Abstract
Purpose
To compare the toxicity and biochemical outcomes of intensity-modulated radiation therapy (IMRT) and 125I transperineal permanent prostate seed implant (125I) for patients with low-risk prostate cancer.
Methods and Materials
Between 1998 and 2004, a total of 374 low-risk patients (prostate-specific antigen < 10 ng/ml, T1c–T2b, Gleason score of 6 or less, and no neoadjuvant hormones) were treated at Fox Chase Cancer Center (216 IMRT and 158 125I patients). Median follow-up was 43 months for IMRT and 48 months for 125I. The IMRT prescription dose ranged from 74–78 Gy, and 125I prescription was 145 Gy. Acute and late gastrointestinal (GI) and genitourinary (GU) toxicity was recorded by using a modified Radiation Therapy Oncology Group scale. Freedom from biochemical failure was defined by using the Phoenix definition (prostate-specific antigen nadir + 2.0 ng/ml).
Results
Patients treated by using IMRT were more likely to be older and have a higher baseline American Urological Association symptom index score, history of previous transurethral resection of the prostate, and larger prostate volumes. On multivariate analysis, IMRT was an independent predictor of lower acute and late Grade 2 or higher GU toxicity and late Grade 2 or higher GI toxicity. Three-year actuarial estimates of late Grade 2 or higher toxicity were 2.4% for GI and 3.5% for GU by using IMRT compared with 7.7% for GI and 19.2% for GU for 125I, respectively. Four-year actuarial estimates of freedom from biochemical failure were 99.5% for IMRT and 93.5% for 125I (p = 0.09).
Conclusions
The IMRT and 125I produce similar outcomes, although IMRT appears to have less acute and late toxicity.
Keywords: Prostate cancer, Radiation therapy, IMRT, Brachytherapy, Toxicity
INTRODUCTION
Patients with low-risk prostate cancer can be treated effectively by using surgery, external beam radiation therapy (RT), or low-dose-rate transperineal permanent prostate seed implants (1, 2). Permanent prostate implants have been used for many years and conceptually are an elegant method to obtain a highly conformal radiation dose delivery. Low-risk patients are ideally suited for this procedure because of a low chance of extracapsular extension or spread to seminal vesicles or lymph nodes. A number of series published outcomes equivalent to surgery with long-term follow-up (3–5). External beam RT as a definitive treatment for patients with low-risk prostate cancer using conformal techniques also was used for many years with durable clinical results (6). It now generally is accepted that cancer control outcomes are equivalent among surgery, brachytherapy, and external beam RT, and patients should make their treatment decision based in part on their own bias, with consideration of toxicities (4, 5, 7). Although intensity-modulated RT (IMRT) is now common in the United States, there are limited reports of the toxicity and outcomes achievable by using this technique (8, 9).
At Fox Chase Cancer Center (FCCC), Philadelphia, PA, the use of magnetic resonance imaging (MRI) simulation to improve target delineation, daily prostate localization to reduce setup uncertainties, and IMRT have been in practice for more than 8 years. These methods should improve the toxicity profile of external beam RT (10–12). Permanent seed implants as a treatment option for patients with low-risk prostate cancer was introduced at FCCC in 1998. Seed implants are a more convenient treatment for patients compared with the logistics of daily external beam RT for up to 8 weeks, but with improvements in external beam RT, a direct comparison is necessary. This study is designed to compare the outcomes and morbidity between prostate implants and IMRT for the treatment of patients with low-risk prostate cancer.
METHODS AND MATERIALS
This study included all patients treated at our institution with low-risk prostate cancer defined by using American Joint Committee on Cancer Clinical Stage (13) T1C–T2B, prostate-specific antigen (PSA) level of 10 ng/ml or less, and Gleason score of 6 or less who were treated by using either 125I permanent prostate implant alone (May 1998–August 2004) or IMRT (August 2001–June 2004). Patients were excluded if they had any neoadjuvant androgen deprivation therapy, were treated with a combination of external beam RT and seed implant, or had less than 15 months’ follow-up. All pathology slides for cases diagnosed in referring institutions were centrally reviewed at FCCC for Gleason scoring.
Our policy has been to offer permanent seed implants to patients with prostate volumes of 20–60 ml, baseline urinary function using the American Urological Association (AUA) scoring system of less than 15, and no significant anesthesia risk. Although not an absolute contraindication, we have been cautious about placing implants in patients with previous transurethral resection of the prostate (TURP) or diabetes.
