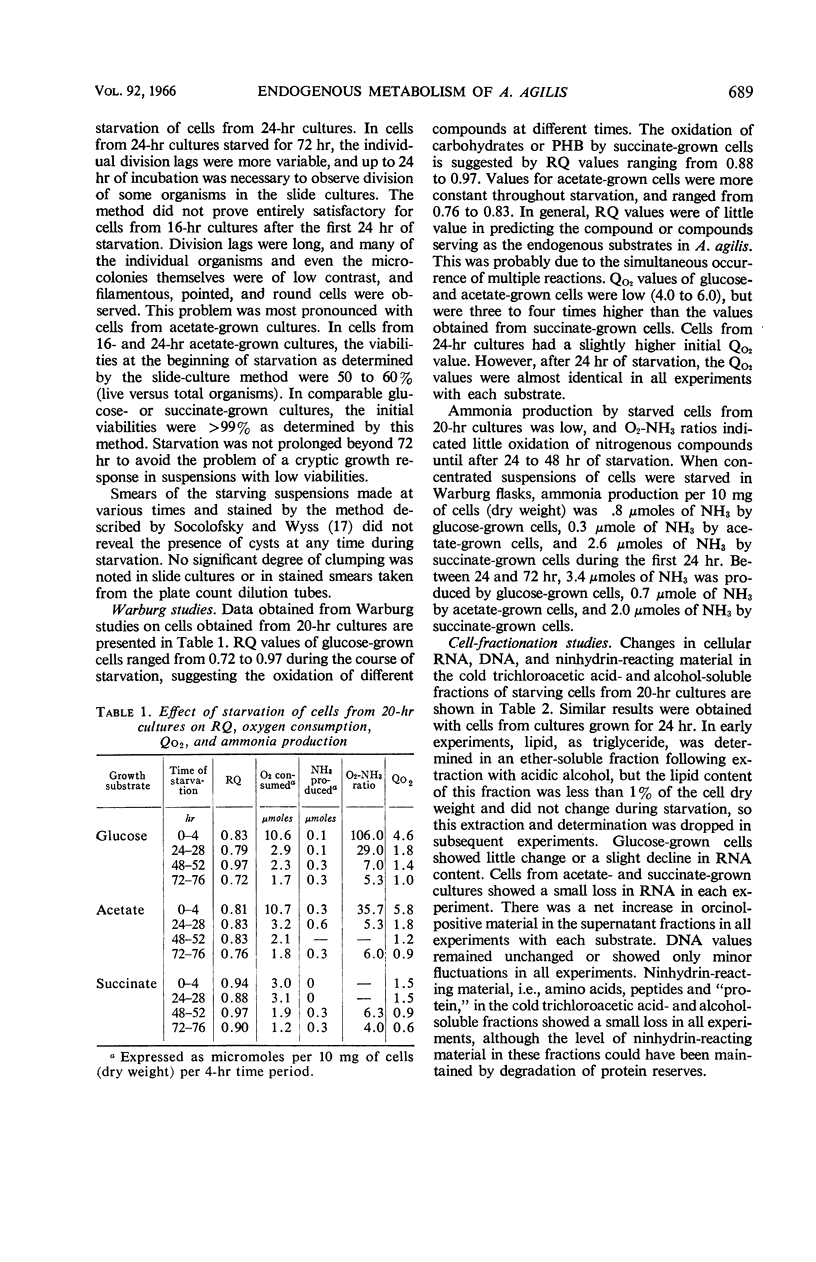
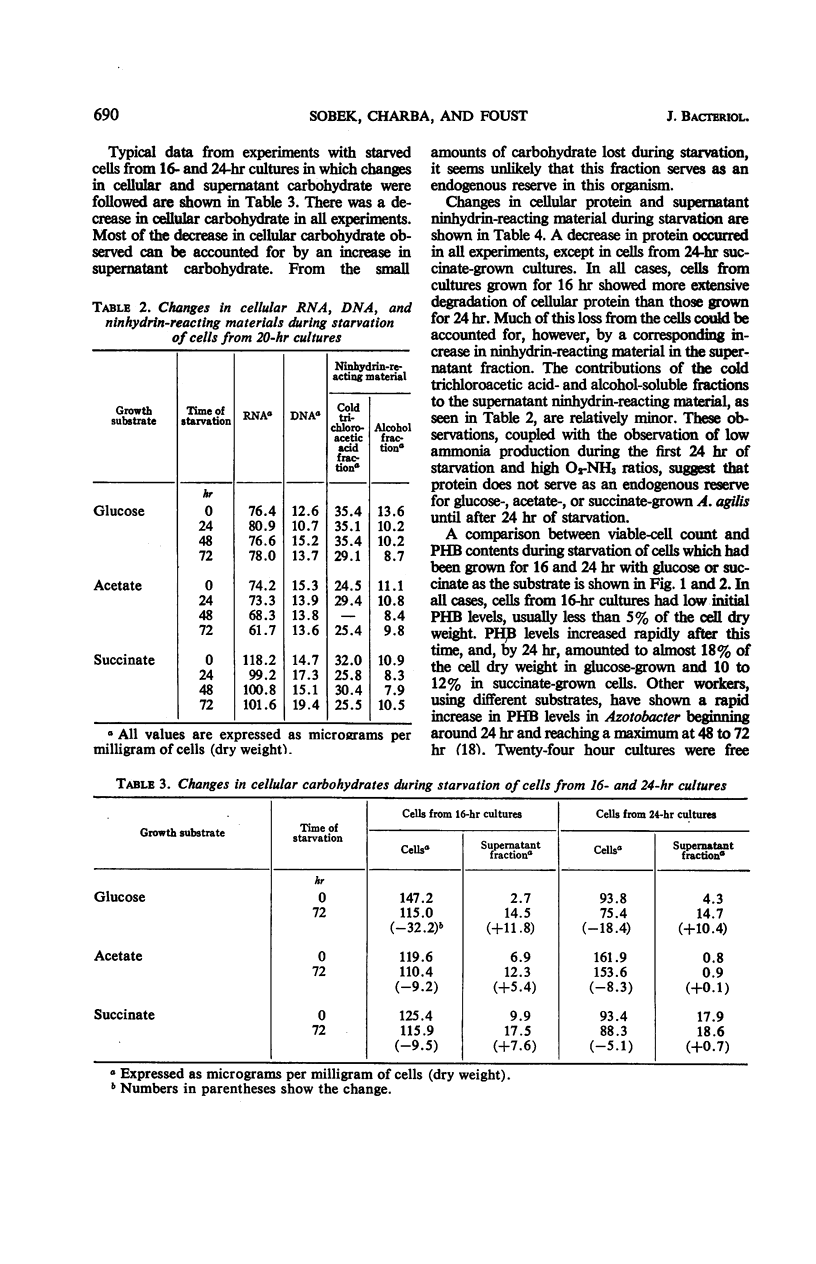
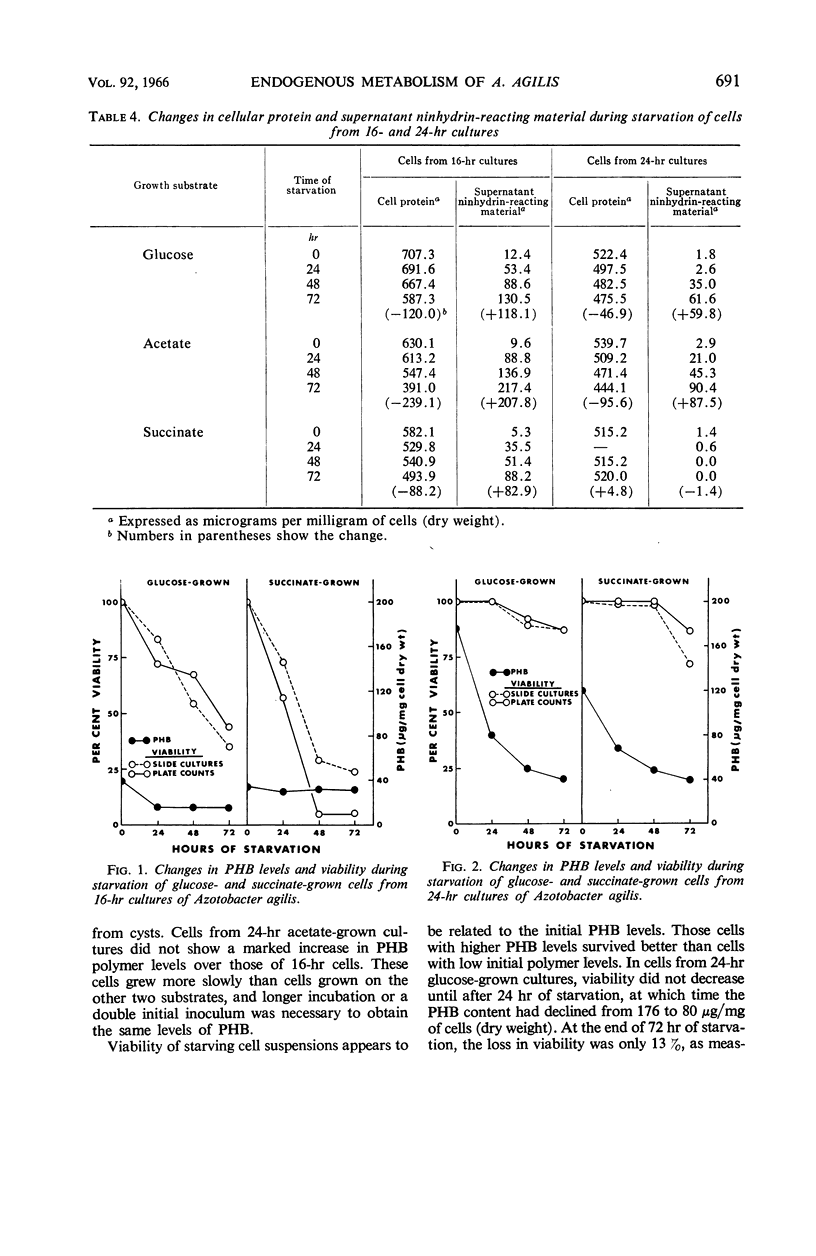
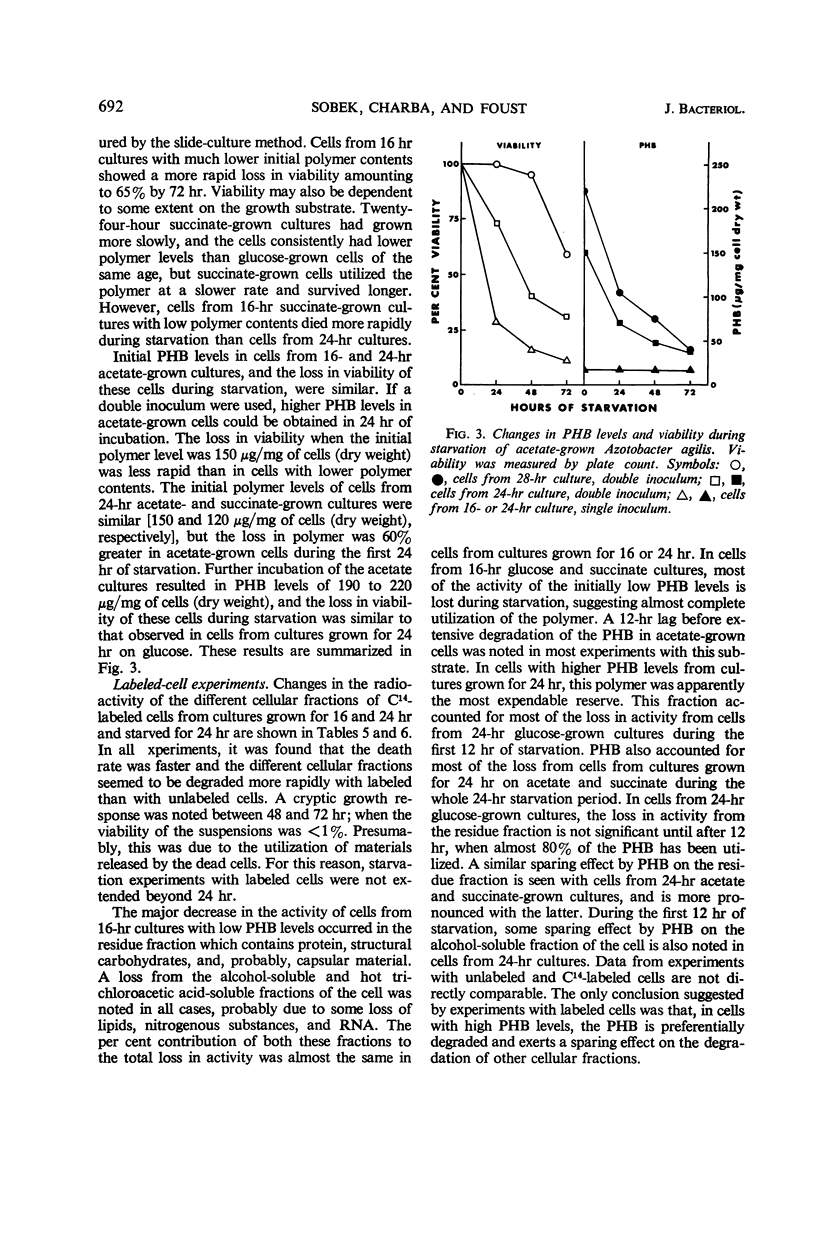
Abstract
Sobek, J. M. (University of Southwestern Louisiana, Lafayette), J. F. Charba, and W. N. Foust. Endogenous metabolism of Azotobacter agilis. J. Bacteriol. 92:687–695. 1966—Ribonucleic acid, deoxyribonucleic acid, cellular carbohydrate, and the cold trichloroacetic acid and acidic alcohol fractions of the cell do not appear to function as endogenous reserves for Azotobacter agilis. The immediate endogenous reserve of cells grown on glucose, acetate, or succinate was poly-β-hydroxybutyric acid (PHB). Viability of the cells during starvation