Abstract
Purpose
We previously reported that protein kinase A type I (PKARIα) overexpression was predictive of outcome in prostate cancer patients treated with radiotherapy (RT) ± short-term androgen deprivation (STAD) on Radiation Therapy Oncology Group (RTOG) protocol 86-10. Here, we attempt to verify our prior findings and test the hypothesis that the relationship of the length of AD to patient outcome is affected by PKARIα overexpression.
Experimental Design
There were 313 cases in the RTOG 92-02 study cohort with available tissue and suitable staining by immunohistochemistry. Median follow-up was 10.1 years. The intensity of PKARIα staining intensity was quantified manually and by image analysis. Multivariate analyses were done for overall mortality using Cox proportional hazards models and for local failure, biochemical failure, distant metastasis, and cause-specific mortality using Fine and Gray’s regression models.
Results
The expression levels of PKARIα, determined by manual and image analysis, were strongly correlated (P < 0.0001). In the multivariate analyses, manual-quantified and image analysis–quantified PKARIα staining intensities were independent predictors of distant metastasis (P < 0.01), local failure (P < 0.05), and biochemical failure (P ≤ 0.01). Furthermore, the benefit of long-term AD over STAD was much less when PKARIα expression was high.
Conclusions
PKARIα overexpression has been shown in two RTOG trials to be associated with an increased risk of failure after AD + RT. In this series of contemporary high-risk patients, PKARIα overexpression was associated with diminished response to LTAD + RT relative to STAD + RT, suggesting that such patients would be ideal for a PKARIα knockdown strategy.
The protein kinase A (PKA) protein belongs to a family of cyclic AMP–dependent holoenzymes that is related to cell proliferation and malignant transformation (1). Preliminary results (2) indicate that PKA type I (PKARIα) knockdown using an antisense strategy significantly inhibits prostate cancer cell growth in vitro and in vivo when combined with androgen deprivation (AD) and radiation. Thus, PKARIα is a therapeutic target with the potential to enhance response in high-risk men treated with AD and radiation, which currently represents the standard of care for men with high-risk disease. A knockdown approach may be most beneficial for prostate cancers that overexpress PKARIα, if such over-expression were associated with worse patient outcome.
PKA overexpression has been associated with poor patient outcome for colorectal, breast, and lung cancers (3–5). Recently, we reported that the intensity of PKARIα expression quantified by manual or image analysis methods was significantly related to a higher rate of distant metastasis (DM) in men treated with radiotherapy (RT) alone or RT + short-term AD (STAD) on Radiation Therapy Oncology Group (RTOG) trial 86–10. In this analysis, we attempt to validate and extend our findings in an independent cohort of men treated with RT + STAD or RT + long-term AD (LTAD) as part of RTOG trial 92-02. Our results indicate that PKARIα expression predicts for prostate cancer patient outcome and that overexpression may reduce the gains expected from LTAD + RT relative to STAD + RT.
Translational Relevance
In this report, we document in a relatively contemporary population of men with high-risk clinically localized prostate cancer treated with radiation plus androgen deprivation (AD) that protein kinase A type I (PKARIα) overexpression is associated with a poor prognosis. The other finding that has significant clinical relevance is that high levels of PKARIα are related to reduced effects of long-term AD (LTAD) over that of short-term AD when combined with radiation. These data suggest that patients with PKARIα overexpression might benefit from a PKARIα knockdown strategy, which is hypothesized to restore response to LTAD plus radiation. In the absence of a PKARIα knockdown strategy, patients with PKARIα overexpression are poor candidates for LTAD plus radiation only and should be considered for clinical trials.
Materials and Methods
Patient characteristics
RTOG protocol 92-02 was a phase III trial comparing RT + STAD (4 mo) with RT + LTAD (28 mo) in men with high-risk prostate cancer (6). AD was started 2 mo before RT. The patients had locally advanced prostate cancer (T2c-T4) and were treated with external beam RT (65–70 Gy to the prostate and 44–50 Gy to the pelvic lymph nodes). The current study examined PKA expression in 313 cases with sufficient tissue and suitable staining out of a parent cohort of 1,521 patients. In 21 cases (7%), the tissue was obtained from transurethral resection, and in 292 (93%), the tissue was obtained from needle core biopsies.