IMRT
Patients underwent simulation supine in an α-cradle (Smithers Medical Products, Inc., North Canton, OH), stabilizing their pelvis with an acrylic holder to immobilize their feet. Patients were instructed to have a moderately full bladder and an enema per rectum before simulation. Patients underwent simulation using computed tomography (CT) and MRI unless there was a contraindication to performing an MRI. A slice thickness of 3 mm was used for both scans. Scans were fused in the planning computer based on bony and soft-tissue anatomy by using either chamfer matching or maximization of mutual information methods. Structures outlined on MRI included the prostate, seminal vesicles, rectum (from ischial tuberosity to sigmoid flexure), entire bladder, and bilateral femoral heads. Clinical tumor volume was defined as the prostate and proximal seminal vesicles (the first 9 mm of the seminal vesicles). Clinical tumor volume was expanded by 5–6 mm posteriorly and 8 mm in all other directions to produce the planning target volume (PTV). Prescription dose range was 74–78 Gy, delivered with 6 MV or higher photons in daily fractions of 2.0 Gy. The PTV was required to receive at least 95% of the prescription dose or higher. Rectal volume receiving greater than 65 Gy and greater than 40 Gy were limited to less than 17% and less than 35%, respectively. Bladder volume receiving greater than 65 Gy and greater than 40 Gy were limited to less than 25% and less than 50%, respectively. Step-and-shoot inverse planning was performed using the Corvus (NOMOS, Cranberry Township, PA) treatment planning system. Daily prostate localization was performed using B-mode acquisition and targeting ultrasound alignment (NOMOS).
125I implant
Preoperative ultrasound volume studies were performed, and pre-plans were obtained before 2000. With the introduction of the MRI simulator, patients underwent preimplantation MRI to measure volume. Real-time intraoperative treatment planning was used. Prophylactic α-blocker therapy was begun for all patients within the week before implantation and continued with nonsteroidal anti inflammatory agents after implantation. A 3–5-mm margin was placed around the ultrasound-defined prostate volume to create the PTV, except posterior, where no margin was added. A modified peripheral-loading plan was calculated with a prescription dose of 145 Gy by using Variseed software (Varian Medical Systems, Palo Alto, CA). Eight patients had prescribed doses of 160 Gy before incorporation of the American Association of Physicists in Medicine Task Group No. 43 recommendations (14). Patients underwent implantation transperineally under transrectal ultrasound guidance with preloaded needles. Postimplantation dosimetry was performed for patients on Day 0 and then 3–4 weeks after implantation by using CT and MRI data. Urethral dosimetry was calculated from the Day 0 CT by using the indwelling catheter to define the prostatic urethra. From Feb 2000, dosimetry was calculated from MRI and CT fusion using our MRI simulator. Seeds were localized on CT by using the Variseed software.
Biochemical and toxicity end points
Freedom from biochemical failure (FFBF) was defined by using the Phoenix (PSA nadir + 2.0 ng/mL) definition (15). The lowest PSA level after completion of treatment was defined as the nadir. Acute and late gastrointestinal (GI) and genitourinary (GU) toxicity was recorded at each follow-up by using a modification of the Radiation Therapy Oncology Group (RTOG) and Late Effects Normal Tissue Task Force criteria (Appendix 1) (16, 17). In this modification, any use of an α-blocker for urinary obstructive symptoms was coded as Grade 1. Statistical differences in acute toxicity were determined by using Fisher’s exact test. Late effects were initially defined as longer than 3 months from completion of RT. To account for the half-life of 125I, late effects were also reanalyzed as occurring longer than 12 months postimplantation in brachytherapy patients. Kaplan-Meier survival curves, logistic regression, and Cox proportional hazards analyses were used to assess univariate and multivariate differences, hazard ratios (HRs), and 95% confidence intervals (CIs) (18, 19). Variables included in the multivariate analysis of FFBF were clinical stage (T2 vs. T1c), initial PSA level (continuous), and treatment type (125I vs. IMRT). Gleason score was not included because there were only 14 patients with a Gleason score other than 6. Variables included in the multivariate analysis of toxicity were treatment modality (125I vs. IMRT), age (continuous), diabetes, previous TURP, prostate size (continuous), and initial AUA score. Median AUA score was used as the cutoff value for this analysis (≤5 vs. 6–35).
APPENDIX 1.
