Abstract
The double-stranded RNA (dsRNA)-activated protein kinase (PKR) plays a major role in the innate immune response in humans. PKR binds dsRNA non-sequence specifically and requires a minimum of 15 bp dsRNA for one protein to bind and 30 bp dsRNA to induce protein dimerization and activation by autophosphorylation. PKR phosphorylates eIF2α, a translation initiation factor, resulting in the inhibition of protein synthesis. We investigated the mechanism of PKR activation by an RNA hairpin with a number of base pairs intermediate between these 15 to 30 bp limits: HIV-I TAR RNA, a 23 bp hairpin with three bulges that is known to dimerize. To test whether RNA dimerization affects PKR dimerization and activation, TAR monomers and dimers were isolated from native gels and assayed for RNA and protein dimerization. To modulate the extent of dimerization, we included TAR mutants with different secondary features. Native gel mixing experiments and analytical ultracentrifugation indicate that TAR monomers bind one PKR monomer and that TAR dimers bind two or three PKRs, demonstrating that RNA dimerization drives the binding of multiple PKR molecules. Consistent with functional dimerization of PKR, TAR dimers activated PKR while TAR monomers did not, and RNA dimers with fewer asymmetrical secondary structure defects, as determined by enzymatic structure mapping, were more potent activators. Thus, the secondary structure defects in the TAR RNA stem function as antideterminants to PKR binding and activation. Our studies support that dimerization of a 15–30 bp hairpin RNA, which effectively doubles its length, is a key step in driving activation of PKR and provide a model for how RNA folding can be related to human disease.
Keywords: protein kinase, RNA folding, innate immunity, analytical ultracentrifugation, RNA-protein interaction
Introduction
The dsRNA-activated protein kinase, PKR, is induced by interferon and plays a pivotal role in the innate immunity response to viral infection. The importance of this antiviral pathway is highlighted by the diverse mechanisms that viruses have evolved to combat PKR.1–3 The enzyme is synthesized in a latent state, but upon binding dsRNA PKR undergoes autophosphorylation at multiple serine and threonine residues resulting in activation. The best-characterized cellular substrate of PKR is the alpha subunit of initiation factor eIF2. Phosphorylation of eIF2α inhibits the initiation of translation. Thus, production of dsRNA that occurs during viral infection results in PKR activation and subsequent inhibition of viral protein synthesis.4
PKR contains an N-terminal double-stranded RNA binding domain (dsRBD) and a C-terminal kinase domain. The dsRBD consists of two tandem copies of the ~70 amino-acid dsRNA binding motif. In the NMR structure of the PKR dsRBD, each motif has the canonical αβββα fold.5 A crystal structure of a complex of the PKR kinase domain and eIF2α has also been reported.6 The catalytic domain adopts a bilobal structure typical of protein kinases and forms a dimer mediated by interactions between residues within the N-terminal lobes. Helix αC comprises a portion of the dimer interface and may allosterically link dimerization with enzymatic activation and substrate recognition. PKR contains a large (~90 residue) region between the dsRBD and kinase that appears unstructured by NMR,7 and small angle scattering measurements indicate that the enzyme is flexible in solution and adopts multiple conformations.8
It has long been recognized that PKR is capable of dimerizing, and several lines of evidence support a key role for dimerization in the activation of PKR by dsRNA.9 A defining feature of PKR is the “bell-shaped” curve for activation, where low concentrations of dsRNA activate but higher concentrations are inhibitory.10,11 These data have generally been interpreted to indicate that low concentrations of dsRNA drive assembly of PKR dimers on a single dsRNA whereas higher dsRNA concentrations dilute PKR monomers onto separate molecules of dsRNA.12 Although PKR can bind to dsRNAs as short as 15 bp,13–15 a minimum of 30 bp is required for activation.11 The ability of dsRNAs to function as PKR activators is correlated with binding of two or more PKR monomers,16 supporting a model where the role of the dsRNA is to bring two or more PKR monomers in close proximity to enhance dimerization via the kinase domain. PKR alone exists in a weak monomer-dimer equilibrium and dimerization of PKR in the absence of dsRNA is sufficient to activate PKR.17
PKR is regulated by a variety of RNAs, but the structural features that distinguish activators of PKR from those that fail to activate are not yet well understood. Incorporation of G-I mismatches in the context of poly(rI)-poly(rC) homopolymeric dsRNA blocks PKR activation,10 while G•U wobble pairs also inhibit dsRNA-mediated activation.18 At the same time, not all non-Watson-Crick motifs interfere with PKR activation. For instance, PKR binds to and is activated by RNAs containing tandem AG mismatches and noncontiguous helices, provided the RNA adopts an overall A-form geometry.19 In addition, bulges do not interfere with activation by PKR aptamers,20 and certain pseudoknotted RNAs can activate PKR.21
The HIV-1 transactivation-responsive region (TAR) RNA consists of a stem-loop interrupted by three bulges and has served as a model system to investigate regulation of PKR by complex RNAs.22–32 The HIV Tat protein specifically recognizes the trinucleotide bulge in TAR.33 Although the interaction of PKR with TAR has been extensively studied, the PKR binding and activation properties of TAR have not yet been clearly defined. The TAR RNA hairpin is known to dimerize,34 which complicates analysis of protein-RNA interactions. In the present study, we have developed methods to prepare homogeneous TAR RNA monomer and dimer and characterized their secondary structures and their ability to bind and activate PKR using a combination of gel mobility shift measurements, autophosphorylation assays, analytical ultracentrifugation, and enzymatic structure mapping. Our results indicate that TAR RNA dimerization leads to a greatly enhanced ability to activate PKR, and that the secondary structure defects in TAR function as antideterminants of PKR binding.
Results
Optimization of RNA monomer and dimer formation
In order to define the contribution of TAR RNA dimerization to PKR binding and activation, we prepared a series of TAR-related variant RNAs that could dimerize to differing extents, optimized conditions for monomer and dimer formation, and purified monomer and dimer species on a native gel. Similar approaches were used by Chen and co-workers to investigate the role of pRNA in DNA translocation machinery.35 A prior study by Kjems and co-workers investigated the extent to which various mutants of TAR dimerize, some of which are shown in Figure 1a.34 They reported that in the presence of HIV-1 nucleocapsid (NC) protein, the A34U single mutant forms only monomer, TARwt forms appreciable dimer, and the A34U:U37A double mutant forms mostly dimer. Using these observations, we prepared TAR variants on the basis of their potential to dimerize. These RNAs included TARwt, the single mutants A34U and U37A, the double mutant A34U:U37A, and an equimolar mixture of the A34U and U37A single mutants, denoted ‘[A34U + U37A]’, which is predicted to form a stable dimer.