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tissue from the pretreatment diagnostic biopsies was sectioned and stored at 4°C, for more than 5 y in the majority of cases, before being processed for immunohistochemical staining by the LSAB (labeled streptavidin-biotin) method. This method has been previously described in detail (7–9). The primary monoclonal antibody PKARIα (1:100 dilution; BD Biosciences) was visualized using chromogen 3,3′-diaminobenzidine (Research Genetics) and commercially prepared hematoxylin (Dako Corp.) for counterstaining. All staining was done on a Dako autostainer (DakoCytomation). Positive controls of human brain and prostate carcinoma tissue sections were used for comparison during tissue analysis. Staining for negative controls was also done on prostatic carcinoma tissue by omitting the primary antibody.
Two investigators (L-Y.K. and T.A-S.) reviewed the slides under conventional light microscopy, without knowledge of patient outcome. As described previously, only cytoplasmic staining was analyzed (9, 10). The intensities were scored manually as 0 (negative staining), 1 (light staining), 2 (moderate staining), or 3 (dark staining). Normal prostate epithelium in each sample consistently stained an intensity of “1” and served as an internal control. The results of negative versus positive (0–1 versus 2–3) and low versus high (0–2 versus 3) were compared.
The intensity of staining was also quantified by an image analysis system (ACIS, Clarient, Inc.). A color threshold for brown (positive) cytoplasmic staining was set for every slide analyzed using version 2.4.3.0 software supplied by the vendor. Where possible, at least six areas of interest in the tissue visualized at ×40 magnification were quantified. The intensity of staining was scored on a grayscale of 0 to 255 arbitrary units. A mean intensity score (MIS), the ACIS intensity, was generated by proprietary software.
Definition of outcomes
The outcomes examined were biochemical failure (BF), local failure (LF), DM, cause-specific mortality (CSM), and overall mortality (OM). The failure event for OM is defined as death due to any cause. The following is considered a failure event in assessing CSM: death certified as due to prostate cancer, death due to complications of treatment, and death from unknown causes with DM or bone metastasis. An LF event is defined as clinical evidence of local recurrence by any methods or persistent disease (tumor regrowth by 25% or stable disease beyond 18 mo). A DM event is defined as clinical evidence of distant disease (by any method). The failure event for BF is defined as three consecutive rises or the initiation of hormone treatment for a rising prostate-specific antigen (PSA) or a posttreatment PSA nadir level of >4.0 ng/mL. Time to BF is from the randomization date to the one of the following three dates whichever comes first: the midpoint of date of last nadir and the date of first PSA rise beyond the nadir, date of salvage hormone therapy, or date of PSA nadir level >4.0 ng/mL. The patient was censored at the date of last PSA if PSA was declining or at the nadir. Time to event measurements were made from the date of randomization to the first reported date of failure, death, or to the last follow-up date in the absence of failure events.
Statistical analysis
The goals were to determine if PKARIα overexpression was related to patient outcome, whether semiautomated image analysis results were stronger than the manual determinations, and if the division of PKARIα values at the median versus other quartile-based cutpoints was optimal.
There were 313 patients who had adequate tissue for PKA staining and quantification, out of a parent cohort of 1,521 patients. The median follow-up of all of those in the PKA cohort was 10.1 y (range, <0.5–13.4 y), whereas for those living the median follow-up was 11.17 y (range, 1.8–13.4 y). The following covariates were considered in the multivariate analyses: age (continuous), pretreatment initial PSA (continuous), Gleason score {2–6 [reference level (RL)] versus 7 versus 8–10}, clinical T-stage [T2 (RL) versus T3/T4], and assigned treatment [STAD + RT (RL) versus LTAD + RT]. The Gleason score and clinical T-category were based on the original protocol stratification.