MODIFIED RTOG RADIATION TOXICITY SCALE
| Lower GI toxicity | |
|---|---|
| Acute GI toxicity | |
| I | Increased frequency or change in quality of bowel habits needing ≤2 antidiarrheals/wk |
| Rectal discomfort not requiring analgesics | |
| Mild rectal bleeding needing occasional steroid suppositories or other medications | |
| II | Diarrhea needing > 2 antidiarrheals/wk. |
| Mucous discharge requiring ≤ 1 sanitary pad/d | |
| Rectal pain needing occasional narcotics | |
| Rectal bleeding needing regular steroid suppositories or other medication. | |
| Rectal bleeding or other GI symptoms requiring a treatment break ≤1 week | |
| III | Diarrhea needing > 2 antidiarrheals/d or parenteral support |
| Severe mucous discharge requiring >1 sanitary pad/d | |
| Rectal pain requiring regular narcotics | |
| GI bleeding requiring 1 transfusion | |
| Rectal bleeding or other GI symptoms requiring a treatment break > 1 wk | |
| IV | Acute or subacute obstruction |
| Fistula or perforation | |
| GI bleeding requiring > 1 transfusion | |
| Abdominal pain or tenesmus requiring bowel diversion | |
| Late GI toxicity | |
| I | Excess bowel movements twice baseline or need for occasional antidiarrheal use |
| Slight rectal discharge or bleeding not requiring pads | |
| Temporary steroids per suppositories or enemas for proctitis/ulceration of ≤ 1 mo | |
| II | Regular antidiarrheal use |
| Coagulations ≤ 2 for bleeding | |
| Steroids per suppositories or enema for proctitis/ulceration > 1 mo | |
| Mucous discharge requiring sanitary pads < 2/d | |
| Occasional narcotic for pain | |
| III | Hospitalization for treatment-related dehydration |
| One blood transfusion or > 2 coagulations for bleeding | |
| Hyperbaric oxygen treatment for ulceration or bleeding | |
| Sanitary pads ≥ 2/d for > 1 mo | |
| Regular narcotic use | |
| IV | Fistula or obstruction requiring surgery |
| More than 1 blood transfusion | |
| Urinary toxicity | |
| Acute GU toxicity | |
| I | Frequency or nocturia twice pretreatment habit or medication (e.g., α-blocker) over baseline |
| Dysuria or pain requiring non-narcotic medication | |
| Microscopic or infrequent gross hematuria not needing intervention | |
| II | Frequency or nocturia hourly |
| Pain requiring anesthetic or occasional narcotics | |
| Regular use of antispasmodic | |
| Hematuria or GU symptoms requiring a treatment break ≤ 1 wk | |
| Urinary obstruction requiring temporary catheterization (including Foley or self-catheterization) for ≤ 1 wk | |
| III | Frequency or nocturia > 1 time/h |
| Requiring regular narcotics | |
| Hematuria or GU symptoms requiring a treatment break > 1 wk | |
| Gross hematuria requiring 1 transfusion | |
| Urinary obstruction requiring catheterization (including Foley, self-catheterization, or suprapubic) for > 1 wk | |
| IV | Hematuria needing > 1 transfusion |
| Hospitalization for sepsis due to obstruction, ulceration, and/or necrosis of the bladder | |
| Late GU toxicity | |
| I | Nocturia twice baseline or medication (e.g., α-blocker) increase over baseline |
| Hematuria not requiring intervention | |
| Light mucosal atrophy and minor telangiectasia | |
| Dysuria or pain requiring occasional non-narcotic medication | |
| Incontinence or dribbling not requiring sanitary pad (over baseline) | |
| II | Frequency less than every hour, nocturia > 2 times baseline |
| Generalized telangiectasias | |
| Hematuria requiring ≤ 2 cauterizations | |
| Pain requiring regular anti-inflammatory agent, anesthetic or antispasmodic, or occasional narcotic | |
| Stricture requiring ≤ 2 dilatations | |
| Foley or self-catheterization for ≤ 2 wk; incontinence requiring ≤ 2 sanitary pads (over baseline) | |
| III | Frequency or nocturia hourly or more |
| Dysuria and/or pain requiring regular narcotic use | |
| Reduction in bladder capacity (150 ml) | |
| At least 1 blood transfusion or > 2 cauterizations for bleeding | |
| Hyperbaric oxygen treatment | |
| Foley or self-catheterization for > 2 wk | |
| Urethrotomy, TURP, or > 2 dilatations | |
| Incontinence requiring > 2 sanitary pads (over baseline) or artificial sphincter | |
| IV | Gross hematuria requiring > 1 blood transfusion |
| Severe hemorrhagic cystitis or ulceration requiring urinary diversion and/or cystectomy | |
RESULTS
A total of 374 patients were included in this study; 216 were treated with IMRT and 158 were treated with 125I. Median follow-up was 43 months (range: 17–61 months) for IMRT patients and 48 months (range: 16–99 months) for 125I patients. Patient demographics are listed in Table 1. There were no statistical differences between groups for initial PSA level or Gleason score. There were no patients treated by using 125I with clinical Stage T2b disease compared with 14 patients (6.5%) treated by using IMRT (p = 0.005). The IMRT patients were more likely to be older (median age, 67.6 vs. 64.7 years; p = 0.001) and have larger prostates (47.8 compared with 38.1 ml; p < 0.001), higher baseline AUA symptom score (6.0 vs. 4.0; p = 0.014), and history of previous TURP (7.9% vs. 1.3%; p = 0.004). There was no significant difference in numbers of patients with diabetes (16.7% vs. 11.4%; p = 0.15). Median dose to 90% of the prostate at Week 3 for 125I patients was 153.6 Gy.
