Abstract
Dendritic cell (DC)-based tumor vaccines have only achieved limited clinical efficacy, underscoring the limitation of stimulatory strategies to elicit effective cytotoxic T lymphocyte (CTL) responses against self tumor-associated antigens. Here we investigate the role of human suppressor of cytokine signaling (SOCS) 1, a feedback inhibitor of the JAK/STAT signaling pathway, in regulating antigen presentation by human DCs. We find that human SOCS1-silenced DCs have an enhanced stimulatory ability to prime self antigen-specific CTLs in vitro and in an SCID-hu mouse model. Human CTLs activated by SOCS1-silenced DCs, but not wild-type DCs, have an active lytic activity to natural antigen-expressing tumor cells. We further find that the capacity of human DCs to prime CTLs is likely controlled by SOCS1 restricted production and signaling of proinflammatory cytokines such as IL-12. These results indicate a critical role of human SOCS1 in negatively regulating the immunostimulatory capacity of DCs and imply a translational potential of this alternative, SOCS1 silencing strategy to develop effective DC vaccines.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells with key regulatory roles in the maintenance of tolerance to self-antigens and in the activation of innate and adaptive immunity (1). They use pattern-recognition receptors such as Toll-like receptors (TLRs) to recognize conserved microbial structures such as lipopolysaccharide (LPS) (2). TLR signaling promotes DC maturation by activating nuclear factor-κB (NF-κB), which mediates the up-regulation of antigenic peptide-loaded MHC molecules and costimulatory molecules, and expression of proinflammatory cytokines, resulting in the induction of innate and adaptive immunity (2). DCs have been demonstrated to be effective in inducing antitumor responses in mice (1, 3). However, the results of DC vaccine trials have been largely disappointing with very low rates of objective clinical responses (4). The major challenge now is to find a novel way to elicit effective T cell responses to self-antigens preferentially expressed by tumor cells.
Several recent studies in mice suggest a critical role of SOCS1-restricted signaling in maintaining self-tolerance and negatively regulating antigen-presenting cells. SOCS1 functions as a negative regulator of signaling by various cytokines, such as IFN-γ, IL-2, IL-6, IL-7, IL-12, and IL-15, by inhibiting the Janus kinases (JAKs)/STAT in T cells and other immune cells (5, 6). Metcalf D. et al. (7) reported that adoptive transfer of bone marrow (BM) cells of neonatal SOCS1-deficient (−/−) mice into irradiated syngeneic mice caused a pathology characteristic of graft-versus-host disease with chronic inflammatory lesions in multiple organs of the recipients, in agreement with earlier findings (6). Hanada T. et al. further demonstrated that SOCS1−/− transgenic mice in which SOCS1 expression had been restored in T and B cells on a SOCS1−/− background developed only mild autoimmune diseases and that SOCS1−/− DCs were hyper-responsive to LPS and IFN-γ and triggered allogeneic T cell expansion (8). Hashimoto M et al. recently revealed that silencing of SOCS1 in macrophages suppressed tumor development by enhancing antitumor inflammation (9). These results clearly suggested an essential role of SOCS1 in maintaining self-tolerance of hematopoietic immune cells. In a recent study, we found that murine SOCS1 critically controled antigen presentation by DCs (10). In support of our study, Hanada et al. discovered that DCs lacking the SOCS1 gene induced hyper Th1 cell-type immune responses (11). Because studies on the role of SOCS1 in regulating immune responses have been limited to mouse models, we sought to investigate the regulatory role of SOCS1 in human (h) monocyte-derived DCs, which have been widely used in the clinic.
Materials and Methods
Western blot and quantitative RT-PCR analysis of human SOCS1 expression
We first used a computer program from Dharmacon RNAi Technologies (Dharmacon Inc, Chicago, IL) to select siRNA sequences targeting human SOCS1: siSOCS1-1 (CACGCACUUCCGCACAUUC.dT.dT), siSOCS1-2 and siSOCS1-3. We then co-transfected 293T cells with a siRNA oligonucleotide duplex (21 bp) or an irrelevant oligo duplex and a flag-tagged human SOCS1 expression vector (pCMV-hSOCS1) we constructed using GenePorter reagent (Genlantis, CA) (10) The relative expression of human SOCS1 in transfected 293T cell or human DCs was evaluated by Western blotting analysis and quantitative real-time RT-PCR (10).
Transfection of human monocyte-derived DCs and in vitro priming of human T cells
Human DCs derived from PBMCs of HLA-A2+ healthy volunteers were generated as described in our previous studies (12, 13). This research was approved by the Institutional Review Board on Human Subjects. Monocyte-derived DCs were transfected with 120 nM siRNA oligonucleotides using GenePorter. The transfected DCs were then pulsed with MAGE3 peptide (20 μg/ml) overnight. A total of 1×106 human T-cells per well were co-cultured with 5×104 MAGE3-pulsed, transfected DC (20:1) in 0.5 ml of RPMI-1640 supplemented with 5% AB human serum, rhIL-2 (50 U/ml), and TNFα(10 ng/ml, R&D Systems). The co-cultured T-cells were re-stimulated weekly. Some cocultures were supplemented with 10 ug/ml monoclonal anti-hIL12 (R&D Systems) to block IL-12 activity.