χ2 Test statistics were used to compare pretreatment characteristics of subjects at study entry. OM rates were estimated by the Kaplan-Meier method (11). Cox proportional hazard multivariate regression models (12) were used to assess the relationship of PKARIα expression to OM after adjusting for other covariates. The rates of LF, DM, and CSM were estimated using the cumulative incidence approach (13) because it specifically adjusts for other competing risks of failure events of interest. Fine and Gray’s regression (14) was used for these outcomes to adjust for other covariates. Manual PKARIα intensities were dichotomized as negative (0, 1) versus low/high (2, 3) or negative/low (0, 1 and 2) versus high (3), as reported previously (9). The ACIS (image analysis)–quantified MISs were treated as continuous or dichotomized variables. The MIS median, first quartile (25%), and third quartile (75%) cutpoints were analyzed. In addition, we tested the significance of the previously established MIS cutpoint of 135.5 (9) in an attempt to validate our prior results; the 135.5 cutpoint was the median MIS for men treated on RTOG trial 86-10. The missing at random assumption is assumed and a multiple imputation method (20 imputed data sets) with Markov chain Monte Carlo estimation is applied to impute missing pretreatment characteristics. Unadjusted and adjusted hazard ratios (HR) were calculated for all covariates using either the Cox proportional hazards model or Fine and Gray’s regression model with associated 95% confidence intervals (95% CI) and P values. All statistical tests were two sided and a P value of <0.05 was considered statistically significant. Statistical Analysis System (SAS Institute) and R software11 were used for all statistical analyses.
Results
The expression of PKARIα was determined in 313 (21%) of 1,521 assessable cases in RTOG 92-02. The patient characteristics are shown in Table 1. We then analyzed whether the PKA cohort was representative of the parent cohort by comparing the distribution of patients with respect to age (<70 versus ≥70 years), pretreatment PSA (≤30 versus >30 ng/mL), Gleason score (2–6 versus 7 versus 8–10), clinical T-category (T2 versus T3/T4), and assigned treatment (STAD + RT versus LTAD + RT). The only statistically significant difference was in Gleason score (P = 0.03) because there were slightly more cases with Gleason score 8 to 10 (28% versus 23%) and fewer with Gleason score 2 to 6 (32% versus 40%) in the PKA cohort compared with those in whom PKA was not available. In terms of the various end points tested (Supplementary Table S1), the only significant difference was that the PKA cohort had more LFs (P = 0.03; HR, 1.35). Thus, some imbalances between those cases that had a PKA available and those that did not were apparent.
Table 1.
Patient characteristics by PKA status
| Characteristics | Missing PKA, n (%) | PKA cohort, n (%) | P* |
|---|---|---|---|
| Age (y) | |||
| <70 | 535 (44) | 146 (47) | |
| ≥70 | 673 (56) | 167 (53) | 0.45 |
| Median | 70 | 70 | |
| Range | 43–88 | 44–88 | |
| Gleason score | |||
| Unknown/missing | 77 (6) | 23 (7) | |
| 2–6 | 483 (40) | 100 (32) | |
| 7 | 373 (31) | 104 (33) | |
| 8–10 | 275 (23) | 86 (28) | 0.03 |
| Clinical stage | |||
| T2 | 552 (46) | 140 (45) | 0.76 |
| T3 or T4 | 656 (54) | 173 (55) | |
| PSA (ng/mL) | |||
| ≤30 | 805 (67) | 216 (69) | |
| >30 | 403 (33) | 97 (31) | 0.43 |
| Median | 19.9 | 20.3 | |
| Range | 0.1–250.0 | 0.4–151.1 | |
| Assigned treatment | |||
| STAD + RT | 602 (50) | 161 (51) | |
| LTAD + RT | 606 (50) | 152 (49) | 0.61 |
P value is from χ2 test statistics.
Table 2 displays a series of comparisons between manual and image analysis estimations of PKARIα intensity. These comparisons were done with the goal of identifying a robust image analysis approach with the potential to be better replicated between laboratories compared with more subjective manual quantification. To limit comparisons, but to identify if image analysis cutpoints other than the median were best, we tested the median, first quartile (Q1, 25%), and third quartile (Q3, 75%) cutpoints as well as a cutpoint identified in the prior analysis of cases from RTOG 86–10. Thus, the image analysis–derived MIS cutpoints tested were at 101.7 (Q1), 111.8 (median), and 128.0 (Q3), as well as a previously tested median cutpoint of 135.5 (9). The grouping of the manual PKARIα determinations into negative (staining classified as level 0 or 1) versus positive (staining classified as level 2 or 3) and negative/low (level 0, 1, or 2 staining) versus high (level 3 staining) was modeled after our prior analysis. The comparisons in Table 2 between the MISs by image analysis and the manual assessments revealed strong correlations between the two for all cutpoints tested.