Table 1.
Patient characteristics for the study group
| IMRT | 125I | p* | |
|---|---|---|---|
| n | 216 | 158 | |
| Median age (y) | 67.6 (26.7–80.6) | 64.7 (42.0–78.3) | 0.001 |
| PSA (ng/ml) | |||
| <5 | 97 (44.9) | 71 (44.9) | 0.38 |
| 5–8 | 103 (47.7) | 69 (43.7) | |
| 8–10 | 16 (7.4) | 18 (11.4) | |
| Median | 5.2 (0.4–9.6) | 5.2 (0.5–9.8) | |
| T1c | 169 (78.2) | 132 (83.5) | 0.005 |
| T2a | 33 (15.3) | 26 (16.5) | |
| T2b | 14 (6.5) | 0 (0) | |
| Gleason score 5 | 8 (3.7) | 6 (3.8) | 0.96 |
| Gleason score 6 | 208 (96.3) | 152 (96.2) | |
| Median prostate size (ml) | 47.8 (12.9–160) n = 199 | 38.1 (22–66.8) n = 158 | 0.001 |
| Median baseline AUA | 6.0 (0–35) n = 167 | 4.0 (0–17) n = 73 | 0.014 |
| Diabetes | 36/216 (16.7) | 18/158 (11.4) | 0.15 |
| TURP before radiotherapy | 17/216 (7.9) | 2/158 (1.3) | 0.004 |
| Median D90 (Gy) | NA | 153.6 | NA |
Abbreviations: IMRT = intensity-modulated radiotherapy; PSA = prostate-specific antigen; AUA = American Urologic Association symptom score; TURP = transurethral resection of the prostate; D90 = dose to 90% of the prostate; NA = not applicable.
Values expressed as median (range) or number (percent) unless noted otherwise.
p from chi-square test.
Acute toxicity
Of 216 patients treated by using IMRT, 5 patients (2.3%) experienced an acute Grade 2 GI toxicity compared with 3 125I patients (1.9%). This difference was not statistically different (p = 1.00). No patient in either group experienced a Grade 3 or Grade 4 acute GI toxicity. Acute Grade 2 or higher GU toxicity was significantly lower in IMRT patients (6.9% vs. 26.6%; p < 0.001). Acute Grade 3 GU toxicity was recorded in 3 IMRT patients (1.4%) and 6 125I patients (3.8%). This difference was not statistically significant (p = 0.18). Catheterization in the first 3 months for urinary obstruction was required in 11 patients (7.0%) treated by using 125I compared with 4 patients (1.9%) treated by using IMRT. In 3 IMRT patients and 6 125I patients, the catheter was required for longer than 7 days, accounting for the Grade 3 toxicity in each group. In 3 patients, all treated by using 125I, long-term intermittent catheterization was required, which may be considered a delayed acute toxicity. There were no Grade 4 acute GU toxicities in either group (Table 2).
Table 2.
Comparison of acute and late toxicity between IMRT and 125I seed monotherapy
| Acute toxicity | IMRT | 125I | Fisher’s exact test |
|---|---|---|---|
| GI Grade 2 | 5/216 (2.3%) | 3/158 (1.9%) | 1.00 |
| GI Grade 3 | 0 (0) | 0 (0) | |
| GU ≥ Grade 2 | 15/216 (6.9%) | 42/158 (26.6%) | <0.0001† |
| GU Grade 3 | 3/216 (1.4%) | 6/158 (3.8%) | 0.1758 |
| Late toxicity* | Log rank | ||
| GI Grade 2 | 2.4% | 7.8% | 0.0278 |
| GI Grade 3 | 0.0% | 0.1% | 0.2276 |
| GU ≥ Grade 2 | 3.5% | 19.2% | <0.001 |
| GU Grade 3 | 0.5% | 5.6% | 0.006 |
| Urethral strictures | 0 | 11/158 (7.0%) | |
Abbreviations: IMRT = intensity-modulated radiotherapy; GI = gastrointestinal; GU = genitourinary.
Three-year actuarial risk.
Boldface = significant.
Late toxicity
Kaplan-Meier estimate of Grade 2 or higher GI toxicity is shown in Fig. 1a. At 3 years, this was 2.4% for IMRT and 7.8% for 125I (p = 0.03). There were no Grade 3 or Grade 4 late GI toxicities in the IMRT group. One patient (0.7% actuarial 3-year risk) had a late Grade 3 GI toxicity in the 125I cohort caused by proctitis, which developed at 30 months after treatment. On multivariate analysis, treatment group (125I vs. IMRT; HR, 3.18; 95% CI, 1.00–10.05; p = 0.05) approached significance, but age (continuous; HR, 1.04; 95% CI, 0.97–1.11; p = 0.29), prostate volume (continuous; HR, 1.00; 95% CI, 0.97–1.03; p = 0.80), and diabetes (HR, 0.66; 95% CI, 0.15–2.90; p = 0.58) were not significant independent predictors of late Grade 2 or higher GI toxicity.
