Abstract
Two new jaspamide derivatives 2 and 3, together with the parent compound jaspamide (1) have been isolated from the marine sponge Jaspis splendens collected in Kalimantan (Indonesia). The structures of the new compounds were unambiguously elucidated based on 1D and 2D NMR spectral data, mass spectrometry and comparison with jaspamide (1). The new derivatives inhibited the growth of mouse lymphoma (L5178Y) cell line in vitro with IC50 values of <0.1 μg/mL.
Keywords: Jaspis splendens, jaspamide Q and R, structure elucidation, cytotoxic activity
1. Introduction
Peptides are well-known bioactive metabolites from marine invertebrates [1,2] including linear peptides [3,4], depsipeptides [5,6], cyclic [7–11] and bicyclic peptides [12,13]. Sponges of the genus Jaspis (family, Jaspidae) have been a rich source of biologically active, structurally novel natural products. After the discovery of the cyclodepsipeptide jaspamide (jasplakinolide, 1) in the sponge Jaspis cf. johnstoni in 1986 by Ireland [14] and Crews [15], which is known for its pronounced biological activities including antifungal [16], anthelmintic, insecticidal [14,15] and cytotoxic activity [17], sponges of the genus Jaspis have received considerable attention, and since then the chemistry of Jaspis sponges has been the subject of more than 90 publications. A wide variety of constituents has been isolated from this genus including several jaspamide derivatives from Jaspis splendens [18–21], isomalabaricane triterpenes from Jaspis stellifera [22–24] and other species [25–29], cytotoxic macrolides from the Okinawan sponge Jaspis sp. [30], bengazoles [31,32] that stand out as unique bis-oxazoles containing a carbohydrate-like polyol side chain, antiparasitic, antimicrobial, and cytotoxic amino acid derivatives known as bengamides [33–35], cytotoxic bromotyrosine derivatives [36,37], and a series of dihydroxystyrene sulphate derivatives [38–42].
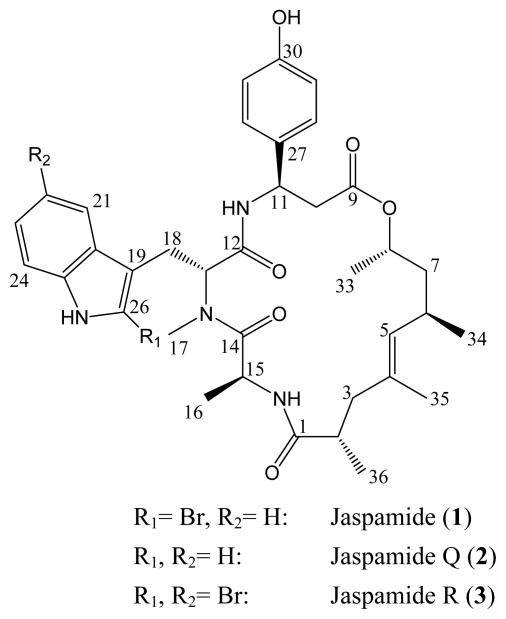
As part of our ongoing studies on bioactive natural products from marine sponges, we investigated a specimen of Jaspis splendens collected in Kalimantan (Indonesia). The crude methanolic extract exhibited considerable in vitro cytotoxic activity against mouse lymphoma L5178Y cells. Chromatographic separation of the extract yielded jaspamide (1) as the major constituent. Through bioactivity-guided chemical investigation of the ethyl acetate soluble fraction minor analogues of jaspamide, including the new natural products jaspamide Q (2), and jaspamide R (3) (Figure 1) were obtained. In this paper, we describe isolation, structural elucidation, and biological activity of the new jaspamide derivatives, both of which carry a modified 2-bromoabrine (N-methyltryptophan) residue compared to jaspamide (1).
Figure 1.
New jaspamide derivatives 2 and 3, together with the parent jaspamide (1).