Fig. 1.
TAR variants used in this study. (a) Secondary structure for TARwt, with changes (boxed) used to make the single mutants A34U and U37A, and the double mutant A34U:U37A. Also shown are truncations at the base of the stem present in the three shortened hairpin TARs: shTAR51, shTAR47, and shTAR43, which remove 3, 5, and 7 base pairs, respectively. A34U can dimerize with U37A (denoted ‘[A34U + U37A]’), while alone each forms a stable monomer; A34U:U37A forms a self-complementary dimer. (b) Secondary structure for self-complementary TAR (scTAR). This variant deletes the three bulges present in TAR, while retaining the two GU wobbles at positions 6 and 9; in addition, it is mutated on the 3’-side of its loop to add perfect self-complementarity. Also shown are changes used to make perfectly self-complementary TAR (pscTAR), in which the two GU wobbles are converted to Watson-Crick GC base pairs.
Large quantities of TAR monomer and dimer were required for the various native gel, activation, AUC, and structure mapping assays. We therefore screened conditions for RNA monomer and dimer formation of these TAR variants using native PAGE (Fig. 2a).i Wild-type, A34U, and A34U:U37A TAR variants at 3 µM concentrations with trace 5’-end radiolabeled RNA were treated according to several different renaturation and under various salt conditions. Following renaturation, these RNAs were fractionated on a 0.5×TBE native gel to determine the extent of monomer and dimer formation.ii Three bands were observed and tentatively assigned as monomer, dimer, and multimer (Fig. 2a); their identities were later confirmed by AUC and structure mapping (see below). When renatured at 3 µM in 1×TEK100, TARwt formed a small amount of dimer (~2%), A34U formed no observable dimer, while A34U:U37A formed the most dimer (up to ~30%) (Fig. 2a). These trends parallel those reported by Kjems and co-workers.34 It is also clear from both TARwt and A34U:U37A samples that either 100 mM KCl or a mixture of 100 mM KCl and 4 mM MgCl2 favors dimer formation. In addition, the presence of Mg2+ significantly reduces the formation of the species that migrates slower than dimer, collectively referred to herein as ‘multimer’.
Fig. 2.
Optimization of TAR monomer and dimer formation on native gels. (a) TARwt, A34U, and A34U:U37A variants dimerize to different extents under the same renaturation conditions. 3 µM RNA was renatured in 1×TE and 1×TEK100 by incubating at 90 °C for 3 min, room temperature for 10 min, and 55 °C for 10 min. The sample was then either cooled at room temperature for 10 min (cooling condition 1) or on ice for 10 min (cooling condition 2). In the third set of salt conditions, MgCl2 was added to 4 mM in the 1×TEK100 samples, prior to the 55 °C step. Gel loading buffer contained 10 mM NaCl and 5% glycerol (b) Optimizing dimer formation in the A34U:U37A variant. 50 or 3 µM A34U:U37A was renatured in 1×TE, 1×TEK100, or 1×TEK500 by incubating at 90 °C for 3 min, followed by one of three cooling conditions: 1) ice for 10 min, 2) room temperature for 10 min, or 3) 55 °C for 10 min followed by room temperature for 10 min. The presence of salt and high RNA concentration drives dimer formation. All gels shown are 10% polyacrylamide native gels (0.5×TBE) fractionated for ~2 h at 16 °C. The percent of strands in dimer is provided below the gel.
Next, conditions for RNA dimer formation were optimized (Fig. 2b). We chose A34U:U37A for optimization of dimer formation because it showed optimal dimerization in the initial screens (Fig. 2a). The extent of dimerization was tested at 3 and 50 µM RNA in 1×TE and a background of 0, 100, and 500 mM KCl. Dimer formation was promoted by higher RNA concentration and the presence of salt. At 3 µM A34U:U37A, almost no dimer was detected for 1×TE,iii while ~25% dimer was observed in 1×TEK100 and 1×TEK500. Upon increasing the RNA concentration to 50 µM, the extent of dimer improved for all salt concentrations, with 1×TEK100 and 1×TEK500 giving optimal RNA dimer formation near ~60%. Varying sample cooling conditions did not significantly affect dimer to monomer ratios. We chose to perform subsequent TAR dimer renaturations at 50 or 100 µM RNA in 1×TEK100 by incubating at 90 °C for 3 min followed by incubation at room temperature for 10 min. The amount of dimer formed depended on the particular TAR variant used and ranged from approximately 10 to 60%.
Purification of RNA monomers and dimers from native gels
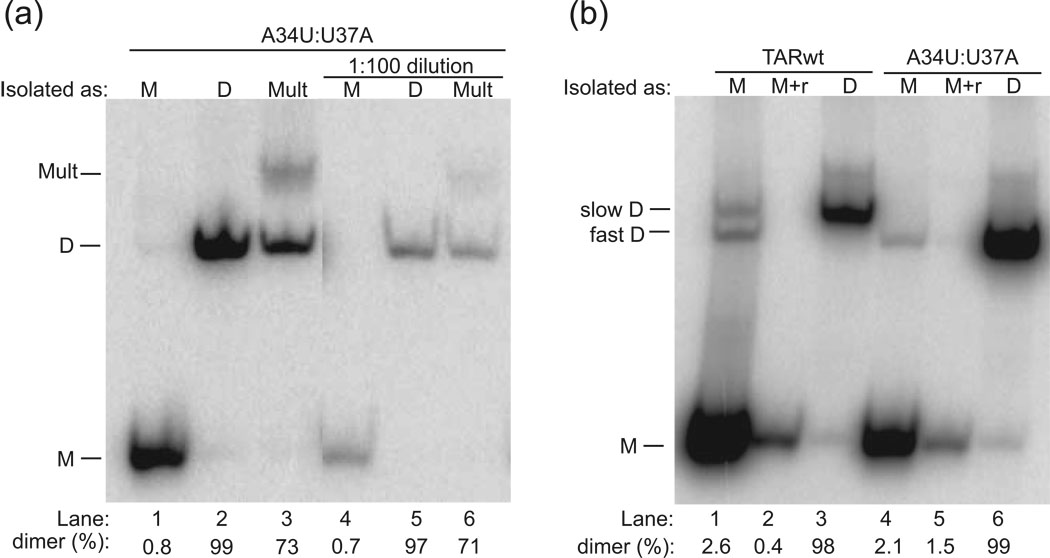
Once variants and experimental conditions for RNA monomer and dimer formation had been identified and optimized, RNA monomer and dimer species were purified from native gels. The monomer, dimer, and multimer species from A34U:U37A containing trace 5’-end radiolabeled RNA were isolated from native gels by UV shadowing. The purified monomer, dimer, and multimers were re-fractionated on a second native PAGE gel to check whether they retained their identity (Fig. 3). We observed that monomer and dimer species were largely unaffected by purification (Fig. 3a, Lanes 1 and 2), suggesting that they are kinetically stable under the conditions tested. The multimer species, however, largely migrated with the dimer upon purification (Fig. 3a, compare Lanes 3 and 2), suggesting that it is less stable. Next, the A34U:U37A native-gel purified monomer, dimer, and multimer species were diluted 1:100 and fractionated on a second native gel. Dilution did not affect the stability of monomer or dimer (Fig. 3a, compare Lanes 4–6 with Lanes 1–3). These observations support the conclusion that the dimer species is kinetically trapped.