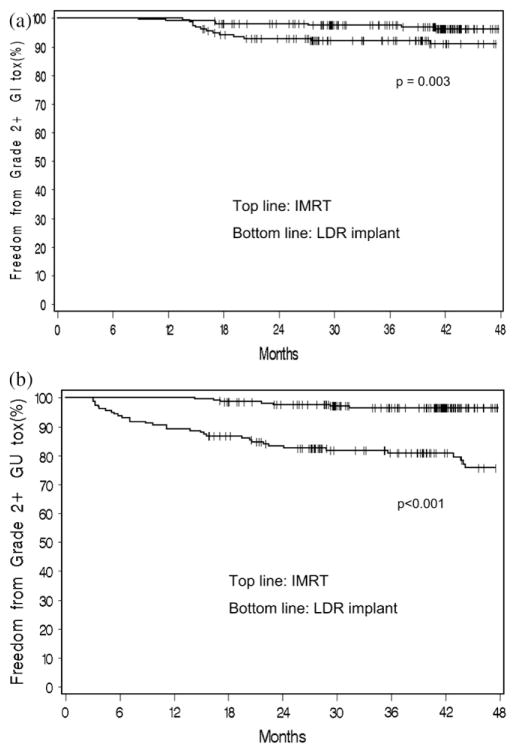
Fig. 1.
Actuarial estimate of late toxicity of Grade 2 or higher for intensity-modulated radiotherapy (IMRT) (top line) and 125I (bottom line). (a) Gastrointestinal (GI) and (b) genitourinary (GU) toxicity. Late toxicity defined as occurring longer than 3 months posttreatment. LDR = low dose rate.
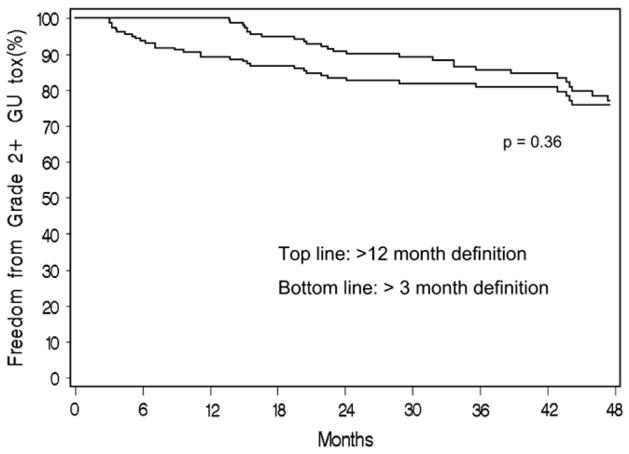
Figure 1b shows the actuarial estimate of Grade 2 or higher GU toxicity. Three-year estimates were 3.5% for IMRT and 19.2% for 125I (p < 0.001). When late GU toxicity was redefined as occurring longer than 12 months postimplantation, the 3-year actuarial estimate of Grade 2 GU or higher toxicity decreased to 14.3% (Fig. 2), which remained significantly greater than for IMRT patients (p < 0.001). Actuarial 3-year estimates of late GU Grade 3 toxicity were 0.5% for IMRT and 5.6% for 125I patients (p = 0.006). Only 1 IMRT patient had a Grade 3 late GU toxicity, which was caused by increased urinary frequency.
Fig. 2.
Comparison of 125I late genitourinary (GU) Grade 2 or higher toxicity with late toxicity defined as (top line) longer than 12 months or (bottom line) longer than 3 months posttreatment.
There were 11 125I patients with a late Grade 3 GU toxicity. In 4 patients, this was caused by obstructive symptoms requiring catheterization for longer than 2 weeks (in 3 of these patients, this occurred 5.8–9 months after implantation and may be considered a delayed acute toxicity). Five patients developed urethral strictures requiring more than two dilatations, 1 patient developed a bladder neck contracture requiring urethrotomy, and 1 patient reported persistent dysuria. There were 11 strictures reported in the study group, all occurring in 125I patients. Median time to developing a stricture was 2.3 years (range, 0.7–4.1 years). One patient had a stricture diagnosed during cystoscopy 7 months after implantation. The cystoscopy was performed to follow up an early bladder cancer. Detailed urethral dosimetry was recorded in 6 of 11 patients with strictures. Median urethral dose to 90% of the prostate and 10% of the prostate in these 6 patients were 100 (range: 80–140 Gy) and 210 Gy (range: 165–271 Gy), respectively (Table 2).