Cytokine ELISA, enzyme-linked immunospot (ELISPOT) assays and tetramer staining
Levels of various proinflammatory cytokines were quantitated from the supernatant of DC cultures by ELISA analysis (BD Biosciences) according to the manufacturer’s instructions. An HLA-A2-restricted human melanoma antigen MAGE3 CTL peptide (FLWGPRALV), an HLA-A2-restricted human herpesvirus EBV latent membrane protein 2 (LMP2) CTL peptide (CLGGLLTMV), a control ovalbumin (OVA) peptide (SIINFEKL), and a control human hepatitis C virus (HCV) E2 protein peptide (RLWHYPCTI) were synthesized and purified by HPLC to >95% purity by Genemed Synthesis Inc. (South San Francisco, CA, USA) (14). Human MAGE3/HLA-A2 tetramers were synthesized at the Baylor College of Medicine Tetramer Core Facility (Houston, TX, USA). ELISPOT assays and tetramer staining were performed as described (10).
DC immunization of HLA-A2 transgenic mice
HLA-A2.1 transgenic mice were purchased from the Jackson Laboratory (Maine, USA) and maintained in the pathogen-free mouse facility according to institutional guidelines. BM-derived DCs were prepared from HLA-A2.1 transgenic mice and transduced with lentiviral vector, as described in our previous study (10). DCs were pulsed with peptides, stimulated with TNFα(10 ng/ml) for 24 hr, and then injected into transgenic mice via a foot-pad. In some mice, LPS (30 μg/mouse) or murine IL-12 (1 μg/mouse, Peprotech) was administered i.p. on indicated days.
CTL assays
CD8+ CTL responses were assessed with a standard chromium release assay (10) or LDH release assay (Roche Diagnostics). Splenocytes pooled from 2–3 immunized mice were restimulated in vitro in RPMI-1640 containing MAGE3 or LMP2 peptide for 4–6 days. Human MAGE3+, HLA-A2+ melanoma cells (SK-Mel-37) and control human MAGE3+, HLA-A2− melanoma cells (NA-6-Mel) were labeled with sodium 51Cr chromate solution for 90 min at 37°C. LMP2-specific CTL response was tested using LDH release assay according to the manufacturer’s instructions.
SCID-Hu mouse model
siSOCS1-transfected DC in vivo priming human T cells was performed in a severe combined immunodeficient (SCID)-hu mouse model as described previously (15). PBMCs were obtained from the blood of healthy HLA-A2+ and HLA-A2−negative donors. Monocyte-derived DCs were transfected with siRNA oligo and loaded with MAGE3 or LMP2 peptide as described above. The transfected DCs (5×105/mouse) and autologous T cells (3×107/mouse) were administered i.p. into SCID mice (Charles River) and followed by in vivo injections (i.p.) with LPS (30 μg/mouse) daily for three times. 10 days after the immunization, mice were sacrificed, and cells were recovered from the peritoneum of different groups of mice and pooled for immune assays.
Statistical analysis
For statistical analysis, we used Student’s t test, and a 95% confidence limit was taken to be significant, defined as p < 0.05. Results are typically presented as means ± standard errors (SE).
Results
SOCS1 negatively regulates hDCs in response to LPS stimulation
To investigate the role of SOCS1 in hDCs, we first identified small interfering RNA (siRNA) with the ability to specifically downregulate hSOCS1 (Fig. 1A). The specificity of hSOCS1 mRNA down-regulation by hSOCS1 siRNA 1 (siSOCS1) was confirmed by the inability of a scrambled siRNA1 oligonuleotide duplex (siMut) to down-regulate SOCS1 mRNA. Human siSOCS1 (1) was therefore selected for further study. Synthetic siRNA duplexes were transfected by Geneporter into DCs derived from human monocytes with a transfection efficiency of about 85.5% (Fig. 1B). As verified by quantitative RT-PCR assays, the level of hSOCS1 mRNA in the total DC population transfected with the siSOCS1 duplexes was specifically decreased by approximately 60%, compared with levels in mock-transfected DCs (Fig. 1C, p<0.01). The siRNA efficiency and SOCS1 RNA reduction were similar to those in our previous study using siRNA duplexes to target mouse SOCS1 in the total mouse DC population (10).
Figure 1. Silencing of hSOCS1 in human monocyte-derived DCs.

(A) Identification of hSOCS1 siRNA. 293T cells were cotransfected with a plasmid expressing hSOCS1 (pCMV-hSOCS1) (0.4 μg) and synthetic siSOCS1 oligo duplexes (120 nM) using GenePorter (9). 48 hrs later, the cell lysates were analyzed by Western blotting. Relative levels of hSOCS1 and actin in each cell lysate are presented.
(B) Transfection of human monocyte-derived DCs by siRNA oligonucleotides. Human monocyte-derived DCs were transfected with Cy3-labeled or unlabeled siRNA oligonucleotide duplexes by GenePorter. Fluorescent micrographs (lower panel) and flow cytometric histograms (upper panel) of the transfected DCs are presented from one of three independent experiments.
(C) hSOCS1 RNA levels in siRNA-transfected human monocyte-derived DCs. The relative expression of hSOCS1 mRNA in human monocyte-derived DCs transfected with siSOCS11 oligonucleotides or scrambled siRNA1 oligo was determined after 24 hr of stimulation with IFN-γ(10 ng/ml) by real-time quantitative RT-PCR. hSOCS1 levels were normalized to those of mock-transfected DCs. The data are representative of three independent experiments. *P < 0.01, vs. hSOCS-siRNA DCs.