Fig. 3.
Analysis of native gel-purified RNAs. The form in which the band was isolated is provided following ‘Isolated as:’. (a) Analysis of native gel-purified A34U:U37A. Purification as well as dilution does not affect the integrity of A34U:U37A monomer (M) (lanes 1 and 4) or dimer (D) (lanes 2 and 5), but the multimer (Mult) is converted mostly to dimer (lanes 3 and 6). (b) Analysis of native gel-purified TARwt and A34U:U37A. Occasionally, bands isolated as monomer contained one or more trace dimer bands when re-fractionated (Lanes 1 and 4), but this could be removed by further renaturing these samples (incubating at 90 °C for 3 min/ room temperature 10 min) in 1×TE at concentrations of 1–3 µM (denoted ‘M+r’) (Lanes 2 and 5). In addition, migration of TARwt dimer is slower than A34U:U37A dimer (Lanes 3 and 6). Analysis of native-gel-purified single mutants is provided in the Supplementary Fig. 1. All gels are 10% polyacrylamide native gels (0.5×TBE) fractionated for ~2 h at 16 °C. The percent of strands in dimer is provided below the gel.
Because PKR can potentially be activated by even small amounts of TAR RNA dimer, as discussed above, we examined whether the monomer lanes contained trace amounts of dimer. As shown in Fig. 3b, Lanes 1 and 4, the TARwt and A34U:U37A monomer lanes contained 2.6 and 2.1% dimer. In an effort to remove trace dimer, the RNA was renatured at lower concentrations (1–3 µM) in 1×TE. These treatments removed most dimer as confirmed by native PAGE (Fig. 3b, compare Lanes 1 and 2, and Lanes 4 and 5). Unexpectedly, U37A and especially A34U monomer-isolated samples also contained dimer after purification from native gels (Supplementary Fig. 1a, Lanes 1 and 2). The A34U and U37A isolated from native gels as monomers were therefore diluted and renatured and then fractionated on a native gel, as described for TARwt and A34U:U37A. This dilution-renaturation treatment was even more effective for A34U and U37A than for TARwt and A34U:U37A, as no dimer was detectable for the single mutants (Supplementary Fig. 1b, lanes 1 and 2). This is consistent with the documented monomeric behavior of A34U34 (Fig. 2a) and the expected behavior of U37A. On the basis of these findings, all RNA desired to be monomeric was renatured at 3 µM or less in 1×TE at 90 °C prior to any given experiment.iv
Lastly, it can be noted that isolated dimers contained at most 5% monomer (Fig. 3 and Fig. S1). This small amount of monomer is not expected to significantly interfere with activation assays because monomer is a (modest) inhibitor (see below); therefore further purification of the dimer was not pursued. In summary, screening of TAR variants and optimization of solution and renaturation conditions allowed preparation of RNA bands that are highly purified in monomer or dimer species.
PKR activation assays using RNA monomers and dimmers
As described above, native gel assays were developed that allowed TAR RNA monomers and dimers to be isolated with high purity. Next, we wanted to test how these species function in PKR activation assays. PKR activation assays were therefore performed on native gel-purifiediv RNA monomer and dimers (Fig. 4). Prior to activation assays, analytical quantities of monomer and native gel-purified dimer species were analyzed on native gels to examine whether freeze-thaw handling affected the integrity of monomer or dimer. We observed that both monomers and dimers retained their expected form (Fig. 3 and Fig. S1).
Fig. 4.
RNA hairpin dimerization promotes PKR activation. Activation and inhibition assays (10% SDS-PAGE) were performed at various concentrations of PKR, as indicated to the right of each gel. (a) Activation assays with native gel-purified TARwt monomer and dimer in the presence of 5 µM PKR. Higher PKR concentration (5 µM) promotes activation by TARwt dimer, with just weak activation by monomer. (b) Activation assays with native gel-purified A34U:U37A monomer and dimer in the presence of 0.8 µM PKR. (c) Activation assays with native gel-purified A34U monomer, U37A monomer, and [A34U + U37A] dimer in the presence of 0.8 µM PKR. For both panels (b) and (c), lower PKR concentration (0.8 µM) achieves activation by dimer with (at most) weak activation by monomer. (d) Inhibition assay with native gel-purified TARwt TAR monomer and dimer. All lanes have 0.1 µM PKR and 0.01 µM 79 bp dsRNA, which is a potent activator of PKR. Increasing the concentration of TARwt monomer from 0.01 to 1.2 µM inhibits activation ~1.5-fold, while the same increases in wtTAR dimer concentration inhibits activation ~2-fold. For all panels, a no-RNA lane (−) is provided, and phosphorylation activities are normalized to 0.01 µM 79 bp RNA. In order to provide RNA-dependent activation values, the no-RNA lane was subtracted from each lane. These background-subtracted values are provided, as are RNA-dependent fold-effects for dimer over monomer (panels a and b) or for inhibition relative to 79 bp RNA (panel d).
PKR activation and inhibition assays performed in the presence of monomer and dimer species from different TAR variants are provided in Figure 4. PKR is present at 5 µM in TARwt activation assays and at 0.8 µM for all other activation assays. Differences in optimal protein concentration are attributed to variations in RNA secondary structures as probed using native gel migration and structure mapping (see below). Increasing the concentration of PKR increased background activation in the no-RNA lane (Fig. 4a), which was expected on the basis of the ability of PKR to dimerize in an RNA-independent fashion and self-phosphorylate.17 With this background corrected, at an RNA concentration of 0.5 µM activation of PKR by TARwt dimer was much higher (at least 23-fold) than by TARwt monomer. Moreover, percent activation by TARwt dimer was within two-fold that of 79 bp RNA, albeit requiring a higher concentration of RNA, indicating that TARwt dimer is capable of high potency activation of PKR. (Fig. 4a).
