Abstract
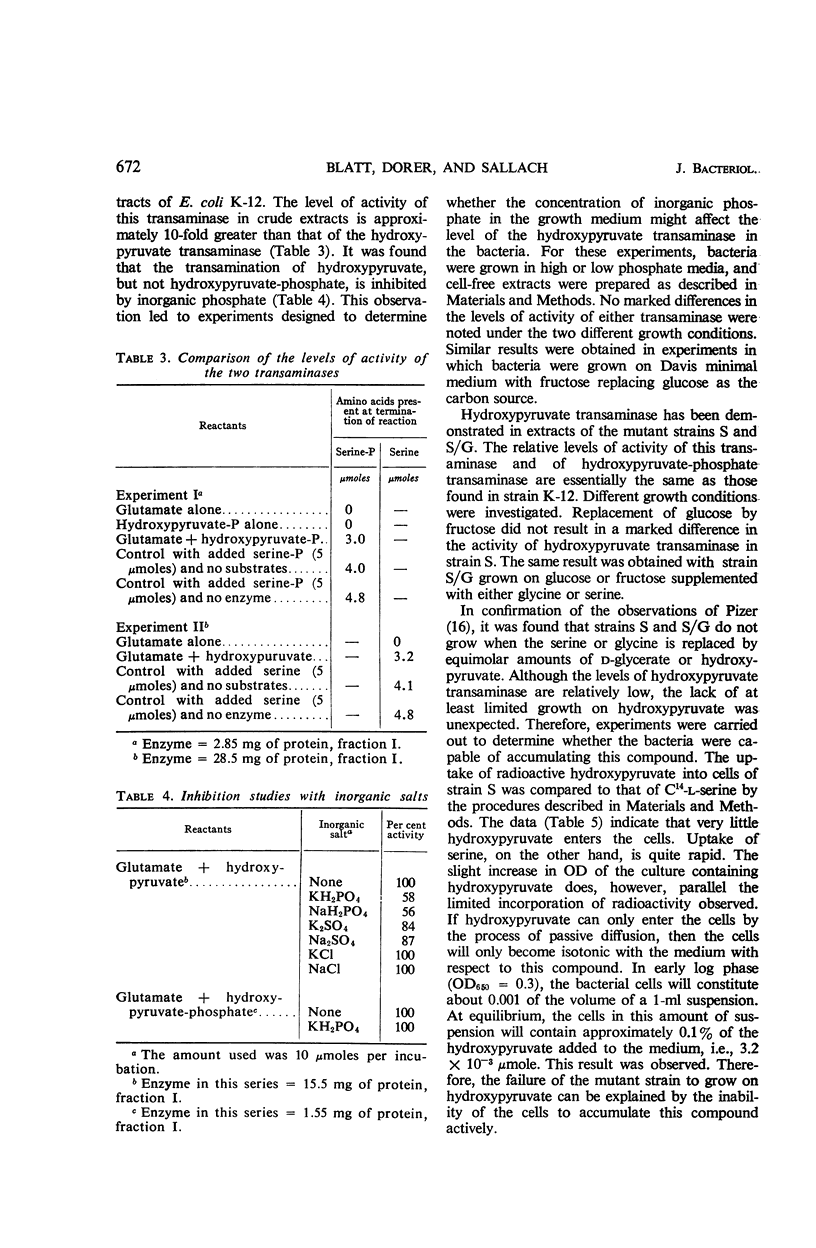
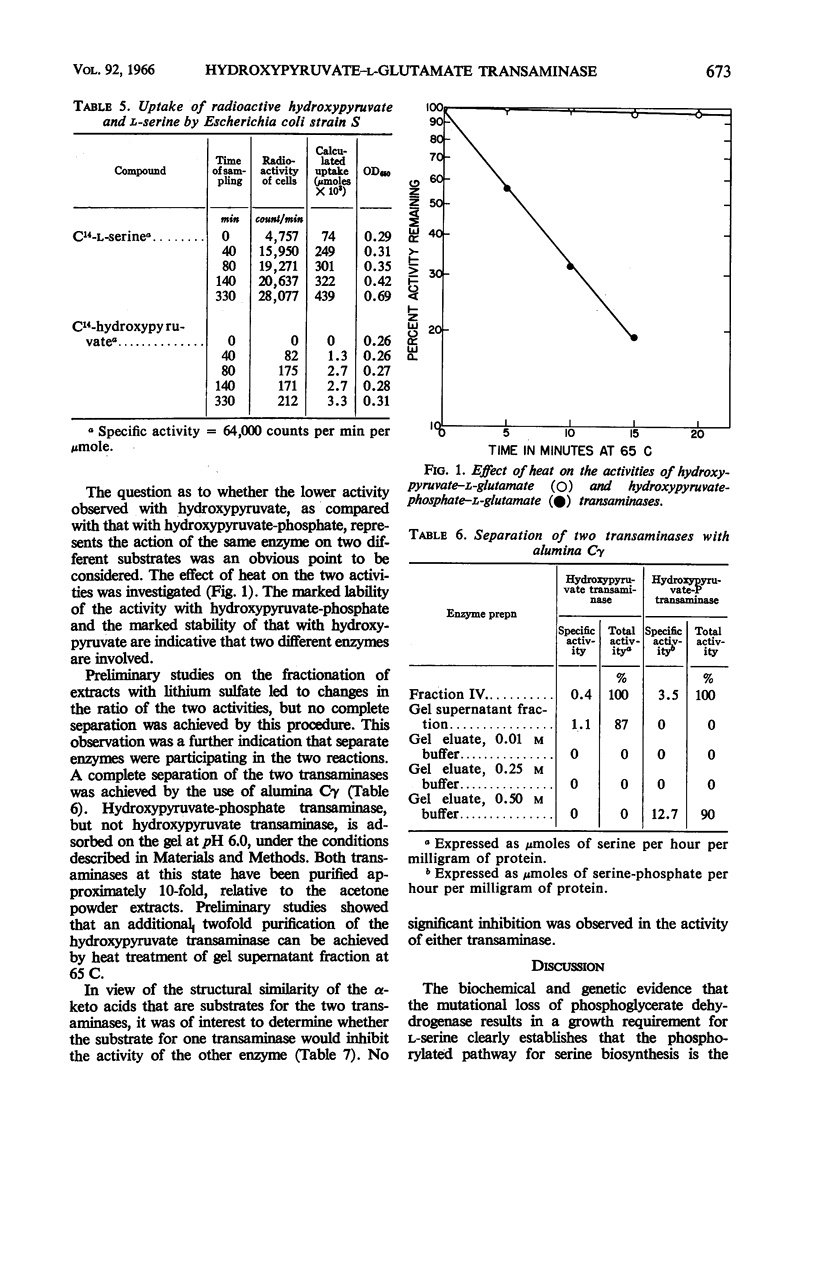
Blatt, L. (University of Wisconsin, Madison), F. E. Dorer, and H. J. Sallach. Occurrence of hydroxypyruvate–l-glutamate transaminase in Escherichia coli and its separation from hydroxypyruvate-phosphate–l-glutamate transaminase. J. Bacteriol. 92:668–675. 1966.—The formation of l-serine from hydroxypyruvate by a transamination reaction with l-glutamate has been demonstrated in extracts of Escherichia coli. The level of activity with hydroxypyruvate is approximately one-tenth that observed with hydroxypyruvate-phosphate in cell-free extracts. The transamination of hydroxypyruvate, but not hydroxypyruvate-phosphate, is inhibited by inorganic phosphate. No marked differences in the levels of activity with hydroxypyruvate were observed in extracts from bacteria grown under different conditions. Heat treatment of enzyme preparations at 65 C rapidly destroys the activity with hydroxypyruvate-phosphate, but not that with hydroxypyruvate. Fractionation of extracts with lithium sulfate and alumina Cγ resulted not only in a 10-fold purification, but also in a complete separation of the two activities, thereby establishing that two different enzymes are involved in the transamination of hydroxypyruvate and hydroxypyruvate-phosphate. Hydroxypyruvate transaminase is present in two mutants that require serine for growth. The inability of hydroxypyruvate to replace the growth requirement for serine, even to a limited extent, was shown to be due to the inability of the bacteria to accumulate this compound actively.