Multivariate analysis was performed for both definitions of late GU toxicity. When the longer-than-3-month definition was used as the end point, treatment group (125I vs. IMRT; HR, 9.90; 95% CI, 3.70–26.47; p < 0.001) and prostate volume (continuous [higher vs. lower]; HR, 1.03; 95% CI, 1.01–1.04; p < 0.001) were significant independent predictors of late Grade 2 or higher GU toxicity. Age (continuous; HR, 0.97; 95% CI, 0.93–1.01; p = 0.12), previous TURP (HR, 2.02; 95% CI, 0.47–8.65; p = 0.35), diabetes (HR, 1.84; 95% CI, 0.85–4.03; p = 0.12) and pretreatment AUA score (6–35 vs. ≤5; HR, 1.12; 95% CI, 0.47–2.68; p = 0.81) were not statistically significant. When toxicity was defined as 12 months or longer, treatment group (HR, 6.7; 95% CI, 2.6–17.8; p < 0.001) and prostate volume (HR, 1.025; 95% CI, 1.01–1.04; p = 0.004) remained significant. Age (HR, 0.97; 95% CI, 0.93–1.02; p = 0.12), previous TURP (HR, 2.25; 95% CI, 0.52–9.69; p = 0.28), diabetes (HR, 1.69, 95% CI, 0.74–3.88, p = 0.21), and pretreatment AUA score (HR, 0.76; 95% CI, 0.29–2.01; p = 0.58) were not statistically significant.
Biochemical failure
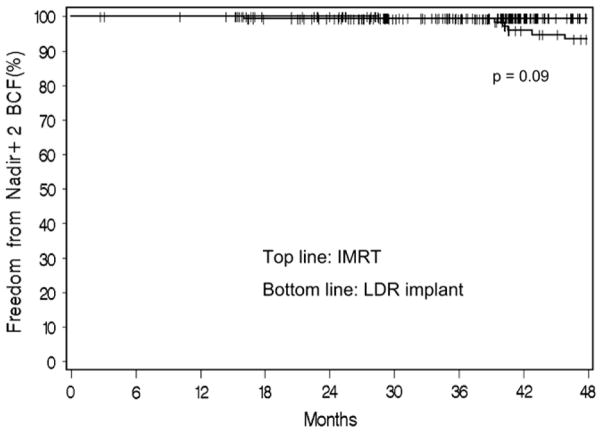
Kaplan-Meier estimates for FFBF for IMRT and 125I patients using the Phoenix definition are shown in Fig. 3. Four-year actuarial estimates of FFBF were 99.5% for IMRT and 93.5% for 125I (p = 0.09). There were no significant independent predictors of FFBF on multivariate analysis, which included initial PSA level (continuous; HR, 1.22; 95% CI, 0.92–1.63; p = 0.16), clinical stage (T2 vs. T1c; HR, 1.88; 95% CI, 0.49–7.20; p = 0.35), or treatment group (125I vs. IMRT; HR, 4.0; 95% CI, 0.80–20.03, p = 0.09).
Fig. 3.
Actuarial estimate of freedom from biochemical failure (BCF) using the nadir + 2 definition for (top line) intensity-modulated radiotherapy (IMRT) and (bottom line) 125I seed implant. LDR = low dose rate.
DISCUSSION
In this study, toxicity and biochemical outcomes between patients with low-risk prostate cancer treated with IMRT or an 125I permanent prostate implant were compared. Both treatment modalities produced excellent PSA control, whereas IMRT appeared to have fewer acute and late toxicities compared with patients with implants. Comparisons of toxicity among brachytherapy, external beam RT, and surgery have been difficult because of retrospective reporting and a lack of consensus on appropriate toxicity scales. Many investigators do not report late toxicity using the RTOG or Common Toxicity Criteria scales and prefer symptom-specific criteria, e.g., catheterization rates, time to return of urinary function scores, or stricture formation, which do not capture such symptoms as dysuria or hematuria. This information is reflected in our data.
The definition of late radiation toxicity can also differ between publications (20). Because the half life of 125I is approximately 60 days, some reported late toxicities may be resolving acute effects. In this study, we used two different times to measure chronic toxicity, one at longer than 3 months and a second at longer than 12 months to account for the half-life of 125I. This change in late-effects classification (Fig. 2) resulted in a decrease of Grade 2 or higher GU toxicity at 3 years from 19.2% to 14.3% (p = 0.36).
How bothersome late RTOG Grade 2 or even some Grade 3 toxicities are to a patient is unclear. More detailed quality-of-life studies attempted to quantify bother (21–23). The conclusion of these studies was that brachytherapy was well tolerated and without a significant disruption in quality of life. However, our data show that with follow-up approaching 4 years, such significant late toxicities as strictures are evidenced, which should have an impact on urinary quality of life.