2. Results and Discussion
Total methanolic extract of the sponge J. splendens was partitioned according to the scheme previously described by Ebada et al. [43]. The bioactive ethyl acetate soluble fraction was further chromatographed by Sephadex LH20 column chromatography followed by reversed-phase preparative HPLC to give jaspamide (1, 0.0013%, dry weight), jaspamide Q (2, 0.00001%, dry weight), and jaspamide R (3, 0.00001%, dry weight).
Jaspamide Q (2) was obtained as a white amorphous solid, and the ESIMS spectrum showed a pseudomolecular ion peak at m/z 631.3 [M+H]+, which was 79 amu smaller than that of jaspamide (1), the parent compound. This difference was assigned to the absence of the bromine atom at C-26 in jaspamide (1). The molecular formula of jaspamide Q (2) was C36H46N4O6, based on HRFTMS (m/z 631.3491 [M+H]+, Δ + 1.0 ppm), therefore jaspamide Q (2) was identified as the debromo analogue of 1. 1H-NMR spectral data (Table 1) revealed that the resonances of 2 were superimposable with those of 1 with only one additional proton resonance at δH 6.87 (1H, br s) that was ascribed to H-26. The complete structure of jaspamide Q (2) was unambiguously elucidated and assigned on the basis of 1H–1H COSY, TOCSY, ROESY, and HMBC spectra.
Table 1.
1H-NMR data of jaspamide Q (2), and R (3), (500 MHz, CDCl3-d).
| Position | Jaspamide Q (2) |
Jaspamide R (3) |
|---|---|---|
| δH mult. (J Hz) | δH mult. (J Hz) | |
| 2 | 2.51 (1H, m) | 2.49 (1H, m) |
| 3 | A) 2.37 (1H, dd, 11.5, 15.8 Hz) | A) 2.37 (1H, dd, 11.5, 15.8 Hz) |
| B) 1.90 (1H, d, 15.8 Hz) | B) 1.90 (1H. d, 15.8 Hz) | |
| 5 | 4.79 (1H, d, 6.7 Hz) | 4.74 (1H, d, 6.7 Hz) |
| 6 | 2.23 (1H, m) | 2.23 (1H, m) |
| 7 | A) 1.32 (1H, m), B) 1.10 (1H, m) | A) 1.32 (1H, m), B) 1.10 (1H, m) |
| 8 | 4.64 (1H, m) | 4.60 (1H, m) |
| 10 | A) 2.67 (1H, dd, 4.7, 15.0 Hz) | A) 2.67 (1H, dd, 4.7, 15.0 Hz) |
| B) 2.61 (1H, dd, 5.7, 14.8 Hz) | B) 2.61 (1H, dd, 5.7, 14.8 Hz) | |
| 11 | 5.25 (1H, dd, 5.3, 8.6 Hz) | 5.25 (1H, dd, 5.3, 8.6 Hz) |
| 13 | 5.63 (1H, dd, 6.3, 10.0 Hz) | 5.73 (1H, dd, 6.3, 10.0 Hz) |
| 15 | 4.77 (1H, m) | 4.72 (1H, m) |
| 16 | 1.04 (3H, d, 6.6 Hz) | 0.81 (3H, d, 6.7 Hz) |
| 17 | 2.97 (3H, s) | 2.98 (3H, s) |
| 18 | A) 3.43 (1H, dd, 6.3, 15.5 Hz) | A) 3.33 (1H, dd, 6.3, 15.5 Hz) |
| B) 3.17 (1H, dd, 10.4, 15.2 Hz) | B) 3.18 (1H, dd, 10.4, 15.2 Hz) | |