Full text
PDF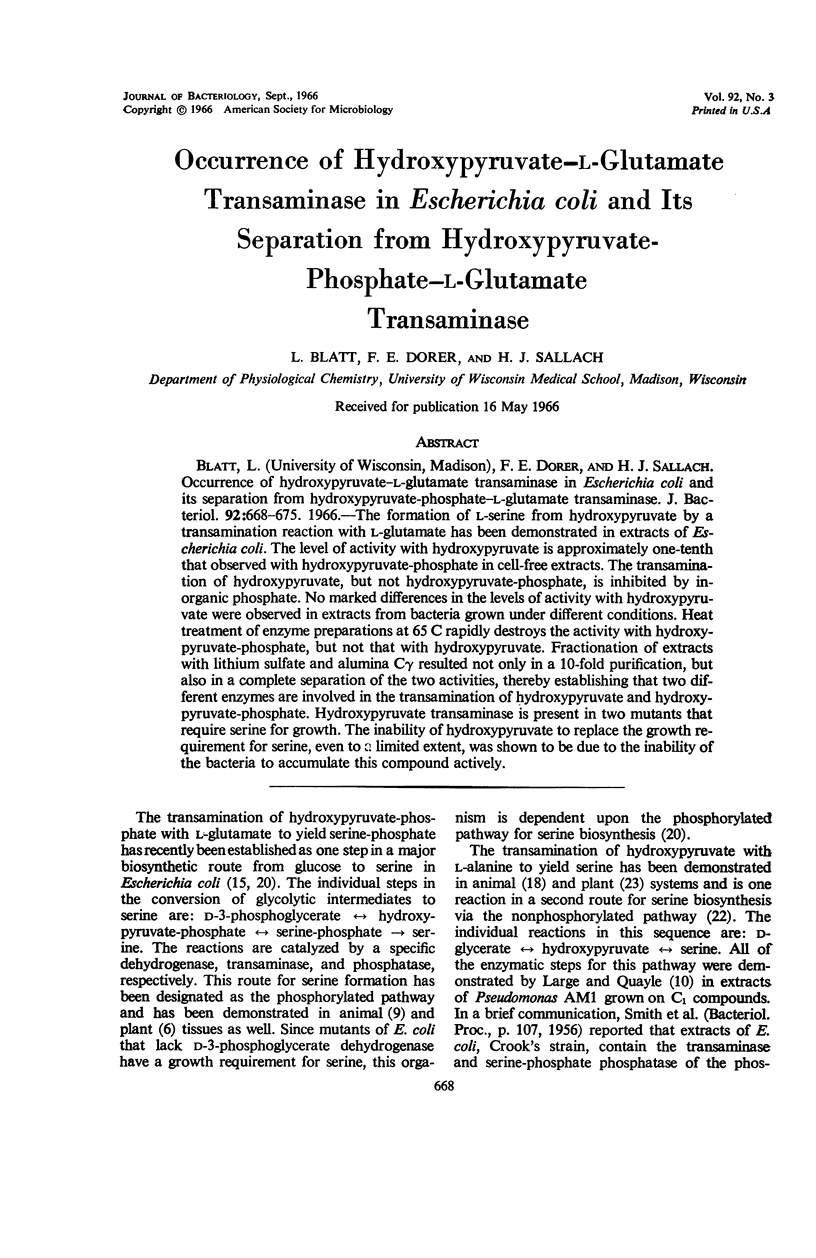





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. The preparation and properties of lithium hydroxypyruvate and hydroxypyruvic acid. Biochem J. 1958 Jan;68(1):74–81. doi: 10.1042/bj0680074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GREENBERG D. M., ICHIHARA A. Further studies on the pathway of serine formation from carbohydrate. J Biol Chem. 1957 Jan;224(1):331–340. [PubMed] [Google Scholar]
- HEDRICK J. L., SALLACH H. J. The metabolism of hydroxypyruvate. I. The nonenzymatic decarboxylation and autoxddation of hydroxypyruvate. J Biol Chem. 1961 Jul;236:1867–1871. [PubMed] [Google Scholar]
- HOLZER H., HOLLDORF A. Isolierung von D-Glycerat-dehydrogenase, einige Eigenschaften des Enzyms und seine Verwendung zur enzymatisch-optischen Bestimmung von Hydroxypyruvat neben Pyruvat. Biochem Z. 1957;329(4):292–312. [PubMed] [Google Scholar]
- LEVINE E. M., SIMMONDS S. Metabolite uptake by serineglycine auxotrophs of Escherichia coli. J Biol Chem. 1960 Oct;235:2902–2909. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., POTOCHNY M. L. NUTRITIONAL AND REGULATORY ASPECTS OF SERINE METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1964 Sep;88:611–619. doi: 10.1128/jb.88.3.611-619.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., WAGNER R. P., SNELL E. E. Biosynthesis of valine and i43soleucine, 3. alpha-Keto-beta-hydroxy acid reductase and alpha-hydroxy-beta-Keto acid reductoisomerase. J Biol Chem. 1960 Aug;235:2322–2331. [PubMed] [Google Scholar]
- SALLACH H. J. Formation of serine hydroxypryuvate and L-alanine. J Biol Chem. 1956 Dec;223(2):1101–1108. [PubMed] [Google Scholar]
- STAFFORD H. A., MAGALDI A., VENNESLAND B. The enzymatic reduction of hydroxypyruvic acid to D-glyceric acid in higher plants. J Biol Chem. 1954 Apr;207(2):621–629. [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIS J. E., SALLACH H. J. Evidence for a mammalian D-glyceric dehydrogenase. J Biol Chem. 1962 Mar;237:910–915. [PubMed] [Google Scholar]
- Walsh D. A., Sallach H. J. Purification and properties of chicken liver D-3-phosphoglycerate dehydrogenase. Biochemistry. 1965 Jun;4(6):1076–1085. doi: 10.1021/bi00882a015. [DOI] [PubMed] [Google Scholar]



