year 20 Exam), N01-HC-45204 from the National Heart, Lung and Blood Institute. The work on this manuscript was supported by the NIMH T32-MH19925 and UCLA Friends of the Semel Institute Fellowship. Dr. Duk-Hee Lee is gratefully acknowledged for her valuable contribution to the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Valdini AF. Fatigue of Unknown Aetiology -- a Review. Fam Pract. 1985;2:48–53. doi: 10.1093/fampra/2.1.48. [DOI] [PubMed] [Google Scholar]
- 2.Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, Wessely SC. Population based study of fatigue and psychological distress. BMJ. 1994;308:763–766. doi: 10.1136/bmj.308.6931.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curt G. Impact of fatigue on quality of life in oncology patients. Semin Hematol. 2000;37:14–17. doi: 10.1016/s0037-1963(00)90063-5. [DOI] [PubMed] [Google Scholar]
- 4.Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler. 2001;7:340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J Nerv Ment Dis. 1997;185:359–367. doi: 10.1097/00005053-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the US Workforce: Prevalence and Implications for Lost Productive Work Time. J Occup Environ Med. 2007;49:1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- 7.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost Productive Time and Cost Due to Common Pain Conditions in the US Workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 8.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of Lost Productive Work Time Among US Workers With Depression. JAMA. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Kullberg BJ, Vonk AG, Verschueren I, Joosten LA, van der Meer JW. Increased voluntary exercise in mice deficient for tumour necrosis factor-alpha and lymphotoxinalpha. Eur J Clin Invest. 2007;37:737–741. doi: 10.1111/j.1365-2362.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 10.Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, et al. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1109–1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, et al. Acute Effects of Recombinant Human Interleukin-6 on Endocrine and Central Nervous Sleep Functions in Healthy Men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- 12.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 13.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao CC, Janoff EN, Hu SX, Thomas K, Gallagher M, Tsang M, et al. Altered cytokine release in peripheral blood mononuclear cell cultures from patients with the chronic fatigue syndrome. Cytokine. 1991;3:292–298. doi: 10.1016/1043-4666(91)90497-2. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald D, Wener MH, Pearlman T, Kith P. Markers of inflammation and immune activation in chronic fatigue and chronic fatigue syndrome. J Rheumatol. 1997;24:372–376. [PubMed] [Google Scholar]
- 17.Cannon JG, Angel JB, Abad LW, Vannier E, Mileno MD, Fagioli L, et al. Interleukin-1ß, Interleukin-1 Receptor Antagonist, and Soluble Interleukin-1 Receptor Type II Secretion in Chronic Fatigue Syndrome. J Clin Immunol. 1997;17:253–261. doi: 10.1023/a:1027314713231. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Aggarwal S, See D, Starr A. Cytokine production by adherent and nonadherent mononuclear cells in chronic fatigue syndrome. J Psychiatr Res. 1997;31:149–156. doi: 10.1016/s0022-3956(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 19.Cannon JG, Angel JB, Ball RW, Abad LW, Fagioli L, Komaroff AL. Acute Phase Responses and Cytokine Secretion in Chronic Fatigue Syndrome. J Clin Immunol. 1999;19:414–421. doi: 10.1023/a:1020558917955. [DOI] [PubMed] [Google Scholar]
- 20.Moss RB, Mercandetti A, Vojdani A. TNF-a and Chronic Fatigue Syndrome. J Clin Immunol. 1999;19:314–316. doi: 10.1023/a:1020595709352. [DOI] [PubMed] [Google Scholar]
- 21.LaManca JJ, Sisto SA, Zhou X, Ottenweller JE, Cook S, Peckerman A, et al. Immunological Response in Chronic Fatigue Syndrome Following a Graded Exercise Test to Exhaustion. J Clin Immunol. 1999;19:135–142. doi: 10.1023/a:1020510718013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Zhou XD, Denny T, Ottenweller JE, Lange G, LaManca JJ, et al. Changes in immune parameters seen in Gulf War veterans but not in civilians with chronic fatigue syndrome. Clin Diagn Lab Immunol. 1999;6:6–13. doi: 10.1128/cdli.6.1.6-13.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashipaz MRA, Swinden D, Todd I, Powell RJ. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clin Exp Immunol. 2003;132:360–365. doi: 10.1046/j.1365-2249.2003.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer-Conna U, Cameron B, Hadzi-Pavlovic D, Singletary K, Davenport T, Vernon S, et al. Postinfective Fatigue Syndrome Is Not Associated with Altered Cytokine Production. Clin Infect Dis. 2007;45:732–735. doi: 10.1086/520990. [DOI] [PubMed] [Google Scholar]
- 25.ter Wolbeek M, van Doornen LJP, Kavelaars A, van de Putte EM, Schedlowski M, Heijnen CJ. Longitudinal analysis of pro- and anti-inflammatory cytokine production in severely fatigued adolescents. Brain Behav Immun. 2007;21:1063–1074. doi: 10.1016/j.bbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Giovannoni G, Thompson AJ, Miller DH, Thompson EJ. Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology. 2001;57:676–681. doi: 10.1212/wnl.57.4.676. [DOI] [PubMed] [Google Scholar]
- 27.Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler. 2004;10:165–169. doi: 10.1191/1352458504ms991oa. [DOI] [PubMed] [Google Scholar]
- 28.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77:34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 30.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 32.Hurst NP, Ruta DA, Kind P. Comparison of the MOS short form-12 (SF12) health status questionnaire with the SF36 in patients with rheumatoid arthritis. Rheumatology (Oxford) 1998;37:862–869. doi: 10.1093/rheumatology/37.8.862. [DOI] [PubMed] [Google Scholar]
- 33.Han C, Pulling CC, Telke SE, Hullsiek KH. Assessing the utility of five domains in SF-12 Health Status Questionnaire in an AIDS clinical trial. AIDS. 2002;16:431. doi: 10.1097/00002030-200202150-00015. [DOI] [PubMed] [Google Scholar]
- 34.Powers DA, Xie Y. Statistical methods for categorical data analysis. Academic Press; San Diego: 2000. [Google Scholar]
- 35.Marmot M, Brunner E. Cohort Profile: The Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 36.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, et al. Quality of Life at the End of Primary Treatment of Breast Cancer: First Results From the Moving Beyond Cancer Randomized Trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 37.Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of Fatigue in Cancer Patients: Development and Validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 38.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of Inflammation and Cardiovascular Disease Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 39.Parker ED, Schmitz KH, Jacobs DR, Dengel DR, Schreiner PJ. Physical Activity in Young Adults and Incident Hypertension Over 15 Years of Follow-Up: The CARDIA Study. Am J Public Health. 2007;97:703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr., Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A Comparison of Methods to Test Mediation and Other Intervening Variable Effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 44.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrineimmune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Corwin EJ, Klein LC, Rickelman K. Predictors of Fatigue in Healthy Young Adults: Moderating Effects of Cigarette Smoking and Gender. Biol Res Nurs. 2002;3:222–233. doi: 10.1177/109980040200300407. [DOI] [PubMed] [Google Scholar]
- 47.Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 48.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 51.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, et al. Polymorphisms within the C-Reactive Protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of Early Life Stress and Psychological Functioning to Adult C-Reactive Protein in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 56.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 57.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. 2003. pp. 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howren MB, Lamkin DM, Suls J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J. Statistical power analysis for the behavioral sciences. 1988 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.