| 21 | 7.61 (1H, d, 8.0 Hz) | 7.41 (1H, br s) |
| 22 | 7.11 (1H, t, 8.0 Hz) | |
| 23 | 7.18 (1H, t, 8.0 Hz) | 7.20 (1H, d, 8.2 Hz) |
| 24 | 7.35 (1H, d, 8.0 Hz) | 7.42 (1H, d, 8.2 Hz) |
| 26 | 6.87 (1H, br s) | |
| 28 | 6.90 (1H, d, 8.3 Hz) | 6.98 (1H, d, 8.4 Hz) |
| 29 | 6.69 (1H, d, 8.3 Hz) | 6.71 (1H, d, 8.4 Hz) |
| 31 | 6.69 (1H, d, 8.3 Hz) | 6.71 (1H, d, 8.4 Hz) |
| 32 | 6.90 (1H, d, 8.3 Hz) | 6.98 (1H, d, 8.4 Hz) |
| 33 | 1.06 (3H, d, 6.3 Hz) | 1.05 (3H, d, 6.1 Hz) |
| 34 | 0.83 (3H, d, 6.5 Hz) | 0.81 (3H, d, 6.7 Hz) |
| 35 | 1.59 (3H, s) | 1.57 (3H, s) |
| 36 | 1.15 (3H, d, 6.8 Hz) | 1.13 (3H, d, 6.8 Hz) |
| NH-Tyr | 7.46 (1H, d, 8.6 Hz) | 7.53 (1H, d, 8.6 Hz) |
| NH-Trp | 8.24 (1H, br s) | 8.42 (1H, br s) |
| NH-Ala | 6.73 (1H, d, 6.4 Hz) | 6.65 (1H, d, 6.5 Hz) |
In particular, the similarity of 1H-, and 13C-NMR resonances between jaspamide Q (2) and jaspamide (1) implied that the chiral centers of alanine, abrine (N-methyltryptophan), β-tyrosine, and of the polypropionate fragment had the same relative configurations in both molecules. Therefore, the stereochemistry depicted in Figure 1 was tentatively assigned by analogy with the parent compound together with ROESY spectra that revealed a clear correlation between Me-16 and Me-34; and Me-33 and Me-36. However, an apparent deshielding of Me-16 was noted in 2 [δH 1.04 (3H, d, 6.6 Hz)] compared to 1 [δH 0.70 (3H, d, 6.7 Hz)] resembling that between jaspamide M [δH 1.17 (3H, d, 6.8 Hz)] [21] and jaspamide H [δH 0.72 (3H, d, 6.7 Hz)] [20]. These differences in chemical shifts for the latter congeners were proven to be caused by d-Ala or l-Ala residues, respectively. Anaylsis of the absolute configurations of the amino acids of 2 could not be performed due to the small amount of compound isolated (0.7 mg).
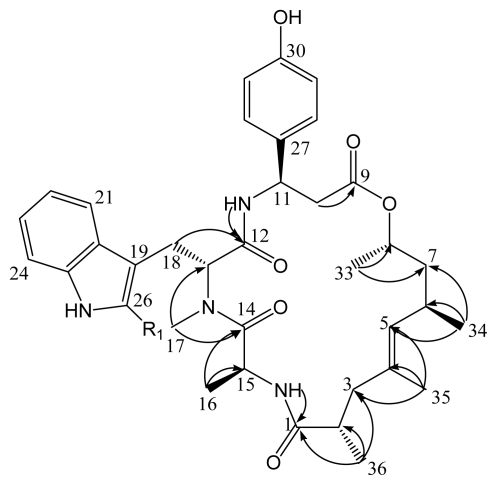
A partial 13C-NMR assignment of jaspamide Q (2) was achieved through HMBC spectra (Figure 2) which revealed clear correlations at δc 174.5, δc 40.4, δc 40.9, δc 133.6, δc 128.2, δc 29.5, δc 43.6, δc 70.4, δc 56.4, δc 173.6, and δc 46.0 ppm that were ascribed to C-1 to C-8, and C-13 to C-15, respectively. Moreover, HMBC spectra evidenced and confirmed the amino acid sequence in jaspamide Q (2) through cross-peaks between NH-Tyr and C-12, Me-17 and C-14, and between NH-Ala and C-1.
Figure 2.
Key HMBC correlations of jaspamide Q (2).