We next tested whether hSOCS1 regulates the expression of costimulatory molecules and MHC class I/II molecules on DCs by flow cytometric analysis. siSOCS1-, siMut-, and mock-transfected DCs showed no apparent difference in their expression of costimulatory molecules CD40, CD80, and CD86, and the CC chemokine receptor CCR7 before and after LPS-induced maturation (Fig. 2A). Comparable levels of MHC-I molecule and MHC-II molecule HLA-DR were detected on siSOCS1 DCs, siMut DCs, and mock DCs (data not shown, and Fig. 2A). In contrast, we found that siSOCS1 DCs were more responsive to stimulation with the toll-like receptor (TLR) agonists such as LPS, CpG, and poly I:C than siMut DCs or mock DCs, as indicated by drastically enhanced secretion of proinflammatory cytokines, such as IL-12p70, IL12p40, IL-1β, IL-6 and TNF-α (Fig. 2B). These data are in agreement with the observations in mouse DCs (9). Gingras et al. (16) recently reported that levels of IL-12 produced by SOCS1−/− BM-derived macrophages were comparable to those produced by wide-type macrophages in response to LPS. However, we found that human monocyte-derived DCs differentiated under the described condition were insensitive to the TLR ligand stimulation unless SOCS1 expression was downregulated. Thus, the different species and cell type used in the study (mouse macrophages vs. human DCs) may contribute to the discrepancy (10, 17).
Figure 2. SOCS1 negatively regulates human monocyte-derived DCs in response to LPS stimulation.
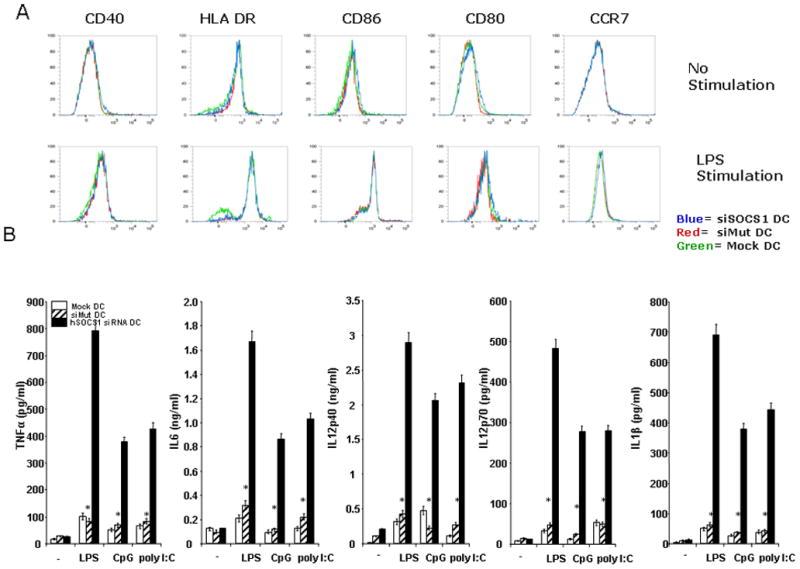
(A). Flow cytometric analysis. Surface representative costimulatory molecules and MHC molecules on transfected human DCs with or without LPS stimulation (100 ng/ml) for 24 hr were analyzed by flow cytometric analysis. Histograms are representative of four independent experiments.
(B). Enhanced production of proinflammatory cytokines by siSOCS1-transfected DCs. Cytokines secreted by transfected DCs after LPS (100 ng/ml), CpG (2.5μM) or poly I:C (1μg/ml) stimulation for 24 hr were measured by ELISA assays. The data are representative of three independent assays. *P < 0.01, vs. hSOCS-siRNA DCs.
SOCS1 negatively regulates stimulatory ability of human DCs in priming autolgous T cells in vitro
To test if silencing SOCS1 can enhance the stimulatory potency of human DCs in priming self-antigen specific CTLs, an HLA-A2-restricted peptide derived from human MAGE3 (14) were used as a model self-antigen. Human monocyte-derived DCs from HLA-A2+ healthy volunteers were transfected with siRNA and then pulsed with MAGE3 peptide overnight. Autologous human T cells were cocultured with MAGE3-pulsed, transfected DC in the presence of TNFα(a maturation stimulus) for 2–3 weeks. In cocultures with siSOCS1 DCs, 13.9% of the CD8+ T cells were positive for the MAGE3-tetramer, compared with only 5.4% and 4.3% in cocultures with siMut DCs or mock DCs, respectively (Fig. 3A). Tetramer staining of unstimulated lymphocytes from the same donors showed a low level of MAGE3 tetramer staining (0.5% of the CD8+ T cells). In agreement, intracellular IFNγ staining showed that siSOCS1 DCs substantially improved MAGE3-specific CTL responses (11.18% of IFNγ+ CD8+ T cells) compared to siMut DC (6.9%) or mock DCs (5.7%) (Fig. 3B). Furthermore, IFNγ ELISPOT assays showed that an increased number of MAGE3-specific CTLs were activated by siSOCS1 DCs (Fig. 3C). Repeated experiments from HLA-A2+ donors showed similar results. Most of the primary human T cells were dead after a two-week coculture with DCs not pulsed with antigens. Collectively, these results indicate that hSOCS1-silenced DCs have enhanced immunostimulatory ability to prime self antigen-specific CTLs. Since SOCS1-silenced DCs produce enhanced amounts of IL-12 (Fig. 2B), a key cytokine in the activation of CTL responses (17) and IL-12 signaling is restricted by SOCS1 (18), we examined the role of IL-12 in priming CTLs. Figs. 3B & C showed that anti-IL12 antibody blocking abrogated the enhanced ability of siSOCS1 DCs to stimulate MAGE3-specific CTLs. Moreover, anti-IL-12 blocked the immunostimulatory activity of siSOCS1 DCs in a dose-dependent manner, as shown by that the increasing concentration of anti-IL-12 (1, 5, 10 ug/ml) led to enhanced inhibitory effects on siSOCS1 DCs (Fig. S1). Anti-IL-6 was also found to block the immunostimulatory activity of siSOCS1 DCs, but the inhibitory effect was less prominent than anti-IL-12, as evidenced by that a higher concentration of anti-IL-6 (2ug/ml) failed to achieve the inhibitory effect mediated by 1 ug/ml anti-IL-12 in the siSOCS DC blocking assay (Supplemental Fig. S1). The result highlighted importance of IL-12 in siSCOS1 DCs priming CTLs.