Table 2.
Concordance of PKA scores obtained from manual and semiautomated image (ACIS) analysis
| Manual | Image analysis |
KS (P)* | |
|---|---|---|---|
| <Q1 (101.7), n (%) | ≥Q1 (101.7), n (%) | ||
| Negative (0, 1) | 77 (25) | 103 (33) | |
| Positive (2, 3) | 1 (<1) | 132 (42) | 0.38 (<0.0001) |
| Low (0, 1, 2) | 78 (25) | 176 (56) | |
| High (3) | 0 | 59 (19) | 0.14 (<0.0001) |
| <Median (111.8) | ≥Median (111.8) | ||
| Negative (0, 1) | 144 (46) | 36 (12) | |
| Positive (2, 3) | 12 (4) | 121 (39) | 0.69 (<0.0001) |
| Low (0, 1, 2) | 156 (50) | 98 (31) | |
| High (3) | 0 | 59 (19) | 0.38 (<0.0001) |
| <Q3 (128.0) | ≥Q3 (128.0) | ||
| Negative (0, 1) | 177 (57) | 3 (1) | |
| Positive (2, 3) | 57 (18) | 76 (24) | 0.59 (<0.0001) |
| Low (0, 1, 2) | 229 (73) | 25 (8) | |
| High (3) | 5 (2) | 54 (17) | 0.72 (<0.0001) |
| <135.5 | ≥135.5 | ||
| Negative (0, 1) | 178 (57) | 2 (<1) | |
| Positive (2, 3) | 80 (26) | 53 (17) | 0.42 (<0.0001) |
| Low (0, 1, 2) | 240 (77) | 14 (4) | |
| High (3) | 18 (6) | 41 (13) | 0.66 (<0.0001) |
KS = κ statistic and P values are from two-sided tests.
The relationships of PKARIα expression to age, initial PSA, Gleason score, T-category, and assigned treatment, using the categorizations used in Table 1, were explored. As described above, manually determined PKARIα expression was dichotomized as intensities of 0 to 1 versus 2 to 3 and 0 to 2 versus 3. Image analysis–determined PKARIα MIS expression was dichotomized by the first quartile, median, third quartile, and the cutpoint from our prior study (9). In none of the permutations tested was PKARIα expression significantly associated with patient- or treatment-related factors.
Multivariate analyses were used to examine the relationship of the estimated PKARIα levels and the clinical outcomes. Multi variate analyses were adjusted for age (continuous), initial PSA (continuous), Gleason score, T-category, and assigned treatment and the results are presented in Table 3. With the exception of OM, in which Cox proportional hazards was used, Fine and Gray’s regression was applied to account for competing risks. Dichotomized PKARIα expression was most significantly associated with outcome using the third quartile cutpoint (MIS of 128.0) as well as the previously established cutpoint (MIS of 135.5) based on an analysis of RTOG 86-10. Using the 92-02–derived third quartile cutpoint or the 86-10–derived cutpoint, high PKARIα expression was significantly correlated with DM, LF, and BF. A borderline relationship (P = 0.07) was seen for CSM. As a continuous covariate, PKARIα expression was only significant for BF (P = 0.005; Table 3).
Table 3.