Jaspamide R (3) was isolated as a white amorphous solid. Its ESI mass spectrum exhibited pseudomolecular ion peaks at m/z 787.1, 789.1, and 791.1 [M+H]+, in a ratio of 1:2:1, supporting the existence of two bromine atoms in the compound. The molecular formula of jaspamide R (3) was determined to be C36H44Br2N4O6 by HRFTMS (m/z 789.1691 [M+H]+, Δ + 1.0 ppm) which exceeds that of jaspamide (1) by 79 amu revealing that jaspamide R (3) is a dibromo analogue of jaspamide Q (2).
This difference was explained by the 1H-NMR spectral data (Table 1), which revealed close similarity between jaspamide R (3) and jaspamide (1), except for the proton resonances corresponding to the indole moiety of the 2-bromoabrine unit. Jaspamide R (3) showed three proton resonances at δH 7.41 (1H, br s), δH 7.20 (1H, d, 8.2 Hz), and δH 7.42 (1H, d, 8.2 Hz) that were assigned to H-21, H-23, and H-24, respectively. Whereas for jaspamide (1), four resonances, at δH 7.23 (1H, d, 8.0 Hz), δH 7.10 (1H, t, 8.0 Hz), δH 7.12 (1H, t, 8.0 Hz), and δH 7.54 (1H, d, 8.0 Hz), ascribed for H-21 to H-24, were observed. Based on this finding, the additional bromine atom of 3 was assumed to be located at C-22. This hypothesis was further confirmed by 2D NMR spectral analyses including 1H–1H COSY, TOCSY, and NOESY spectra that revealed a clear NOE correlation between proton resonance at δH 7.41 (1H, br s), and NH-Tyr at δH 7.53 (1H, d, 8.6 Hz) proving the attachment of the second bromine atom to be at C-22. Moreover, the close resemblance in 1H resonances between jaspamide R (3), and jaspamide (1) supports the notion that the chiral centers of alanine, 2,4-dibromoabrine, β-tyrosine, and the polypropionate fragment have the same relative configurations in both molecules (Figure 1). Again, due to the lack of material isolated of 3 (0.5 mg), analysis of the absolute configurations of the amino acids, e.g. by Marfey’s method, could not be performed.
To the best of our knowledge, fifteen jaspamide congeners (B–P) were hitherto isolated from the genus Jaspis [14,18–21] and all of them show antiproliferative activity with IC50 values ranging from 0.01 to 33 μM against human breast adenocarinoma (MCF-7), and colon carcinoma (H-29) cell lines [21].
Jaspamide Q (2) and R (3) together with the parent jaspamide (1) differ in the bromination pattern of the abrine (N-methyltryptophan) moiety. Since these modifications were claimed as essential for the observed biological activity [44], compounds (2 and 3) together with jaspamide (1) were subjected to a cytotoxicity (MTT) assay against mouse lymphoma (L5178Y) cell lines. They exhibited potent activities with IC50 values in the ng/mL range (<0.1 μg/mL, <0.16 μM), compared to kahalalide F (IC50 = 6.3 μg/mL, 4.3 μM) which was used as a positive control. Further studies aimed at determining the effect of bromination pattern of the abrine residue on cytototoxic activity are in progress.
3. Experimental Section
General experimental procedures
Column chromatography was carried out on Sephadex LH-20 using methanol as an eluent. For analytical HPLC analysis, samples were injected into a HPLC system equipped with a photodiode array detector (Dionex, Munich, Germany). Routine detection was at 235, 254, 280, and 340 nm. The separation column (125 × 4 mm ID) was prefilled with C-18 Eurosphere, 5 μm (Knauer, Berlin, Germany). Separation was achieved by applying a linear gradient from 90% H2O (pH 2.0) to 100% MeOH over 40 min. TLC analysis was carried out using aluminium sheet precoated with silica gel 60 F254 (Merck, Darmstadt, Germany).