Figure 3. Enhanced potency of SOCS1-silenced hDCs to induce antigen-specific T cell response in vitro.
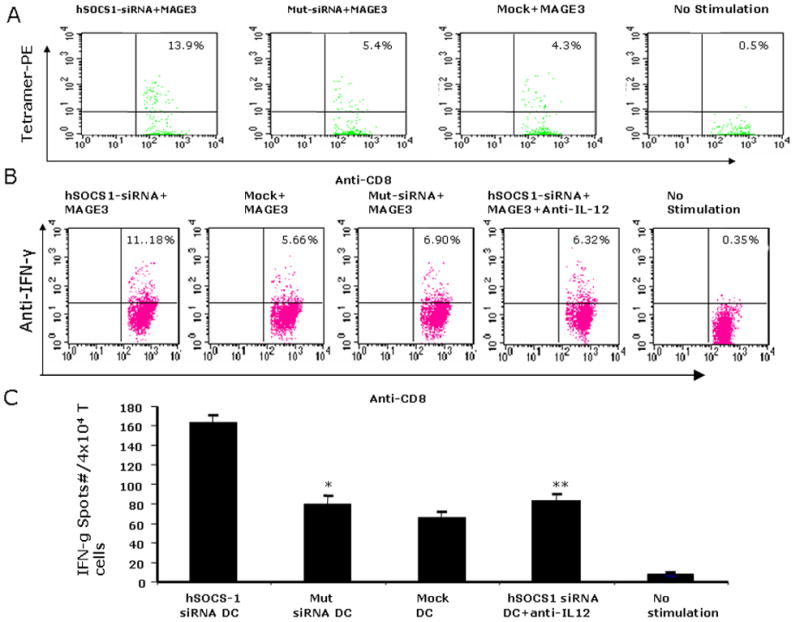
Human monocyte-derived DCs from HLA-A2+ donors were transfected with siSOCS1 oligo, pulsed with MAGE3 peptide, and cocultured with autologous T cells for two wks in the absence or presence of anti-human IL-12 antibodies. MAGE3-PE tetramer+ T cell percentages (A), intracellular IFNγ+ T cell percentages (B) in the gated CD8+ T cell populations, and IFN-γ+ ELISPOT numbers (C) in the cocultures are shown from one of four independent experiments. Unstimulated samples from the same donors were used as controls. *P < 0.01, siMut DCs vs. siSOCS1 DCs; **P < 0.01, siSOCS1 DCs vs. siSOCS1 DCs + anti-human IL-12.
We next determined whether activated T cells possess tumor lytic effector function by using natural MAGE3+ human tumor cells as target cells for CTL assays. Previous studies indicated that the human melanoma cell lines, such as MAGE3-transfected NA8-MEL and SK23-MEL inefficiently processed and presented the MAGE3271–279 epitope due to inappropriate proteasomal cleavage at the COOH terminus of the antigenic peptide (19), and therefore failed to be recognized by MAGE3271–279-specific CTL line (20). However, a recent study demonstrated that the melanoma cell line, SK-Mel-37, could be recognized and killed by CTL responses triggered by MAGE3271–279-pulsed, Akt-expressed DCs (21), implicating SK-Mel-37 does present MAGE3271–279 epitope, albeit less efficiently, on the cell surface. Consistent with the study, our result showed, while human T cells activated by siMut DC or Mock DC showed a very weak cytolytic activity to natural SK-Mel-37 cells, the T cells activated by siSOCS1 DCs had a strong cytolytic activity against the MAGE3+ HLA-A2+ SK-Mel-37 cells (Fig. 4A & B). We further found that the tumor lytic activity of T cells in the coculture with siSOCS1 DCs was significantly compromised by anti-hIL-12 antibody treatment (Fig. 4A & 4B). The tumor cytolytic activity was specifically mediated by CTLs, since the activated human T cells only had a background cytolytic activity against the HLA-A2-negative, MAGE3+ melanoma cells (NA-6-Mel) (Supplemental Fig. S2A). Repeated experiments from different donors showed similar results.
Figure 4. siSOCS1 DCs more potently prime antigen-specific CTL responses in vitro.