Multivariate analyses of dichotomized and continuous PKARIa expression quantified by image analysis
| End point | Image analysis cutpoint |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (101.7) |
Median (111.8) |
Q3 (128.0) |
Prior study (135.5) |
Continuous |
||||||
| Adjusted HR (95% CI) | P* | Adjusted HR (95% CI) | P* | Adjusted HR (95% CI) | P* | Adjusted HR (95% CI) | P* | Adjusted HR (95% CI) | P* | |
| OM | 0.98 (0.68–1.42) | 0.92 | 1.08 (0.79–1.49) | 0.62 | 0.89 (0.62–1.28) | 0.51 | 0.76 (0.50–1.16) | 0.20 | 1.00 (0.997–1.003) | 0.89 |
| CSM | 1.51 (0.76–2.97) | 0.23 | 1.35 (0.80–2.30) | 0.26 | 1.74 (0.96–3.16) | 0.07 | 1.71 (0.93–3.14) | 0.08 | 1.00 (0.997–1.01) | 0.25 |
| DM | 1.50 (0.79–2.84) | 0.22 | 1.73 (1.05–2.83) | 0.03 | 2.49 (1.48–4.19) | 0.0006 | 2.31 (1.37–3.92) | 0.002 | 1.00 (0.998–1.01) | 0.14 |
| LF | 1.31 (0.72–2.39) | 0.37 | 1.49 (0.91–2.41) | 0.11 | 1.74 (1.05–2.87) | 0.03 | 1.77 (1.04–3.00) | 0.03 | 1.00 (0.999–1.01) | 0.13 |
| BF | 1.39 (0.98–1.98) | 0.06 | 1.62 (1.20–2.19) | 0.002 | 1.95 (1.42–2.68) | <0.0001 | 1.94 (1.38–2.73) | 0.0002 | 1.005 (1.001–1.008) | 0.005 |
NOTE: Adjusted for age (continuous), pretreatment initial PSA (continuous), Gleason score (2–6 versus 7 versus 8–10), clinical stage (T2 versus T3/T4), and assigned treatment.
Abbreviations: Q1, 25th percentile; Q3, 75th percentile.
P values are from Fine and Gray’s regression model (CSM, DM, LF, and BF) and Cox regression model (OM).
Because the expression of PKARIα at the third quartile cutpoint (MIS of 128.0) was significantly associated with DM, but not CSM or OM, we examined the power to detect a significant relationship for the 313 analyzable patients in the cohort. For the BF, DM, and LF end points, at HRs of 1.8, 1.56, and 1.34, the statistical power would be at least 92%, 95%, and 72%. In contrast, for CSM and OM at HRs of 1.25 and 1.13, the statistical power would be at least 55% and 17%. These estimates were made using a one-sided log-rank test at the 0.05 significance level. Thus, the study was most adequately powered to detect associations with BF and DM, with relatively low statistical power to detect differences in CSM and OM based on dichotomized PKARIα at the third quartile.
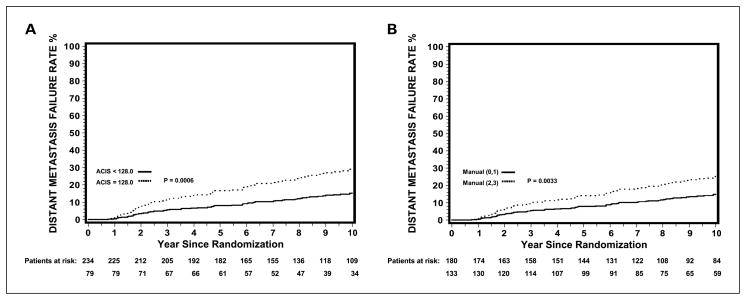
Figure 1 displays adjusted cumulative incidence DM curves for patients subdivided by the third quartile MIS cutpoint of 128.0 (Fig. 1A) and the manual cutpoint of 0 to 1 versus 2 to 3 (Fig. 1B). The 10-year risk of DM was 15.3% for those with a PKARIα of <128.0 and 29.1% for a PKARIα of ≥128.0. Similar results were observed using manual counts with a 10-year risk of DM of 14.8% for those with a manual intensity of 0 to 1 and 25.2% for a manual intensity of 2 to 3. Table 4 shows the multivariate results of PKARIα expression quantified manually to the end points investigated. There was little difference between grouping the manual intensities as 0 to 1 versus 2 to 3 or 0 to 2 versus 3. The results were analogous to those using image analysis; however, the image analysis PKARIα results using the Q3 (75th percentile) cutpoint, and the cutpoint from the prior study, had more significant P values and higher HRs (Table 3).
Fig. 1.