Preparative HPLC separations were performed on a LaChrom-Merck Hitachi HPLC system, pump L-7100, UV detector L-7400 using a C-18 column (Knauer, 300 × 8 mm ID, prefilled with C-18 Eurosphere, flow rate 5 mL/min, UV detection at 280 nm), and the solvent system consisted of a linear gradient of MeOH and nanopure H2O.
Optical rotations were measured on a Perkin-Elmer-241 MC polarimeter. ESIMS were obtained on a ThermoFinnigan LCQ DECA mass spectrometer coupled to an Agilent 1100 HPLC system equipped with a photodiode array detector. HRFTMS was recorded on a LTQ FT-MS-Orbitrap (ThermoFinnigan, Bremen, Germany). 1D and 2D NMR spectra were recorded at 300 ºK on a Bruker ARX-500. Samples were dissolved in deuterochloroform.
Biological material
In August 2008, specimens of J. splendens were collected on three neighboring Islands from East Kalimantan (Indonesia), namely Samama, Panjang, and Shoal Islands, at 10 meter depths. Numbers of voucher specimens are RMNH Por. 4234, 4266 and 4299, respectively. They were taxonomically identified as Jaspis splendens (order Astrophorida, family Ancorinidae) at the National Museum of Natural History, Leiden, Netherlands. HPLC and LCMS analyses of the three samples revealed that they were identical with regard to their peptide derivatives. Hence, the material was combined in order to obtain sufficient amounts of compounds for subsequent structure elucidation.
Extraction and isolation
The animal was freeze-dried, and the material (500 g) was extracted with methanol (3 × 2 L) and filtered. The extract was then combined, evaporated to dryness, and partitioned as follows. The methanolic extract (80 g) was dissolved in water and partitioned against n-hexane, ethyl acetate, and then n-butanol. The bioactive ethyl acetate soluble fraction (2 g) was chromatographed by CC using Sephadex LH20 as stationary phase and eluted with methanol followed by reversed-phase (C18 Eurosphere 100) HPLC using gradient elution of MeOH:H2O to yield 105 mg of jaspamide (1), 0.7 mg of jaspamide Q (2), and 0.5 mg of jaspamide R (3).
Jaspamide Q (2) was obtained as a white amorphous solid: [α]20D −62.0° (c 0.01, CHCl ); UV (MeOH) λmax 226, 280 nm; 1H-NMR see Table 1; ESI-MS pos m/z 631.3 [M+H]+ (100), ESI-MS neg m/z 629.3 [M-H]− (100), HRFTMS m/z 631.3491 [M+H]+ (calcd for C36H47N4O6, 631.3490), and m/z 653.3307 [M+Na]+ (calcd for C36H46N4O6Na, 653.3310).
Jaspamide R (3) was obtained as a white amorphous solid: [α]20D −100.0° (c 0.01, CHCl ); UV (MeOH) λmax 231, 283 nm; 1H-NMR see Table 1; ESI-MS pos m/z 787.1, 789.1, 791.1 [M+H]+, 1:2:1; ESI-MS neg m/z 785.2, 787.1, 789.0 [M-H] −, 1:2:1; HRFTMS m/z 789.1691 [M+H]+ (calcd for C36H45N4O679Br2, 789.1680), and m/z 811.1507 [M+Na]+ (calcd for C36H44N4O679Br2Na, 811.1499).
Cell proliferation assay
Cytotoxicity was tested against L5178Y mouse lymphoma cells using the microculture tetrazolium (MTT) assay as described earlier [11,45]. All experiments were carried out in triplicate and repeated three times. As controls, media with 0.1% EGMME/DMSO were included in the experiments.
Acknowledgements
A scholarship granted and financed by the Egyptian Government (Ministry of High Education) to S.S.E. is gratefully acknowledged. This project was supported by a grant of BMBF (to P.P.) and MOST (to W.L.). We are indebted to Prof. Dr. W.E.G. Mueller (University of Mainz) for performing the cytotoxicity (MTT) assay.