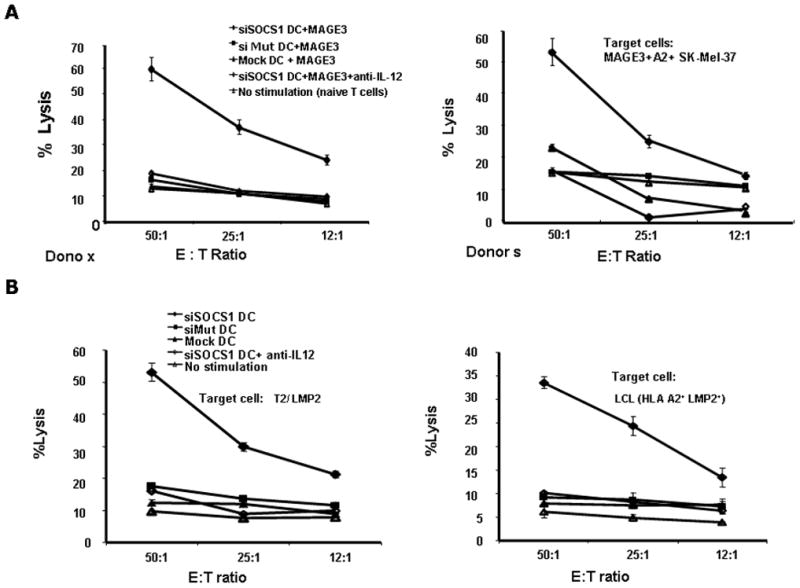
(A) & (B), MAGE3-specific tumor lytic activities. Cytolytic activities against human MAGE3+, HLA-A2+ melanoma cells (SK-Mel-37) after a two-week coculture of DCs and autologous T-cells in the absence or presence of anti-human IL-12 antibodies are shown from two of five independent experiments.
(C) & (D), LMP2-specific tumor lytic activities. Cytolytic activities against LMP2-pulsed, HLA-A2+ lymphoblastoma T2 (C) or EBV transferred, HLA-A2+ B-lymphoblastoid cell lines (LCLs) (D) after a two-week coculture of DCs and autologous T-cells in the absence or presence of anti-human IL-12 antibodies are shown from one of two independent experiments. P < 0.01, siMut DCs vs. siSOCS1 DCs; P <0.01, siSOCS1 DCs vs. siSOCS1 DCs + anti-human IL-12 antibodies.
Due to the inefficiency of SK-Mel-37 in processing and presenting MAGE3271–279 (19,20), it is not excluded that the observed siSOCS1 DC-induced CTL activity against SK-Mel-37 may be caused by some level of non-specific activation of lytic effectors (CTL or NK), to which SK-MEL-37 might be more sensitive than the other cell lines. To verify the ability of siSOCS1 DC inducing antigen-specific CTL response, we pulsed the transfected DCs with EBV-LMP2 CTL peptide, and co-cultured the DCs with autologous human T cells as described above. We found that activated T cells by siSOCS1 DCs exhibited a superior CTL activity against LMP2-pulsed, HLA-A2+ lymphoblastoma cell line T2 and EBV-transferred, HLA-A2+ B- lymphoblastoid cell lines (LCLs), compared with the activated T cells by siMut DCs or mock DCs (Fig. 4C & D). The CTL response induced by siSOCS1 DC is the LMP2-specific, as the activated human T cells by siSOCS1 DC displays much lower efficiency in killing the irrelative peptide HCV E2 peptide-pulsed, HLA-A2+ T2 cell or renal carcinoma A498 cell (Supplemental Fig. S2B & C). Once again, siSOCS1 DC-induced, T cell-mediated tumor lytic activity was significantly compromised by addition of anti-hIL-12 antibody (Fig. 4C & D).
SOCS1 negatively regulates stimulatory ability of human DCs in SCID-hu mice
To additionally evaluate the in vivo immunostimulatory capacity of hSOCS1-silenced DCs, we used an SCID-hu mouse model (15). Groups of SCID mice were xenotransplanted with human HLA-A2+ or HLA-A2− monocyte-derived DCs that were transfected with siSOCS1, siMut or mock and loaded with MAGE3 or EBV-LMP2 peptide, and autologous T cells, followed by in vivo injections with LPS for three times, as administration of LPS was demonstrated to boost the immunostimulatory efficacy of siSOCS1 DC (10) owed to the large number of pro-inflammatory cytokines it induces, many of which are regulated by SOCS1. Mice immunized with siSOCS1 DCs showed more robust MAGE3-specific T cell responses than did siMut DC or mock DC immunized mice, as manifested by IFN-γ Elispot assay (Fig. 5A). Consistently, mice immunized with siSOCS1 DCs also showed an enhanced LMP2-specific T cell response, compared with siMut DC or mock DC immunized mice (Fig. 5B). The T cell responses were antigen-specific, since HLA-A2− DCs that were transfected with siSOCS1 and loaded with MAGE3 did not induce MAGE3-specific T cell responses (data not shown). Intracellular IFNγ staining also showed the enhanced T cell responses induced by siSOCS1 DCs (data not shown). The results of the SCID-hu mouse experiments indicate that human SOCS1-silenced DCs have an enhanced ability to prime CTL responses in vivo. Collectively, our results indicate that human SOCS1-silenced DCs may possess a unique ability to fully activate CTLs that have an active lytic effector function against natural tumor cells.
Figure 5. SOCS1-silenced DCs more potently prime T cell responses in SCID-hu mice model.
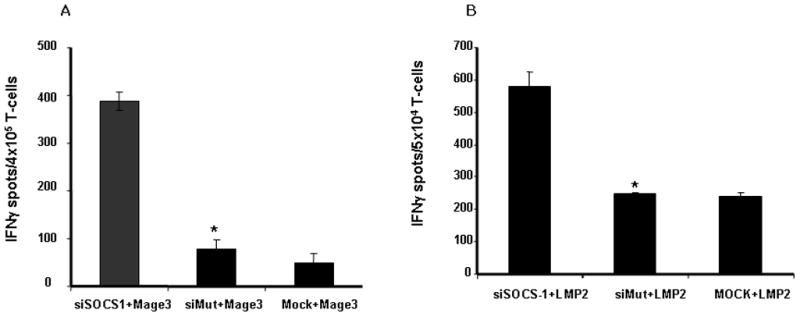
Groups of SCID mice (4 mice/group) were immunized with human DCs that were transfected with siRNA oligos and pulsed with MAGE3 peptide (A) or LMP2 peptide (B), and autologous T cells. 10 days after immunization, cells were recovered from the peritoneum andsubjected to IFNγ ELISPOT assays. IFNγ+ spot numbers specific for MAGE3 or LMP2 after subtracting the background spots of the SCID mice immunized with siSOCS1 oligo DCs without peptide pulsing are presented from one of two assays. *P < 0.01, siMut-DCs vs. sihSOCS1-DCs.