Adjusted DM cumulative incidence curves by PKARIα expression quantified by image analysis (ACIS MIS cutpoint of 128.0; A) and manually (manual intensity of 0–1 versus 2–3; B).
Table 4.
Multivariate analyses of dichotomized PKARIα expression by manual scoring
| End point | Cutpoint |
|||||
|---|---|---|---|---|---|---|
| (0, 1) vs (2, 3) |
(0, 1, 2) vs (3) |
|||||
| Fail/total (0, 1) vs (2, 3) | Adjusted HR* (95% CI) | P† | Fail/total (0, 1, 2) vs (3) | Adjusted HR* (95% CI) | P† | |
| OM | 93/180 | 0.92 (0.66–1.27) | 0.60 | 127/254 | 1.05 (0.71–1.54) | 0.82 |
| 68/133 | 34/59 | |||||
| CSM | 27/180 | 1.42 (0.83–2.41) | 0.20 | 39/254 | 1.83 (0.99–3.37) | 0.053 |
| 26/133 | 14/59 | |||||
| DM | 28/180 | 2.10 (1.28–3.45) | 0.004 | 45/254 | 2.21 (1.26–3.87) | 0.006 |
| 36/133 | 19/59 | |||||
| LF | 31/180 | 1.78 (1.10–2.88) | 0.02 | 51/254 | 1.47 (0.85–2.54) | 0.16 |
| 36/133 | 16/59 | |||||
| BF | 96/180 | 1.45 (1.07–1.97) | 0.02 | 142/254 | 1.47 (1.06–2.04) | 0.02 |
| 86/133 | 40/59 | |||||
Adjusted for age (continuous), pretreatment initial PSA (continuous), Gleason score (2–6 versus 7 versus 8–10), clinical stage (T2 versus T3/T4), and assigned treatment.
P values are from Fine and Gray’s regression model (CSM, DM, LF, and BF) and Cox regression model (OM).
We then asked whether PKARIα overexpression had a differential effect on outcome when the patients were subdivided by assigned treatment. Table 5 displays this analysis and reveals that, in general, the gain from extending AD for an additional 2 years (LTAD + RT arm) was most pronounced when PKARIα expression was low. Manual PKARIα intensities of 0 to 1 were associated with HRs for CSM, DM, LF, and BF of 0.25 (P = 0.005), 0.23 (P = 0.003), 0.31 (P = 0.007), and 0.54 (P = 0.0003) when RT + LTAD was referenced to RT + STAD. In contrast, the only statistically significant treatment-related difference was for BF (HR, 0.45; P = 0.003) when the manual PKARIα intensities were 2 to 3. A very similar pattern was seen using the image analysis data (MIS cutpoint of 128.0). These results hold when the HRs were adjusted for other covariates.
Table 5.
Subgroup analyses for treatment effect [LTAD + RT versus STAD (RL) + RT]
| Group for PKA | End point | HR (95% CI) of RX | P* | Adjusted HR† (95% CI) of RX | P* |
|---|---|---|---|---|---|
| (0, 1) (n = 180) | OM | 0.90 (0.60–1.36) | 0.61 | 0.95 (0.62–1.47) | 0.83 |
| CSM | 0.25 (0.09–0.65) | 0.005 | 0.28 (0.10–0.80) | 0.02 | |
| DM | 0.23 (0.09–0.60) | 0.003 | 0.27 (0.09–0.75) | 0.01 | |
| LF | 0.31 (0.14–0.73) | 0.007 | 0.30 (0.13–0.70) | 0.006 | |
| BF | 0.54 (0.36–0.81) | 0.003 | 0.56 (0.36–0.86) | 0.008 | |
| (2, 3) (n = 133) | OM | 0.91 (0.57–1.47) | 0.71 | 0.80 (0.48–1.32) | 0.38 |
| CSM | 1.13 (0.52–2.45) | 0.75 | 1.12 (0.52–2.44) | 0.77 | |
| DM | 0.63 (0.33–1.22) | 0.17 | 0.62 (0.31–1.22) | 0.16 | |
| LF | 0.52 (0.26–1.02) | 0.058 | 0.48 (0.25–0.95) | 0.04 | |
| BF | 0.45 (0.30–0.69) | 0.0003 | 0.43 (0.27–0.69) | 0.0005 | |
| <128.0 (n = 234) | OM | 0.87 (0.60–1.26) | 0.46 | 0.87 (0.60–1.27) | 0.47 |
| CSM | 0.50 (0.25–1.01) | 0.055 | 0.53 (0.25–1.12) | 0.10 | |
| DM | 0.32 (0.15–0.68) | 0.003 | 0.33 (0.15–0.73) | 0.007 | |
| LF | 0.36 (0.18–0.71) | 0.003 | 0.35 (0.18–0.70) | 0.003 | |
| BF | 0.55 (0.39–0.78) | 0.0009 | 0.57 (0.40–0.82) | 0.003 | |
| ≥128.0 (n = 79) | OM | 0.96 (0.53–1.76) | 0.90 | 0.89 (0.46–1.71) | 0.72 |
| CSM | 0.69 (0.27–1.76) | 0.43 | 0.79 (0.29–2.17) | 0.65 | |
| DM | 0.59 (0.27–1.28) | 0.18 | 0.76 (0.33–1.75) | 0.51 | |
| LF | 0.52 (0.23–1.20) | 0.12 | 0.48 (0.21–1.08) | 0.08 | |