One of the more detailed brachytherapy toxicity reports used the multifactorial Rectal Function Assessment Score quality-of-life questionnaire. A comparison between Rectal Function Assessment Scores and a modified RTOG scale for the same patient showed the more detailed quality-of-life questionnaire was required to identify rectal toxicities related to dose–volume constraints (24). A similar comparison for GU morbidity was not reported to date. Because institutions with the most experience are likely to have fewer complications, the rate of morbidity from brachytherapy across all institutions may be greater than that published. Likewise, advances in imaging, seed placement, and planning are likely to result in a decrease in the current rate of toxicity compared with older published series (25).
Both acute and late toxicity in our IMRT patients remains very low, with only 2.4% and 3.5% actuarial risk at 3 years of Grade 2 GI and GU toxicity, respectively. Of note is that only 1 patient to date reported a Grade 3 late toxicity. Similar low toxicity was reported by Zelefsky et al. (8) at Memorial Sloan-Kettering Cancer Center in 772 IMRT patients treated to similar doses. The decrease in late rectal toxicity with IMRT should be emphasized because the 125I patients in our series had a greater incidence of Grade 2 rectal side effects than those treated with IMRT (7.8% vs. 2.4% at 3 years). In comparison, late Grade 2 or higher GI toxicity greater than 20% was reported when doses to the isocenter of 78 Gy were used with a three-dimensional conformal technique (26, 27). The low rate of late GI toxicity may be caused by a combination of improved target delineation using MRI simulation, daily online setup correction, and the highly conformal dose delivery produced with IMRT. Our planning protocol places a point at the high dose end of the dose–volume histogram (Volume of rectum receiving 65 Gy [V65] < 17%), but also reduces the lower doses to large volumes of the rectum (Volume of rectum receiving 35 Gy [V35] < 40%). The potential importance of decreasing the circumferential dose to the rectum was reported previously (28), and our data further support this principle.
Despite the increase in GI toxicity in 125I patients relative to IMRT, GI toxicity was fairly low overall. This supports the evidence of a dose–volume relationship predictive of rectal toxicity (24, 29) because 125I brachytherapy also produces a highly conformal dose distribution with a low rectal dose. However, inexact seed placement or movement of seeds closer to the rectal wall over time (30) can produce higher rectal doses than initially predicted and the potential for late GI toxicity in some patients. Urinary toxicity appears to have a more complex cause, and a combination of patient factors (baseline urinary function, transitional zone size, and diabetes) and treatment factors (urethral and bladder base dosimetry) likely are involved.
In this selected cohort of low-risk patients, the FFBF for both treatment groups remains excellent. Although it may appear that there is a trend toward improved FFBF in the IMRT group, follow-up was short and there was no significant difference. The Phoenix definition of biochemical failure is more reliable with shorter follow-up than the American Society for Therapeutic Radiology and Oncology definition (31), but early results may be unfairly weighted against brachytherapy because of the greater incidence of a PSA bounce (32). Longer follow-up is needed to see whether this difference will truly be borne out. The findings more clearly establish a pattern of increased acute and late toxicity for low-dose-rate brachytherapy compared with IMRT.
CONCLUSIONS
This is the first comparison between IMRT and 125I prostate brachytherapy in the treatment of patients with low-risk prostate cancer. Both treatment modalities produced excellent PSA outcomes. IMRT appears to have less acute and late toxicity compared with 125I prostate brachytherapy.
Acknowledgments
Supported in part by Grants No. CA-006927 and CA101984-01 from the National Cancer Institute, and a grant from Varian Medical Systems. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Note—An online CME test for this article can be taken at http://asro.astro.org under Continuing Education.
Presented at the 48th Annual Meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), Philadelphia, PA, November 5–9, 2006.
Conflict of interest: none.