SOCS1 negatively regulates stimulatory ability of DCs in humanized HLA-A2.1 mice
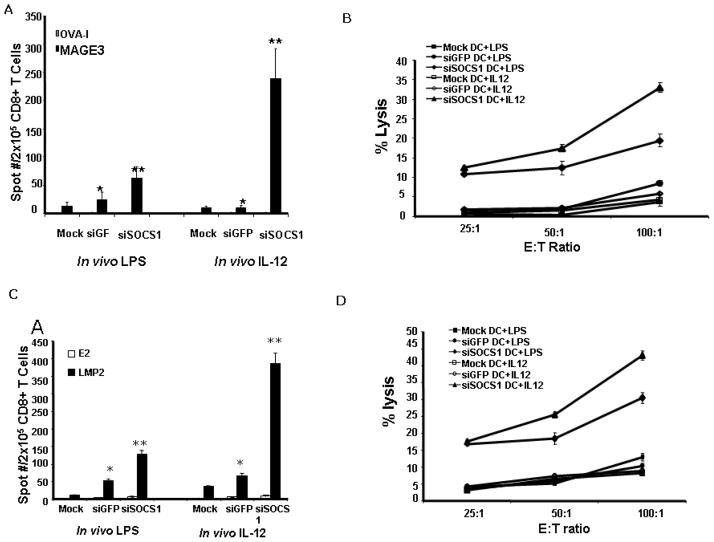
We used humanized HLA-A2.1 transgenic mice to further define SOCS1 regulatory function in DCs. BM-derived DCs from HLA-A2.1 transgenic mouse were transduced with LV-mSOCS1 siRNA or LV-GFP siRNA, which were previously generated (10), and pulsed with A2-restricted MAGE3 peptide. After maturation with TNFα, transduced DCs were administered into the transgenic mice twice at a weekly interval. After each DC immunization, the mice were stimulated in vivo three times with either LPS or a low dose of recombinant IL-12 cytokines. Here, LPS served as a positive control for in vivo stimulation. Consistent with our previous study (10), with in vivo LPS stimulations, 70 IFNγ+ spots per 2 × 105 T cells were detected in siSOCS1 DC immunized spleen, compared with only 20 or 10 IFNγ+ spots per 2 × 105 T cells in siGFP DC or mock DC immunized spleen (Fig. 6A). In vivo LPS stimulations also preferentially augmented the CTL responses against the natural SK-Mel-37 in siSOCS1 DC immunized mice compared with those in control DC immunized mice, as shown in Fig. 6B. However, in vivo IL-12 stimulation was more effective than LPS stimulation to enhance the immunostimulatory potency of siSCOS1 DCs, as manifested by a higher frequency of IL-12- stimulated T cell producing IFN-γ in response to MAGE3 stimulation than LPS stimulated T cells (239 vs 70 IFNγ+ spots per 2 × 105 T cells) (Fig. 6A) and more robust CTL responses against the natural SK-Mel-37 in IL-12 stimulated, siSOCS1 DC immunized mice (P<0.01) (Fig. 6B). In vivo IL-12 stimulation also preferentially boosted the stimulatory ability of siSOCS1 DC compared with boosting control DCs, as evidenced by that, with in vivo IL-12 stimulations, 239 IFNγ+ spots per 2 × 105 T cells were detected in siSOCS1 DC immunized splenocytes, but only 10 or 9 IFNγ+ spots per 2 × 105 T cells in control DC immunized splenocytes (Fig. 6A). IL-12 preferentially stimulating siSOCS1 DC was also demonstrated by more active cytolytic response against SK-Mel-37 triggered by siSOCS1 DC immunization compared with immunization by siGFP DCs or mock DCs (Fig. 6B). Furthermore, the CTL response is HLA-A2 restricted, as the isolated splenocytes from either LPS- or IL-12-stimulated, siSOCS1 DC-immunized mice failed to kill HLA-A2-negative NA-6-Mel tumor cell line, as shown in Supplemental Fig. S3A. These results are in agreement with the results shown in Fig. 4.
Figure 6. SOCS1-silenced DC induces more potent CTL responses in humanized HLA-A2.1 transgenic mice.
(A) & (B), SOCS1-silenced DC induces more potent MAGE3-specific T cell response. BM-derived DCs from HLA-A2.1 transgenic mice were transduced with LV-mouse SOCS1 siRNA or LV-GFP siRNA, pulsed with MAGE3 peptide (50 μg/ml), and then matured with TNFα. HLA-A2.1 transgenic mice were immunized with the transduced DCs weekly for two weeks. Following each DC immunization, the mice were stimulated i.p. with murine IL-12 (1μg/mouse) or LPS (30 μg/mouse) on days 1, 3 and 5. Two wk later, CD8+ T-cells isolated from the pooled splenocytes of immunized mice (2–3 per group) were subjected to IFN-γELISPOT assays. An irrelevant OVA-I peptide was used as a negative control (A). Cytotoxicities against human SK-Mel-37 cells were determined (B). The data are representative of two independent assays.