| BF | 0.34 (0.20–0.58) | <0.0001 | 0.28 (0.16–0.50) | <0.0001 |
P values are from Fine and Gray’s regression model (CSM, DM, LF, and BF) and Cox regression model (OM).
Adjusted for age (continuous), pretreatment initial PSA (continuous), Gleason score (2–6 versus 7 versus 8–10), clinical stage (T2 versus T3/T4), and assigned treatment.
Discussion
The results described herein show that PKARIα expression is a significant predictor of prostate cancer patient outcome when primary treatment is RT + AD. PKARIα overexpression is associated with biochemical (BF) and clinical failure (LF and DM). To our knowledge, the analysis of RTOG 92-02 described here and our prior report of an analysis of RTOG 86-10 (9) are unique in that there are no other published descriptions of the relationship of PKARIα expression to outcome after RT ± AD. The PKARIα expression results were concordant overall between the RTOG 86-10 and 92-02 series in that manual-quantified and image analysis–quantified PKARIα were related to each other and to patient outcome. In the current study of RTOG 92-02, which involved more patients and had greater power as a consequence, the association of PKARIα overexpression to DM, LF, and BF was more consistent and significant. Moreover, the median MIS was 111.8 in the 92-02 cohort, a contemporary high-risk group, and was 135.5 in the 86-10 cohort, a pre-PSA era locally advanced group. There was consistency in terms of PKARIα expression and tumor aggressiveness within and between each study population.
We tested PKARIα expression as both dichotomous and continuous covariates. As a continuous covariate, the only relationship that was significant in multivariate analysis was with BF. As a dichotomous covariate, PKARIα was associated with more generalized failure (biochemical and clinical failure). The data imply that a threshold level of PKARIα is what most influences tumor response. Because the image analysis HRs for BF, LF, and DM at the Q3 cutpoint of 128.0 were higher overall than for the median or Q1 image analysis cutpoints (Table 3), the manual cutpoints (Table 4), and the prior image analysis median cutpoint from RTOG 86-10 of 135.5 (Table 3), we recommend that it be used in future studies.
A drawback of the study is that only 21% of the original study population had tissue samples for analysis. As a consequence, the study group was not entirely representative of the parent cohort in terms of the distribution of patients by Gleason score and the proportion experiencing LF. A diagnostic needle biopsy specimen retrieval rate of 50% is considered excellent in the RTOG experience. In a recent analysis of samples from two Medical Research Council trials (15), a sample analysis rate of 32% was reported. In the current study, the original sample retrieval was close to 50%, but several biomarkers have been analyzed, leaving fewer cases for analysis. As a consequence, unknown variables might not be evenly distributed. Nonetheless, the analysis of 313 prostate cancer cases for PKA expression is the largest in the literature and the findings are substantive, even if there are some differences in attributes between those in whom PKARIα levels were measured and those in whom they were unavailable. That PKA expression was related to BF, LF, and DM in this analysis and there was consistency with the findings in another independent patient group analyzed previously (9) affirm the clinical potential of this biomarker. The current study was not powered to assess an association between PKARIα overexpression and CSM and OM.