Next, we examined activation of PKR in the presence of monomer and dimer RNA from single and double mutant TAR RNAs. In these assays, PKR is present at a lower concentration of 0.8 µM, where reasonable activation could be obtained. Activation by A34U:U37A dimer at 0.05 µM RNA was 17-fold higher than by A34U:U37A monomer RNA (Fig. 4b). A decrease in fold-activation with increasing RNA concentration was observed (down to 6-fold) (Fig. 4b), but is due to an increase in activation by the RNA prepared as monomer, probably because A34U:U37A can slightly dimerize at these higher RNA concentrations (Fig. 3b, Lane 5). Likewise, activation by the [A34U + U37A] dimer was much greater than by A34U or U37A monomer (Fig. 4c). Thus, the data in Figure 4 (a)–(c) show that RNA dimer is a strong activator of PKR, and provide no support for RNA monomer being an activator.
Lastly, the ability of TARwt monomer and dimer to inhibit PKR was tested using a background of 0.01 µM 79 bp RNA (a potent activator of PKR) and 0.1 µM PKR (Fig. 4d). The concentration of RNA monomer and dimer ranged from 0.01 µM (the same as 79 bp) to 1.2 µM. We observed ~1.5-fold inhibition at 1.2 µM monomer and ~2-fold inhibition at 1.2 µM by dimer. Consistent with this behavior, TARwt dimer has approximately twice the number of base pairs as monomer. In addition, the dependence of PKR activation on TARwt dimer is bell-shaped, also consistent with high concentrations of TARwt dimer being an inhibitor (Supplementary Fig. 2).
Stoichiometry between p20 and RNA: Native gel analysis
As described in the previous section, native gel-purified dimer RNAs activate PKR while monomers do not. One possibility is that there is a correlation between RNA dimerization and PKR dimerization. The stoichiometry between RNA and p20, the dsRBD of PKR, was explored by native gel analysis (this section) and between RNA and PKR by analytical ultracentrifugation (AUC) (next section).
To test whether one protein binds to a TAR monomer, we performed mobility-shift assays with p20v and a fusion protein of p20, p20-VP16vi, where VP16 is the ~80 amino acid activation domain from herpes simplex virus type 1.36 The p20-VP16 has a retarded electrophoretic mobility relative to p20. Four conditions were analyzed in the native gel: TAR alone, TAR + p20, TAR + p20 + p20-VP16, and TAR + p20-VP16 (Fig. 5a). The TAR RNA is 5’-end labeled and present in trace amounts, while p20 and p20-VP16 are present at 1 and 4 µM concentrations, respectively. Also, the TAR RNA was renatured prior to electrophoresis to form monomer. As shown in Figure 5a Lane 1, free TAR RNA migrates as a single band, consistent with a single species, and shifts to an electrophoretically distinct species when incubated with p20 or p20-VP16 (Fig. 5a, Lanes 2 and 4) consistent with a single favored complex. When TAR, p20, and p20-VP16 are mixed, bands are observed that co-migrate with TAR•p20 and TAR•p20-VP16 with no intermediate bands (Fig. 5a, Lanes 3), suggesting only one protein binds TAR.
Fig. 5.
Stoichiometry and affinity between p20 and TAR by native PAGE. Native gel assays were performed using electrophoretically unique variants of p20 and TAR RNA. For p20, a VP16 peptide was fused to the C-terminus (p20-VP16), while 30 adenosines were fused to the 3’-end of TAR (TAR-A30). All RNAs were renatured by incubating at 90 °C to populate the monomer. Trace (~2 nM) 5’-end labeled RNA was incubated with 1 µM p20, 4 µM p20-VP16, or both proteins for 1 h at room temperature. Native gels are 10% polyacrylamide, 0.5×TBE, and were run at 4 °C. (a) p20, p20-VP16, or both were incubated with TAR and fractionated by native PAGE. Free TAR was also run on the gel. Data are consistent with a 1:1 complex. (b) TAR, TAR-A30, or both were incubated p20 or alone, and fractionated by native PAGE. Data are consistent with a 1:1 complex. (c) Truncated TAR variants, shTAR51, shTAR47, and shTAR43, were tested for binding to p20. Data show that all three truncated TARs bind p20, but less strongly as RNA length decreases. Percent RNA shifted is provided below the gel.
Using a similar strategy, we addressed the possibility of two TAR RNA hairpins binding to a single p20. Mixing experiments were performed with TAR and a longer TAR containing 30 adenosines at the 3’-end, TAR-A30. Five conditions were analyzed: TAR alone, TAR + p20, TAR + TAR-A30 + p20, TAR-A30 + p20, and TAR-A30 (Fig. 5b). As shown in Figure 5b Lanes 1 and 5, free TAR and TAR-A30 RNAs migrate as single, electrophoretically distinct bands, consistent with a single species. Moreover, each RNA completely shifts to a single electrophoretically distinct species when incubated with p20 (Fig. 5b, Lanes 2 and 4), consistent with a single favored complex. When TAR, TAR-A30, and p20 are mixed, bands are observed that co-migrate with TAR•p20 and TAR-A30•p20, and no intermediate bands are observed (Fig. 5b, Lane 3), suggesting that the stoichiometry between TAR and p20 is 1:1.vii Moreover, site-directed mutants in dsRBM1 (K60A) and dsRBM2 (K150A) do not bind to TAR or to 16 or 22 bp perfect dsRNAs (Supplementary Fig. 3), supporting both dsRBMs being required for this 1:1 complex. These observations are consistent with affinity cleavage experiments on p20 and TAR, which found that both dsRBMs contact TAR.30 Together with the TAR inhibition assays in Fig. 4d, these data suggest that some PKR antagonists including TAR monomer interact with both of PKR’s dsRBMs.