(C) & (D), SOCS1-silenced DC induces more potent LMP2-specific T cell responses. The transduced DCs from HLA-A2.1 transgenic mice were pulsed with LMP2 peptide (50 μg/ml), and then matured with TNFα. HLA-A2.1 transgenic mice were immunized and stimulated as described above. Two wk later, CD8+ T-cells isolated from the pooled splenocytes of immunized mice (2–3 per group) were subjected to IFN-γ ELISPOT assays (C). Cytotoxicities against human EBV-transferred, HLA-A2+ LCLs were determined (D). The data are representative of two independent assays. P <0.01, SOCS1-siRNA DCs vs. GFP-siRNA DCs; P <0.01, SOCS1-siRNA DCs vs. SOCS1-siRNA DCs + IL-12.
To confirm the superior ability of siSOCS1 DC in inducing antigen-specific immune response in the mouse model, groups of HLA-A2.1 transgenic mice were immunized with LMP2-pulsed, transduced DCs followed by in vivo stimulation of LPS or IL-12 as described above. As shown in Fig. 6C, in comparison with siGFP DC or Mock DC immunization, siSOCS1 DC immunization enhanced the frequency of IFNγ+ T cells in response to LMP2 stimulation under in vivo administration of either LPS or IL-12, and augmented LMP2-specific CTL response against HLA-A2+, EBV-transferred LCL under in vivo administration of either LPS or IL-12 (Fig. 6D). Consistent with the above result, in vivo IL-12 stimulation showed more effective than LPS stimulation to elevate the immunostimulatory potency of siSCOS1 DCs (Fig. 6C & D). siSOCS1 DC immunization induced the vigorous CTL response against the LCL line, which is the LMP2-specific, as the splenocytes from siSOCS1 DC immunized, LPS- or IL-12-stimulated mice failed to kill HLA-A2+ A498 tumor cells (Supplemental Fig. S3B).
Discussion
The results of this study, for the first time, demonstrate a critical role of SOCS1 in negatively regulating the immunostimulatory ability of human DCs to prime antigen-specific CTLs. Significantly, we found that hSOCS1-silenced DCs had a unique ability to fully activate human CTLs that possessed a robust lytic function against natural antigen-expressing tumor cells. It has been frequently observed in the clinic and laboratory studies that self antigen-specific T cells can be activated by DC vaccination or in vitro sensitization, as determined by various immune assays such as tetramer staining and ELISPOT assays. However, although such activated T cells can effectively kill artificial antigen-pulsed tumor cells or tumor cells modified to overexpress self-antigens, they usually show a weak cytolytic activity against natural tumor cells, which has been considered to be a main reason for the poor efficacy of current tumor vaccines (22, 23). Our results suggest that enhanced antigen presentation provided by SOCS1-silenced DCs may be required to fully activate self-reactive, low affinity T cells and endow them with active lytic effector function against natural tumor cells.
We further demonstrated that the superior ability of hSOCS1-silenced DCs to prime antigen-specific CTLs was likely due to the enhanced production and signaling of proinflammatory cytokines such as IL-12 (signal 3), which is different from SOCS1−/− disease caused primarily by unrestricted IFN-γ signaling (5, 6) and LPS-induced toxicity in SOCS1−/− mice through unrestricted IFN-α/β signaling (16). This conclusion is based upon the following observations: 1) hSOCS1-silenced DCs produce enhanced levels of IL-12 in response to stimuli; 2) antibody blocking of IL-12 compromises the immunostimulatory capability of SOCS1-silenced DCs; and 3) in vivo administration of a low dose of IL-12 more efficiently enhances antigen-specific CTL responses induced by SOCS1-silenced DCs than those induced by wild-type DCs. The argument that hSOCS1-silenced DCs had an enhanced IL-12 signaling is mainly derived from the observation 3) and in agreement with our previously finding in which murine SOCS1-silenced DCs cotransduced with Ad-IL-12 induced significantly more potent antigen-specific T cell responses than Ad-IL-12 transduced wild type DCs, and knockout of IL-12 receptor deprived the Ad-IL-12-transduced SOCS1-silenced DCs of the superior stimulatory ability (24). The argument is also supported by the study from Nishimura’s group that murine SOCS1 negatively regulated IL-12 signaling (18). However, we should point out that this study does not rule out the roles of other proinflammatory cytokines such as IFN-γ, IL-6, etc. in the induction of CTLs (11). Indeed, addition of anti-IL-6 into the DC:T cell coculture also blocked the immune stimulatory function of siSOCS1 DCs, albeit with a lower efficiency (Fig. S1), implying the other proinflammatory cytokines over-expressed by siSOCS1 DC also play a certain role in up-regulation of DC function.
Early studies suggested the effect of IL-12 on induction of immune response in combination with immunization (25, 26). However, our experimental result showed that siGFP DC or mock DC immunization in combination with IL-12 stimulation only induced a modest CTL immune response (Fig. 4 & 6). To investigate the possible deviation, we examined effect of IL-12 administration on distribution of antitumor effector cells in MAGE3-pulsed, siGFP DC immunized mice. As shown in Supplemental Fig. S4, in vivo IL-12 stimulations led a high frequency of IFNγ+ T cells to respond MAGE3 stimulation in siGFP DC immunized lymph nodes, compared with those in immunized mice without IL-12 stimulation, wherase, IL-12 stimulation only led a low frequency of IFNγ+ T cells to be detected in siGFP DC immunized spleen, although the frequency is also higher than those splenocytes without IL-12 stimulation. With in vivo IL-12 stimulation, siSOCS1 DC immunized lymph nodes persistently exhibited much higher frequency of IFNγ+ cells compared with siGFP DC immunized lymph nodes (data not shown). The result confirmed effect of IL-12 on induction of immune response in combination with immunization, and is also in agreement with the previous studies that IL-12-activated antitumor effector cells preferentially accumulated in peripheral lymph nodes, while they disappeared from spleen in spite of inducing splenomegaly (27). As the IL-12-stimulated, siSOCS1 DC immunized mice harbor active antigen-specific T cell not only in the lymph nodes but also in the spleen (Fig. 6, data not shown), the result supported that silencing SOCS1 in combination with administration of IL-12 would lead to DC more potent in inducing anti-tumor immune responses, and also implied that a critical role of SOCS1-restricted IL-12 signaling in DCs for the induction of antigen-specific T cell responses.