Footnotes
Samples Availability: Available from the authors.
References and Notes
- 1.Blunt JW, Copp BR, Hu W-P, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 2.Hamann MT, Scheuer PJ. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J Am Chem Soc. 1993;115:5825–5826. [Google Scholar]
- 3.Hamada T, Matsunaga S, Yano G, Fusetani N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J Am Chem Soc. 2005;127:110–118. doi: 10.1021/ja045749e. [DOI] [PubMed] [Google Scholar]
- 4.Nakao Y, Masuda A, Matsunaga S, Fusetani N. Pseudotheonamides, serine protease inhibitors from the marine sponge Theonella swinhoei. J Am Chem Soc. 1999;121:2425–2431. [Google Scholar]
- 5.Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, Williams DE, Dilip de Silva E, Lassota P, Allen TM, van Soest R, Andersen RJ, Boyd MR. Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc. 1999;121:5899–5909. [Google Scholar]
- 6.Reese MT, Gulavita NK, Nakao Y, Hamann MT, Yoshida WY, Coval SJ, Scheuer PJ. Kulolide: A cytotoxic depsipeptide from a cephalaspidean mollusk, Philinopsis speciosa. J Am Chem Soc. 1996;118:11081–11084. [Google Scholar]
- 7.Renner MK, Shen YC, Cheng XC, Jensen PR, Frankmoelle W, Kauffman CA, Fenical W, Emil CJ. Cyclomarins A–C, new anti-inflammatory cyclic peptides produced by a marine bacterium Streptomyces sp. J Am Chem Soc. 1999;121:11273–11276. [Google Scholar]
- 8.Clark WD, Corbett T, Valeriote F, Crews P. Cylcocinamide A, an unusual cytotoxic halogenated hexapeptide from the marine sponge Psammocinia. J Am Chem Soc. 1997;119:9285–9286. [Google Scholar]
- 9.Nakao Y, Yeung BKS, Yoshida WY, Scheuer PJ, Kelly-Borges M. Kapakahine B: A cyclic hexapeptide with an α-carboline ring system from the marine sponge Cribrochalina olemda. J Am Chem Soc. 1995;117:8271–8272. [Google Scholar]
- 10.Fusetani N, Sugawara T, Matsunaga S, Hirota H. Orbiculamide A: A novel cytotoxic cyclic peptide from a marine sponge Theonella sp. J Am Chem Soc. 1991;113:7811–7812. [Google Scholar]
- 11.Ashour M, Edrada RA, Ebel R, Wray V, Waetjen W, Padmakumar K, Mueller WEG, Lin WH, Proksch P. Kahalalide derivatives from the Indian sacoglossan mollusk Elysia grandifolia. J Nat Prod. 2006;69:1547–1553. doi: 10.1021/np060172v. [DOI] [PubMed] [Google Scholar]
- 12.Matsunaga S, Fusetani N, Hashimoto K, Walchli M. Theonellamide F: A novel antifungal bicyclic peptide from a marine sponge Theonella sp. J Am Chem Soc. 1989;111:2582–2588. [Google Scholar]
- 13.Ireland C, Scheuer PJ. Ulicyclamide and ulithiacyclamide, two new small peptides from a marine tunicate. J Am Chem Soc. 1980;102:5688–5691. [Google Scholar]
- 14.Zabriskie TE, Klocke JA, Ireland CM, Marcus AH, Molinski TF, Faulkner DJ, Xu C, Clardy JC. Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity. J Am Chem Soc. 1986;108:3123–3124. [Google Scholar]
- 15.Crews P, Manes LV, Boehler M. Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett. 1986;27:2797–2800. [Google Scholar]