To test whether the length of TAR affects p20 binding, we performed mobility-shift experiments with three shortened TAR variants: shTAR51, shTAR47, and shTAR43 (Fig. 1a and Fig. 5c). These variants remove 3, 5, or 7 base pairs from the base of the stem, respectively. Binding of p20 to shTAR51 and shTAR47 remains fairly strong, with 1 µM p20 giving 60% and 40% complex, respectively. This indicates that the last two base pairs are dispensable for binding. The affinity of p20 to shTAR43, a 16 bp RNA, is significantly diminished, however, with only 11% complex formed, which is consistent with previous reports that 16 bp is the minimal length RNA that supports binding of p20, 14 and is consistent with binding to a monomeric TAR.
Affinity and Stoichiometry of PKR binding to TAR: AUC analysis
Stoichiometry between p20 and monomeric TAR was explored by native gel analysis (previous section) and led to the conclusion that these form a 1:1 complex that requires both dsRBMs and ~16 bp in the stem. Next, the interaction of full-length PKR with monomeric and dimeric TAR was investigated by sedimentation velocity AUC to measure the binding affinities and stoichiometries under rigorous equilibrium conditions. Our goal for AUC analysis was to correlate TAR multimerization with PKR activation and binding stoichiometries.
First, the homogeneity and association state of RNAs were analyzed in the absence of PKR. The sedimentation velocity data were analyzed using SEDFIT37 to provide the c(s) continuous sedimentation coefficient distribution function. The presence of contaminants gives rise to additional peaks in the distribution. All the RNA samples were found to be homogeneous by c(s) analysis with less than 5% contaminants present. Fitting of data to a single-species model yielded apparent molecular weights that are within 10% of the expected values for the scTAR monomer (a bulgeless version of TAR (Fig. 1b) that is described fully below) and for all of the RNA dimers, assuming a partial specific volume of ν̄ = 0.55 mL/g (Table 1). However, the apparent molecular weights of the monomers of TARwt, A34U and U37A are about 15% higher than the expected values (Table 1). The origin of this discrepancy is not clear, but may reflect differences in the hydration of the TAR monomers that contain three bulges. We also note that the apparent molecular weights for the TAR dimers are in good agreement with the expected values (Table 1).
Table 1.
RNA hydrodynamic parameters and molecular weights.
| RNA | Mexperimental (kDa)a | Mpredicted (kDa) | sb | RMSc |
|---|---|---|---|---|
| Monomers | ||||
| TARwt | 21.2 (20.9, 22.3) | 18.3 | 3.22 | 0.00678 |
| A34U | 21.4 (20.6, 22.0) | 18.3 | 3.19 | 0.00604 |
| U37A | 21.8 (20.7, 22.6) | 18.3 | 3.20 | 0.00634 |
| scTAR | 18.0 (17.3, 18.6) | 16.7 | 2.98 | 0.00689 |
| Dimers | ||||
| TARwt | 34.7 (31.1,39.0) | 36.6 | 4.25 | 0.00721 |
| A34U:U37A | 39.9 (38.7,41.2) | 36.6 | 4.31 | 0.00708 |
| scTAR | 32.0 (31.3,32.7) | 33.4 | 4.07 | 0.00618 |
Parameters obtained by global nonlinear least-squares analysis of sedimentation velocity experiments.
The values in parentheses represent the 95% joint confidence intervals. All data in this table were collected in AU200 buffer.
Experimental molecular weights obtained by fitting to a single ideal species model and the Svedberg equation.
Uncorrected sedimentation coefficient (Svedbergs).
RMS deviation of the fit in absorbance units.
A representative sedimentation velocity experiment performed with TARwt monomer and PKR is shown in Figure 6. The data were initially analyzed by the time derivative method38 to define the binding mechanism. The normalized g(s*) distributions of PKR binding to TARwt monomer are plotted in Figure 6a. In the absence of PKR, a single feature at s = 3.2 S is observed. As the concentration of PKR increases, the peak maximum shifts to higher S, up to about s = 5 S at the highest PKR concentration. The monotonic increase in the peak position with increasing PKR concentration is consistent with PKR binding to TARwt in rapid exchange on the timescale of the sedimentation velocity experiment. The same trend is observed for PKR binding to all other RNAs used in this study (data not shown). The magnitude of the increase in the sedimentation coefficients is consistent with a single PKR binding to TARwt monomer.
Fig. 6.
Sedimentation velocity analysis of PKR binding to TARwt monomer. (a) Plot of normalized g(s*) distributions for 1 µM TARwt monomer (red), and 1 µM TARwt monomer plus 0.5 µM PKR (green), 1 µM PKR (blue) or 4 µM PKR (black). The distributions are normalized by area under the curve. (b) Global analysis of the sedimentation difference curves. Scans within each dataset were subtracted in pairs to remove time-invariant background and fit to a 1:1 binding model using SEDANAL.63 Top panels show data points and the solid lines represent fitting results using the parameters presented in Table 2. Bottom panels show residuals for each fit. Only every 4th difference curve is shown for clarity purpose. Measurements were performed in AU200 buffer at 20 °C and 40 000 RPM using absorbance detection at 260 nm.
Figure 6b shows a global fit of the sedimentation velocity data for PKR binding to TARwt monomer to a simple one-to-one binding model (Model 1) where R corresponds to TAR RNA, P to protein and RP to the complex.
| Model 1 |
The data fit well to this model with a low RMS deviation and random residuals, yielding a dissociation constant of Kd = 667 nM (Table 2). Sedimentation velocity experiments were also performed using the other three RNA monomers (A34U, U37A, and scTAR). In all cases, the data fit well to a 1:1 binding stoichiometry. As shown in Table 2, PKR binds with similar affinity to TARwt, A34U and U37A monomers. Slightly weaker binding of U37A as compared to A34U is consistent with the former having an AA mismatch in place of a base pair in the upper portion of the stem (see below).
Table 2.
PKR–RNA interaction parameters
| RNA | Modela | Kd1 (nM) | Kd2 (nM) | s (RP)b | s (RP2)b | RMSc |
|---|---|---|---|---|---|---|
| Monomers | ||||||
| TARwt | 1 | 667 (589,752) |
- | 5.10 (5.04,5.16) |
- | 0.00699 |
| A34U | 1 | 752 (641,900) |
- | 5.01 (4.93,5.09) |
- | 0.00729 |
| U37A | 1 | 926 (746,1150) |
- | 4.92 (4.84,5.00) |
- | 0.00637 |
| scTAR | 1 | 188 (148, 236) |
- | 4.97 (4.88,5.07) |
- | 0.00595 |
| Dimers | ||||||
| TARwt | 2 | 404 (257,648) |
2760 (1170,4540) |
5.51 (5.02,5.95) |
7.70 (6.97,8.60) |
0.00794 |
| A34U:U37A | 2 | 331 (226,490) |
1470 (910,2430) |
5.29 (5.13,5.47) |
7.18 (6.86,7.66) |
0.00581 |
| A34U:U37A | 3 | 980 | 2.58×106 | 6.75 | 11.85 | 0.00785 |
Parameters obtained by global nonlinear least-squares analysis of sedimentation velocity experiments.