In summary, our results provide evidence that SOCS1 restricts the signaling of IL-12 in DCs, underscoring the importance of cytokine signaling in determining the efficacy of DC-based tumor therapy. The SOCS1 silencing approach has the ability to enhancing an antigen-specific immune response induced by DCs loaded with tumor-associated antigens, which would be more attractive than blocking of CTLA4 on T cells to non-discriminately overactivates self-reactive T cells (28). Overall, this human SOCS1 study implies a translational potential of this generally applicable, SOCS1 silencing approach to develop more effective tumor vaccines.
Supplementary Material
Acknowledgments
We thank Lisa Rollins, Melissa Aldrich, Natasha Lapteva, Andrew Sharabi, and Lei Shen for technical assistance and valuable suggestions. This work was supported by grants from the US Army Prostate Cancer Research Program (to XFH), the National Institute of Health Research Grants (R01CA100841 to XFH) and (R01CA90427, R0148480, and R01AI48711 to SYC), and the Leukemia and Lymphoma Society Research Grant (to SYC). KEK was supported by a predoctoral NIH training grant (T32-AI07495).
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Gilbo E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–11. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 6.Alexander WS, Hilton DJ. The Role of Suppressors of Cytokine Signaling (SOCS) Proteins in Regulation of the Immune Response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 7.Metcalf D, Mifsud S, Di Rago L, Alexander WS. The lethal effects of transplantation of Socs1−/− bone marrow cells into irradiated adult syngeneic recipients. Proc Natl Acad Sci USA. 2003;100:8436–41. doi: 10.1073/pnas.1032925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanada T, Yoshida H, Kato S, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–50. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Ayada T, Kinjyo I, et al. Silencing of SOCS1 in macrophages suppresses tumor development by enhancing antitumor inflammation. Cancer Sci. 2009;100:730–6. doi: 10.1111/j.1349-7006.2009.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–53. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 11.Hanada T, Tanaka K, Matsumura Y, et al. Induction of hyper Th1 cell-type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. J Immunol. 2005;174:4325–32. doi: 10.4049/jimmunol.174.7.4325. [DOI] [PubMed] [Google Scholar]
- 12.Schroers R, Shen L, Slawin K, Huang XF, Chen SY. Promiscuous MHC Class II-Restricted T-Helper Epitopes from Human Telomerase Reverse Transcriptase. Clinical Can Res. 2003;9:4743–55. [PubMed] [Google Scholar]
- 13.Schroers R, Chen SY. Lentiviral transduction of human dendritic cells. Methods Mol Biol. 2004;246:451–9. doi: 10.1385/1-59259-650-9:451. [DOI] [PubMed] [Google Scholar]
- 14.van der Bruggen P, Bastin J, Gajewski T, et al. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038–43. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 15.Curiel TJ, Morris C, Brumlik M, et al. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J Immunol. 2004;172:7425–31. doi: 10.4049/jimmunol.172.12.7425. [DOI] [PubMed] [Google Scholar]
- 16.Gingras S, Parganas E, de Pauw A, Ihle JN, Murray PJ. Re-examination of the Role of Suppressor of Cytokine Signaling 1 (SOCS1) in the Regulation of Toll-like Receptor Signaling. J Biol Chem. 2004;279:54702–7. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 17.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–21. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yahata T, Watanabe K, Ohta A, et al. Accumulation of IL-12-activated antitumor effector cells into lymph nodes of tumor-bearing mice. Immunol Lett. 1998;61:127–33. doi: 10.1016/s0165-2478(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 19.Valmori D, Gileadi U, Servis C, et al. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen MAGE-3. J Exp Med. 1999;189:895–906. doi: 10.1084/jem.189.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valmori D, Liénard D, Waanders G, Rimoldi D, Cerottini JC, Romero P. Analysis of MAGE-3–specific cytolytic T lymphocytes in human leukocytes antigen–A2 melanoma patients. Cancer Res. 1997;57:735–41. [PubMed] [Google Scholar]
- 21.Park D, Lapteva N, Seethammagari M, Slawin KM, Spencer DM. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24:1581–90. doi: 10.1038/nbt1262. [DOI] [PubMed] [Google Scholar]
- 22.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–8. [PubMed] [Google Scholar]
- 23.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–94. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song XT, Evel-Kabler K, Rollins L, et al. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sangro B, Melero I, Qian C, Prieto J. Gene therapy of cancer based on interleukin-12. Curr Gene Ther. 2005;5:573–81. doi: 10.2174/156652305774964712. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–21. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yahata T, Watanabe K, Ohta A, et al. Accumulation of IL-12-activated antitumor effector cells into lymph nodes of tumor-bearing mice. Immunol Lett. 1998;61:127–33. doi: 10.1016/s0165-2478(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 28.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–77. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.