was dependent upon the initial levels of PHB and the growth substrate. Cells with high initial PHB levels survived longer than cells with lower levels. Cells from succinate-grown cultures had lower PHB levels than cells from glucose-grown cultures, but were capable of maintaining their viability longer. Cellular protein may also serve as a secondary endogenous reserve substrate for this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLIFTON C. E., SOBEK J. M. Endogenous respiration of Bacillus cereus. J Bacteriol. 1961 Aug;82:252–256. doi: 10.1128/jb.82.2.252-256.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON A. P., Jr, LAWRENCE F. R. PHENOTYPIC, GENOTYPIC, AND CHEMICAL CHANGES IN STARVING POPULATIONS OF AEROBACTER AEROGENES. J Bacteriol. 1963 Apr;85:742–750. doi: 10.1128/jb.85.4.742-750.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. J., SOBEK J. M., CLIFTON C. E. Oxidative assimilation by Azotobacter agilis. J Bacteriol. 1958 Dec;76(6):658–661. doi: 10.1128/jb.76.6.658-661.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACRAE R. M., WILKINSON J. F. Poly-beta-hyroxybutyrate metabolism in washed suspensions of Bacillus cereus and Bacillus megaterium. J Gen Microbiol. 1958 Aug;19(1):210–222. doi: 10.1099/00221287-19-1-210. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., CRUMPTON J. E., HUNTER J. R. The measurement of bacterial viabilities by slide culture. J Gen Microbiol. 1961 Jan;24:15–24. doi: 10.1099/00221287-24-1-15. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- SIERRA G., GIBBONS N. E. Role and oxidation pathway of poly-beta-hydroxybutyric acid in Micrococcus halodenitrificans. Can J Microbiol. 1962 Apr;8:255–269. doi: 10.1139/m62-032. [DOI] [PubMed] [Google Scholar]
- Socolofsky M. D., Wyss O. CYSTS OF AZOTOBACTER. J Bacteriol. 1961 Jun;81(6):946–954. doi: 10.1128/jb.81.6.946-954.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson L. H., Socolofsky M. D. Cyst formation and poly-beta-hydroxybutyric acid accumulation in Azotobacter. J Bacteriol. 1966 Jan;91(1):304–310. doi: 10.1128/jb.91.1.304-310.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]