The values in parentheses represent the 95% joint confidence intervals. All data in this table were collected with wild type PKR in AU200 buffer.
Uncorrected sedimentation coefficient (Svedbergs).
RMS deviation of the fit in absorbance units.
Higher PKR binding stoichiometries were observed for the TAR dimers. The sedimentation velocity data for the dimer forms of TARwt and A34U:U37A fit best to a model in which two molecules of PKR bind to one molecule of RNA dimer (Model 2) with stepwise macroscopic binding constants K1 and K2 to form RP and RP2, respectively.
| Model 2 |
Global fitting yields values of Kd1 = 404 nM and Kd2 = 2.76 µM for PKR binding to TARwt dimer (Table 2). Note that the reduced affinity of binding of the second PKR does not indicate negative cooperativity. The stepwise, macroscopic binding constants measured here are approximately equal to the product of the intrinsic binding constant and a statistical factor of 4, related to the number of configurations of the free and bound forms of the RNA. The reduction in the number of accessible configurations upon binding of the first PKR causes an apparent decrease in affinity for the second PKR.14–16,39 This is further addressed in the Discussion. As we observed for the corresponding monomeric constructs, PKR binds to the A34U:U37A and TARwt dimers with very similar affinities (Table 2).
We also examined an alternative binding model that has been proposed in the context of PKR activation by TAR.7,32 In this case, binding of a molecule of PKR to TAR enhances protein dimerization, resulting in formation of an (RP)2 complex (Model 3).
| Model 3 |
This model does not fit the sedimentation velocity data for PKR binding to dimeric TAR constructs. For example, the fit of the A34U:U37A dimer data to Model 3 gives higher RMS deviations relative to Model 2 (Table 2), an exceptionally weak Kd2 as compared to activation requirements, and systematic deviations in the residuals (data not shown).
Secondary structure of RNA monomers and dimmers
The above experiments establish a correlation between RNA dimerization and the dimerization and activation of PKR. However, certain RNA variants bound and activated PKR more effectively than others. In particular, A34U:U37A activated PKR at lower concentrations of PKR than TARwt (Fig. 4), and bound PKR somewhat tighter than TARwt (Table 2). We therefore probed the secondary structures of the RNA monomers and dimers for TARwt and A34U:U37A since these could contribute to these differing activities.
Using native gel analyses, we observed that TARwt dimer migrates slightly slower than A34U:U37A dimer (Fig. 3b, Lanes 3 and 6), suggesting that they have different secondary structures and that TARwt dimer may be more bent than A34U:U37A dimer.40 We used enzymatic structure mapping to probe the secondary structures of native gel-purified TARwt monomer and dimer, as well as A34U:U37A monomer and dimer (Fig. 7). Single-strand-specific RNases A, T1, and T2, and double-strand-specific RNase V1 which also cleaves stacked bases adjacent to a helix,41–43 were used to probe secondary structures. We also note that RNase V1 does not cleave all double-stranded regions of a given RNA, especially regions with 4 or more contiguous stacked bases.42 Regions of the RNA were therefore assigned as double-stranded on the basis of RNase V1 cleavage, or absence of cleavage by any double- or single-strand specific RNases.
Fig. 7.
Enzymatic structure mapping of TARwt and A34U:U37A monomers and dimers. Monomeric and dimeric RNAs were prepared by native gel-purification and renaturations of monomer, as described. Sequencing lanes for G (T1) and all nucleotides (OH−) are provided to the left of each dataset. The secondary structures were determined using RNases A (C, U-specific) orange, T1 (G-specific) blue, T2 (single stranded-specific) green, and V1 (double strand-specific) red. Open triangles represent weaker cleavages and closed triangles stronger cleavages. RNA samples were run on 12% PAGE/1×TBE/8.3 M urea denaturing gels. For simplicity, triangles are displayed only on the lower half of dimer RNA secondary structures. (a) TARwt monomer mapped as expected, 44,45 while mapping of the dimer is consistent with a completely extended duplex with two 3-nt bulges. (b) A34U:U37A monomer data are consistent with an AA mismatch in the upper stem, which destabilizes the monomer. Mapping of the A34U:U37A dimer is consistent with a completely extended duplex with two [2×1] internal loops. The transition from two monomers to one dimer is annotated for both TARwt and A34U:U37A. Two-state hybridization server (UNAFold) by M. Zuker also predicts these two secondary structures well.47
First, the secondary structures of TARwt and A34U:U37A monomers are presented (Fig. 7). The secondary structure of TARwt monomer gave a cleavage pattern in agreement with its published secondary structure44,45, with a 6-nt hairpin loop, a 3-nt UCU bulge, and two 1-nt bulges nearer to the base of the stem (Fig. 7a).viii The secondary structure of the A34U:U37A monomer, on the other hand, revealed intense single-strand cleavages at and near G25, along with RNase V1 cleavages (Fig. 7b). These data are consistent with the secondary structure of A34U:U37A having an AA mismatch in the upper stem, which likely causes local breathing. Moreover, the monomer-specific destabilizing effect of the AA mismatch likely contributes to shifting the equilibrium toward dimer (Fig. 2a).
Next, the secondary structures of TARwt and A34U:U37A dimers were probed (Fig. 7). Structure mapping of TARwt and A34U:U37A dimers revealed 19 bp segments at each end, with each segment containing two 1-nt bulges, resembling the lower stem of the TAR monomer. Larger bulges, which are localized near the middle of the duplex between residues U22 and C38, mapped differently for TARwt and A34U:U37A. This region of the TARwt dimer contained two UCU bulges, two 1-nt A bulges, and GG mismatch (Fig. 7a). The two UCU trinucleotide bulges are the same as in TARwt monomer, and the single GG mismatch is cleaved by RNase V1, suggesting that the GG stacks with the helix and/or forms some interaction, which is consistent with GG being the most stable mismatch and largely A-form.46 It can also be noted that there are regions in the dimer RNA that are cleaved very weakly by single strand-specific ribonucleases (A, T1, and T2) that are also cleaved strongly in the monomer RNA (Fig. 7, compare right-hand lanes to left-hand lanes). This is likely due to the presence of very small amounts of monomer RNA in the dimer RNA preparation (Fig. 3b).