- 16.Scott VR, Boehme R, Matthews TR. New class of antifungal agents: Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob Agents Chemother. 1988;32:1154–1157. doi: 10.1128/aac.32.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inman W, Crews P. Novel marine sponge derived amino acids, 8. conformational analysis of jasplakinolide. J Am Chem Soc. 1989;111:2822–2829. [Google Scholar]
- 18.Zampella A, Giannini C, Debitus C, Roussakis C, D’Auria MV. New jaspamide derivatives from the marine sponge Jaspis splendans collected in Vanuatu. J Nat Prod. 1999;62:332–334. doi: 10.1021/np9803225. [DOI] [PubMed] [Google Scholar]
- 19.Gala F, D’Auria MV, De Marino S, Zollo F, Smith CD, Copper JE, Zampella A. New jaspamide derivatives with antimicrofilament activity from the sponge Jaspis splendans. Tetrahedron. 2007;63:5212–5219. [Google Scholar]
- 20.Gala F, D’Auria MV, De Marino S, Sepe V, Zollo F, Smith CD, Copper JE, Zampella A. Jaspamides H–L, new actin-targeting depsipeptides from the sponge Jaspis splendans. Tetrahedron. 2008;64:7127–7130. [Google Scholar]
- 21.Gala F, D’Auria MV, De Marino S, Sepe V, Zollo F, Smith CD, Keller SN, Zampella A. Jaspamides M–P: New tryptophan modified jaspamide derivatives from the sponge Jaspis splendans. Tetrahedron. 2009;65:51–56. [Google Scholar]
- 22.Ravi BN, Wells RJ, Croft KD. Malabaricane triterpenes from a Fijian collection of the sponge Jaspis stellifera. J Org Chem. 1981;46:1998–2001. [Google Scholar]
- 23.Tsuda M, Ishibashi M, Agemi K, Sasaki T, Kobayashi J. Stelliferins A–F, new antineoplastic isomalabaricane triterpenes from the Okinawan marine sponge Jaspis stellifera. Tetrahedron. 1991;47:2181–2194. [Google Scholar]
- 24.Kobayashi J, Yuasa K, Kobayashi T, Sasaki T, Tsuda M. Jaspiferals A–G, new cytotoxic isomalabaricane-type nortriterpenes from Okinawan marine sponge Jaspis stellifera. Tetrahedron. 1996;52:5745–5750. [Google Scholar]
- 25.Zampella A, D’Auria MV, Debitus C, Menou J-L. New isomalabaricane derivatives from a new species of Jaspis sponge collected at the Vanuatu islands. J Nat Prod. 2000;63:943–946. doi: 10.1021/np000088u. [DOI] [PubMed] [Google Scholar]
- 26.Meragelman KM, McKee TC, Boyd MR. New cytotoxic isomalabaricane triterpenes from the sponge Jaspis species. J Nat Prod. 2001;64:389–392. doi: 10.1021/np000478g. [DOI] [PubMed] [Google Scholar]
- 27.Tang SA, Deng ZW, Li J, Fu HZ, Pei YH, Zhang S, Lin WH. A new isomalabaricane triterpenoid from sponge Jaspis sp. Chin Chem Lett. 2005;16:353–355. [Google Scholar]
- 28.Tang S, Pei Y, Fu H, Deng Z, Li J, Proksch P, Lin W. Jaspolides A–F, six new isomalabaricane-type terpenoids from the sponge Jaspis sp. Chem Pharm Bull. 2006;54:4–8. doi: 10.1248/cpb.54.4. [DOI] [PubMed] [Google Scholar]
- 29.Tang S, Deng Z, Proksch P, Lin W. Jaspolides G and H, unique bisisomalabaricane from the Chinese marine sponge Jaspis sp. Tetrahedron Lett. 2007;48:5443–5447. [Google Scholar]
- 30.Kobayashi J, Murata O, Shigemori H, Sasaki T. Jaspisamides A–C, new cytotoxic macrolides from the Okinawan marine sponge Jaspis sp. J Nat Prod. 1993;56:787–791. doi: 10.1021/np50095a021. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez J, Nieto RM, Crews P. New structures and bioactivity patterns of bengazole alkaloids from a choristid marine sponge. J Nat Prod. 1993;56:2034–2040. doi: 10.1021/np50102a002. [DOI] [PubMed] [Google Scholar]