References
- 1.Potters L, Morgenstern C, Calugaru E, et al. 12-Year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2005;173:1562–1566. doi: 10.1097/01.ju.0000154633.73092.8e. [DOI] [PubMed] [Google Scholar]
- 2.Kupelian P, Kuban D, Thames H, et al. Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: The combined experience of nine institutions in patients treated in 1994 and 1995. Int J Radiat Oncol Biol Phys. 2005;61:415–419. doi: 10.1016/j.ijrobp.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Potters L, Klein EA, Kattan MW, et al. Monotherapy for stage T1–T2 prostate cancer: Radical prostatectomy, external beam radiotherapy, or permanent seed implantation. Radiother Oncol. 2004;71:29–33. doi: 10.1016/j.radonc.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico A, Whittington R, Malkowicz S, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 5.Kupelian PA, Potters L, Khuntia D, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:25–33. doi: 10.1016/s0360-3016(03)00784-3. [DOI] [PubMed] [Google Scholar]
- 6.Hanks GE, Hanlon AL, Epstein B, et al. Dose response in prostate cancer with 8–12 years’ follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 7.Davis JW, Kuban DA, Lynch DF, et al. Quality of life after treatment for localized prostate cancer: Differences based on treatment modality. J Urol. 2001;166:947–952. [PubMed] [Google Scholar]
- 8.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 9.Kupelian PA, Reddy CA, Klein EA, et al. Short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Preliminary results on late toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2001;51:988–993. doi: 10.1016/s0360-3016(01)01730-8. [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker CC, Damyanovich A, Haycocks T, et al. Magnetic resonance imaging in the radiation treatment planning of localized prostate cancer using intra-prostatic fiducial markers for computed tomography co-registration. Radiother Oncol. 2003;66:217–224. doi: 10.1016/s0167-8140(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer. Prostate. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. New York: Springer; 2002. pp. 337–345. [Google Scholar]
- 14.Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22:209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 15.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon AL, Schultheiss TE, Hunt MA, et al. Chronic rectal bleeding after high-dose conformal treatment of prostate cancer warrants modification of existing morbidity scales. Int J Radiat Oncol Biol Phys. 1997;38:59–63. doi: 10.1016/s0360-3016(97)00234-4. [DOI] [PubMed] [Google Scholar]
- 17.Storey MR, Pollack A, Zagars G, et al. Complications from radiotherapy dose escalation in prostate cancer: Preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys. 2000;48:635–642. doi: 10.1016/s0360-3016(00)00700-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–457. [Google Scholar]
- 19.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman & Hall; 1984. [Google Scholar]
- 20.Zelefsky M, Wallner L, Ling C, et al. Comparison of the 5-year outcome and morbidity of three-dimensional conformal radiotherapy versus transperineal permanent iodine-125 implantation for early-stage prostatic cancer. J Clin Oncol. 1999;17:517–522. doi: 10.1200/JCO.1999.17.2.517. [DOI] [PubMed] [Google Scholar]
- 21.Merrick GS, Butler WM, Wallner KE, et al. Long-term urinary quality of life after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:454–461. doi: 10.1016/s0360-3016(02)04600-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee WR, Hall MC, McQuellon RP, et al. A prospective quality-of-life study in men with clinically localized prostate carcinoma treated with radical prostatectomy, external beam radiotherapy, or interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51:614–623. doi: 10.1016/s0360-3016(01)01707-2. [DOI] [PubMed] [Google Scholar]
- 23.Feigenberg SJ, Lee WR, Desilvio ML, et al. Health-related quality of life in men receiving prostate brachytherapy on RTOG 98-05. Int J Radiat Oncol Biol Phys. 2005;62:956–964. doi: 10.1016/j.ijrobp.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 24.Merrick GS, Butler WM, Wallner KE, et al. Rectal function following brachytherapy with or without supplemental external beam radiation: Results of two prospective randomized trials. Brachytherapy. 2003;2:147–157. doi: 10.1016/S1538-4721(03)00131-4. [DOI] [PubMed] [Google Scholar]
- 25.Zelefsky MJ, Yamada Y, Cohen GN, et al. Five-year outcome of intraoperative conformal permanent I-125 interstitial implantation for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:65–70. doi: 10.1016/j.ijrobp.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: Results of the M.D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 27.Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005;61:1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 28.Tucker SL, Zhang M, Dong L, et al. Cluster model analysis of late rectal bleeding after IMRT of prostate cancer: A case-control study. Int J Radiat Oncol Biol Phys. 2006;64:1255–1264. doi: 10.1016/j.ijrobp.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Huang EH, Pollack A, Levy L, et al. Late rectal toxicity: Dose–volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314–1321. doi: 10.1016/s0360-3016(02)03742-2. [DOI] [PubMed] [Google Scholar]
- 30.Taussky D, Yeung I, Williams T, et al. Rectal-wall dose dependence on postplan timing after permanent-seed prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2006;65:358–363. doi: 10.1016/j.ijrobp.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Buyyounouski MK, Hanlon AL, Eisenberg DF, et al. Defining biochemical failure after radiotherapy with and without androgen deprivation for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1455–1462. doi: 10.1016/j.ijrobp.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 32.Pickles T. Prostate-specific antigen (PSA) bounce and other fluctuations: Which biochemical relapse definition is least prone to PSA false calls? An analysis of 2030 men treated for prostate cancer with external beam or brachytherapy with or without adjuvant androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2006;64:1355–1359. doi: 10.1016/j.ijrobp.2005.10.008. [DOI] [PubMed] [Google Scholar]