Analysis of structure mapping data for the A34U:U37A dimer in the U22 to C38 region suggest that it contains two [2×1] internal loops, which replace the UCU bulges, and two AG single mismatches two base pairs from the [2×1] loops (Fig. 7b). The UA and GC base pairs between the [2×1] loop and AG mismatch are cleaved with both single-strand- and double-strand-specific ribonucleases, suggesting that they form only some of the time. In addition, a strong RNase V1 cleavage appears in the dimer RNA at U30, consistent with the proposed dimer RNA secondary structure (Fig. 7b).
We compared the resultant ribonuclease cleavage patterns of TARwt and A34U:U37A dimers with 2-state hybridization predictions47 and found them to be in complete agreement. The secondary structural models of the dimer RNAs that are consistent with both the experimental data and hybridization predictions are shown in Figure 7. Overall, the main differences between the TARwt and A34U:U37A secondary structures is that TARwt has stronger asymmetry in its secondary structural defects, which is consistent with TARwt migrating slower in the native gel.40
We note that both TARwt and A34U:U37A dimer structures differ with the secondary structures reported from Kjems and co-workers for TARwt dimer.34 Our secondary structure mapping revealed a complete duplex having no intramolecular monomer interactions, while the previously reported structure34 has kissing interactions that are not fully resolved in the lower stem. This disparity could be attributed to difference in experimental conditions. For example, we used high concentrations of RNA and 90 °C renaturations to promote dimer formation, which completely melts the lower stem, while Kjems and co-workers used NC protein to drive duplex formation with a low concentration of RNA and incubating at 37 °C.i
Dimerization and activation by self-complementary TAR variants
Results from activation assays (Fig. 4) and structure mapping (Fig. 7) suggested that RNAs with more symmetry are potentially stronger activators of PKR. It was therefore possible that RNAs with no secondary structural defects would show an even stronger dependence of PKR activation on RNA dimerization. In an effort to test this possibility, we examined a final series of RNA variants in which all the bulges were removed and three mutations were incorporated into the loop to promote loop self-complementarity and facilitate formation of a defect-free duplex (Fig. 1b). This variant is referred to as self-complementary TAR (scTAR). A second variant in which the two GU wobbles near the base of the stem were converted to GC Watson-Crick base pairs was also designed, and is referred to as perfectly self-complementary TAR (pscTAR). As with the bulge-containing RNAs, these self-complementary RNAs were analyzed by native gels and activation assays (Fig. 8), and by AUC (Fig. 9).
Fig. 8.
Self-complementary TAR dimerization promotes very strong PKR activation. Native gel and activation assays were performed with scTAR (panels (a) and (b)) and pscTAR (panels (c) and (d)). (a) Conditions promoting self-complementary TAR (scTAR) monomer and dimer formation were optimized and analyzed on a native gel. Monomer (M) was prepared by renaturation of 1 µM RNA in 1×TE at 90 °C for 3 min, followed by incubation at room temperature for 10 min (heat condition 1) (Lane 1). Preparation of dimer (D) was attempted by renaturation of 20 µM RNA in 1×TE, 3.2 M NaCl using heat condition 1 (Lane 2), or incubating at 98 °C for 5 min, followed by slow cooling to ~40 °C over 1.5 h (heat condition 2) (Lane 3). Only the latter conditions gave dimer. (10% polyacrylamide native gel /0.5×TBE). (b) Activation assays using scTAR renatured at 0.8 µM in 1×TE for monomer with heat condition 1, or at 23 µM in 1×TE, 3.2 M NaCl for dimer with heat condition 2, followed by serial dilution to 0.01, 0.05, and 0.2 µM concentrations in 70 mM NaCl to maintain constant ionic conditions for these high-salt prepared RNAs. Gels are 10% SDS-PAGE, and PKR concentration was 0.8 µM. (c) Conditions promoting perfectly self-complementary TAR (pscTAR) monomer and dimer formation were optimized and analyzed on a native gel. Monomer and dimer were prepared by renaturation in 1×TE at 90 °C for 3 min, followed by incubation at room temperature for 10 min. Concentrations of pscTAR ranging from 0.2 to 20 µM were tested to determine optimal RNA conditions for monomer (M) or dimer (D) formation. (10% polyacrylamide native gel /0.5×TBE). (d) Activation assays using RNAs that were renatured in 1×TE at 0.4 µM for monomer or 20 µM for dimer, followed by serial dilution to 0.01, 0.05, and 0.2 µM concentrations. Gels are 10% SDS-PAGE, and PKR concentration was 0.8 µM. A bell-shaped dependence of activation on scTAR and pscTAR concentration is observed, wherein 0.05 µM dimer gives very strong activation of PKR, while scTAR and pscTAR monomers do not significantly activate PKR. For all panels, a no-RNA lane (−) is provided, and phosphorylation activities are normalized to 0.01 µM 79 bp RNA. In order to provide RNA-dependent activation values, the no-RNA lane was subtracted from each lane. These background-subtracted values are provided, as are RNA-dependent fold-effects for dimer over monomer (panels b and d).
Fig. 9.
Sedimentation velocity analysis of PKR binding to scTAR dimer. Plot of normalized g(s*) distributions for 0.3 µM scTAR dimer (red), and 0.3 µM scTAR dimer plus 0.15 µM PKR (magenta), 0.3 µM PKR (cyan), 0.6 µM PKR (green), 1.2 µM PKR (blue) and 2.4 µM PKR (black). The corresponding values of sw obtained by integration of the g(s*) distributions are: 4.0 S (0 eq. PKR), 5.0 S(1 eq.), 6.0 S(2 eq.), 6.8 S(4 eq.) and 7.5 S (8 eq.). Measurements were performed in AU200 buffer at 20 °C and 40 000 RPM.
We screened solution conditions for formation of scTAR monomer and dimer. Monomer scTAR was prepared by heating 1 µM RNA in 1×TE to 90 °C for 3 min, followed by incubation at room temperature for 10 min (heat condition 1) (Fig. 8a, Lane 1), which is similar to conditions used for other TAR variants. Dimer scTAR was more difficult to prepare and required exploration of unusual salt and heating conditions. We were able to prepare a high fraction of dimer scTAR by heating the 20 µM scTAR in 3.2 M NaCl/1×TE to 98 °C for 5 min, followed by slow cooling to ~40 °C over 1.5 h (heat condition 2) (Fig. 8a, Lane 3). Remarkably, related conditions involving heat condition 1 at 20 µM scTAR did not give appreciable dimer (Fig. 8a, Lane 2). Thus, it is possible to obtain highly pure preparations of monomeric and dimeric scTAR without native gel purification.