Another intriguing relationship identified was between PKARIα expression and outcome in response to the length of AD (assigned protocol treatment in RTOG 92-02). Patients with tumors that expressed high levels of PKARIα had less of a benefit from LTAD + RT compared with those with tumors that expressed low levels of PKARIα (Table 5). These data suggest that high levels of PKARIα reduce the effect of LTAD. Our preclinical studies (2), showing that PKARIα knockdown improves LNCaP prostate tumor response in vitro and in vivo to AD, with or without radiation, are consistent with these findings.
PKARIα causes androgen-mediated androgen receptor activation (16–19). Our preclinical results (2) also showed a reduction in androgen receptor and phosphorylated mitogen-activated protein kinase with PKARIα knockdown. The results from the current biomarker investigation provide further evidence that PKARIα has a central role in mediating response to AD and support the rationale for PKARIα knockdown in men with PKARIα overexpression. A PKARIα antisense approach in such patients should enhance response to LTAD + RT and could potentially obviate the need for LTAD.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute grants CA-006927 and CA-101984-01 and Pennsylvania Department of Health.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute and Pennsylvania Department of Health.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cho-Chung YS. Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approaches to therapy. Cancer Res. 1990;50:7093–100. [PubMed] [Google Scholar]
- 2.Hensley HH, Hachem P, Stoyanova R, Kwon H, Pollack A. Protein kinase A induces apoptosis and sensitizes prostate tumors to androgen deprivation and radiation in vivo. Int J Radiat Oncol Biol Phys. 2006;66:S319–20. [Google Scholar]
- 3.Bradbury AW, Carter DC, Miller WR, Cho-Chung YS, Clair T. Protein kinase A (PK-A) regulatory subunit expression in colorectal cancer and related mucosa. Br J Cancer. 1994;69:738–42. doi: 10.1038/bjc.1994.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller WR, Hulme MJ, Cho-Chung YS, Elton RA. Types of cyclic AMP binding proteins in human breast cancers. Eur J Cancer. 1993;29A:989–91. doi: 10.1016/s0959-8049(05)80207-2. [DOI] [PubMed] [Google Scholar]
- 5.Shi XB, Ma AH, Tepper CG, et al. Molecular alterations associated with LNCaP cell progression to androgen independence. Prostate. 2004;60:257–71. doi: 10.1002/pros.20039. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92–02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 7.Khor LY, Desilvio M, Al-Saleem T, et al. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104:962–7. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 8.Pollack A, Moughan J, Khor L, et al. Bcl-2 and Bax predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on RTOG 92–02. Int J Radiat Oncol Biol Phys. 2006;66:S201. doi: 10.1016/j.ijrobp.2006.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khor LY, Bae K, Al-Saleem T, et al. Protein kinase A RI-a predicts for prostate cancer outcome: analysis of radiation therapy oncology group trial 86–10. Int J Radiat Oncol Biol Phys. 2008;71:1309–15. doi: 10.1016/j.ijrobp.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neary CL, Nesterova M, Cho YS, Cheadle C, Becker KG, Cho-Chung YS. Protein kinase A isozyme switching: eliciting differential cAMP signaling and tumor reversion. Oncogene. 2004;23:8847–56. doi: 10.1038/sj.onc.1208165. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–57. [Google Scholar]
- 12.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 13.Kalbfleisch JD, Ross LP. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Vergis R, Corbishley CM, Norman AR, et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–51. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 16.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–7. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 17.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–83. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 18.Kasbohm EA, Guo R, Yowell CW, et al. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Biol Chem. 2005;280:11583–9. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
- 19.Bagchi G, Wu J, French J, Kim J, Moniri NH, Daaka Y. Androgens transduce the Gas-mediated activation of protein kinase A in prostate cells. Cancer Res. 2008;68:3225–31. doi: 10.1158/0008-5472.CAN-07-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.