- 32.Searle PA, Richter RK, Molinski TF. Bengazoles C–G from the sponge Jaspis sp. synthesis of the side chain and determination of absolute configuration. J Org Chem. 1996;61:4073–4079. doi: 10.1021/jo952261a. [DOI] [PubMed] [Google Scholar]
- 33.D’Auria MV, Giannini C, Minale L, Zampella A, Debitus C, Frostin M. Bengamides and related amino acid derivatives from the new Caledonian marine sponge Jaspis carteri. J Nat Prod. 1997;60:814–816. [Google Scholar]
- 34.Groweiss A, Newcomer JJ, O’Keefe BR, Blackman A, Boyd MR. Cytotoxic metabolites from an Australian collection of the sponge Jaspis species. J Nat Prod. 1999;62:1691–1693. [Google Scholar]
- 35.Thale Z, Kinder FR, Bair KW, Bontempo J, Czuchta AM, Versace RW, Philips PE, Sanders ML, Wattanasin S, Crews P. Bengamides revisited: New structures and antitumor studies. J Org Chem. 2001;66:1733–1741. doi: 10.1021/jo001380+. [DOI] [PubMed] [Google Scholar]
- 36.Park Y, Liu Y, Hong J, Lee C-O, Cho H, Kim D-K, Im KS, Jung JH. New bromotyrosine derivatives from an association of two sponges, Jaspis wondoensis and Poecillastra wondoensis. J Nat Prod. 2003;66:1495–1498. doi: 10.1021/np030162j. [DOI] [PubMed] [Google Scholar]
- 37.Shinde PB, Lee YM, Dang HT, Hong J, Lee C-O, Jung JH. Cytotoxic bromotyrosine derivatives from a two-sponge association of Jaspis sp. and Poecillastra sp. Bioorg Med Chem Lett. 2008;18:6414–6418. doi: 10.1016/j.bmcl.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 38.Ohta S, Kobayashi H, Ikegami S. Isojaspisin: A novel styryl sulfate from a marine sponge, Jaspis sp., that inhibits hatching of sea urchin embryos. Tetrahedron Lett. 1994;35:4579–4580. [Google Scholar]
- 39.Tsukamoto S, Kato H, Hirota H, Fusetani N. Narains: N,N-Dimethylguanidinium styryl sulfates, metamorphosis inducers of ascidian larvae from a marine sponge Jaspis sp. Tetrahedron Lett. 1994;35:5873–5874. [Google Scholar]
- 40.Ohta S, Kobayashi H, Ikegami S. Jaspisin, a novel styryl sulfate from the marine sponge, Jaspis species. Biosci Biotech Biochem. 1994;58:1752–1753. [Google Scholar]
- 41.Tsukamoto S, Kato H, Hirota H, Fusetani N. 3,4-Dihydroxystyrene dimmers, inducers of larval metamorphosis in ascidians, from a marine sponge Jaspis sp. Tetrahedron. 1994;50:13583–13592. [Google Scholar]
- 42.Chang YH, Shin D, Na Z, Lee H-S, Kim D-D, Oh K-B, Shin J. Dihydroxystyrene metabolites from an association of the sponge Poecillastra wondoensis and Jaspis sp. J Nat Prod. 2008;71:779–783. doi: 10.1021/np078015z. [DOI] [PubMed] [Google Scholar]
- 43.Ebada SS, Edrada RA, Lin W, Proksch P. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat Prot. 2008;3:1820–1831. doi: 10.1038/nprot.2008.182. [DOI] [PubMed] [Google Scholar]
- 44.Kahn M, Nakanishi H, Su T, Lee JH, Johnson M. Design and synthesis of nonpeptide mimetics of jaspamide. Int J Pept Protein Res. 1991;38:324–334. doi: 10.1111/j.1399-3011.1991.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 45.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of radiosensitivity. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]