Next, we tested how scTAR monomer and dimer function in PKR activation assays. Monomer was prepared by renaturing 0.8 µM scTAR in 1×TE (heat condition 1) while dimer was prepared by renaturing 23 µM scTAR in 1×TE, 3.2 M NaCl (heat condition 2), followed by serial dilution.ix As shown in Figure 8b, scTAR dimer is a potent activator of PKR, whereas scTAR monomer does not significantly activate PKR. In particular, 0.05 µM scTAR dimer gave nearly a 100-fold increase in activation as compared to scTAR monomer. Moreover, 0.05 µM scTAR is only two-fold less potent an activator of PKR as 0.01 µM 79 bp RNA. The decrease in fold-activation at 0.2 µM scTAR dimer is due to the bell-shaped dependence of RNA activation on scTAR dimer concentration, as well as an increase in activation from RNA prepared to be monomer, perhaps because it is slightly dimerizing at these higher RNA concentrations.
PKR binding to the monomeric and dimeric forms of scTAR was characterized using the same sedimentation velocity AUC methodology that we employed with the other TAR variants. As described above, AUC data on scTAR in the absence of PKR showed the RNA to be homogeneous, with apparent molecular weights for the monomer and dimer RNAs to be within 10% of the expected values (Table 1). As observed for TARwt, and A34U and U37A monomers, a single PKR binds to scTAR monomer (data not shown). However, the affinity of PKR for scTAR monomer (Kd = 188 nM) is about 4-fold higher than for the three bulge-containing RNA monomers (Table 2). Figure 9 shows the g(s*) distributions for PKR binding to scTAR dimer. Increasing the ratio of PKR:RNA from 0 to 8 causes a large shift in the distribution from 4 to 7.5 S (see caption, Fig. 9). The magnitude of this shift is greater than that observed for the other two dimers (TARwt and A34U:U37A) over a comparable range of PKR:RNA, where sw increases from about 4.0–4.2 S to 5.8–6.0 S (data not shown), which indicates that three PKRs binds to the self-complementary TAR dimer. Although the stoichiometry of PKR binding to scTAR dimer is well defined by the sedimentation velocity experiments, the binding affinity is too high to be measured accurately at the RNA concentrations used in these measurements, which is dictated by the detection limit of the absorption optics. Based on these experimental conditions, we estimate the upper limit for the first binding constant of PKR to scTAR dimer to be on the order of 10 nM.
To examine activation of PKR by an RNA dimer requiring less severe salt and heating conditions, pscTAR was prepared in which the two GU wobbles near the base of the stem were converted to Watson-Crick GC base pairs (Fig. 1b). In addition, this variant was prepared because recent data from our laboratory indicate that a sufficient number of GU wobbles in the context of dsRNA can abrogate PKR activation.18 To optimize dimer formation, various concentrations of pscTAR were renatured with trace radiolabel and fractionated on a native gel (Fig. 8c). Renaturation of pscTAR in 1×TE at concentrations of 0.4 µM or less yielded greater than 95% monomer, while renaturation at 20 µM yielded 97% dimer. Thus, as with scTAR, it was possible to obtain highly pure preparations of monomeric and dimeric RNA without native gel purification, although in the case of pscTAR this can be done without recourse to extreme salt conditions.
Next, PKR activation by pscTAR monomer and dimer was tested. The pscTAR RNA was renatured at 0.4 µM to afford monomer and at 20 µM to afford dimer, followed by serial dilution. As shown in Figure 8d, pscTAR dimer is a remarkably potent activator of PKR. At a concentration of 0.05 µM pscTAR, there is a nearly a 100-fold increase in activation by dimer as compared to monomer, and 0.05 µM pscTAR activates PKR to the same level as 0.01 µM 79 bp RNA. We also compared the ability of scTAR and pscTAR to activate PKR (compare Fig. 8b to Fig. 8d). As with scTAR, the dimer of pscTAR is a much more effective activator of PKR than the monomer. However, activation by scTAR is approximately 2-fold less potent than for pscTAR and its bell-shaped dependence of activation on RNA concentration is shifted toward a higher concentration, consistent with a slightly deleterious effect of GU wobbles.18 As with scTAR, a decrease in fold-activation at higher concentrations of RNA dimer is likely due to the bell-shaped dependence of RNA activation on RNA dimer concentration, as well as an increase in activation from the RNA prepared to be monomer, likely because it is dimerizing somewhat at these higher RNA concentrations. Overall, data on scTAR and pscTAR (Fig. 8), like that on other TAR variants (Fig. 4), demonstrate that RNA dimer is a very strong activator, and provide no support for RNA monomer activating PKR.
Discussion
Although the TAR RNA hairpin has served as a model system for investigating the regulation of PKR by a structured RNA, the extent to which RNA dimerization contributes to PKR activation and binding has not been investigated. A minimum length of approximately 15 bp of dsRNA is required to bind PKR and inhibit activation by longer dsRNAs,13–15 whereas at least 30 bp are required to induce PKR dimerization and activation.11,16,20 Thus, the 23 bp stem of the TAR monomer is expected to be long enough to bind PKR but too short to function as an activator. Some studies have reported activation of PKR by TAR 7,22–24,27,29,31,32 whereas others have found that TAR does not activate and in fact is capable of inhibiting PKR activation by dsRNA.25,26 These discrepancies may relate to the propensity of the TAR RNA to dimerize.34 Here, we have prepared homogeneous monomer and dimer RNAs and characterized their secondary structures and their ability to bind and activate PKR using a combination of enzymatic structure mapping, autophosphorylation assays, gel mobility shift measurements, and analytical ultracentrifugation.
Our results indicate that TAR RNA dimerization leads to a greatly enhanced ability to activate PKR (Fig. 4 and Fig. 8). Dimerization effectively doubles the length of dsRNA; thus, these data are fully consistent with previous studies demonstrating that at least 30 bp are required for PKR activation.11,16,20 TAR monomer is also capable of inhibiting PKR activation by a longer dsRNA (Fig. 4). Given the potential of short RNA hairpins to form different fractions of monomers and dimers depending on RNA concentration, salt concentration, and temperature, it is important to purify the individual oligomeric forms and ensure that they do not equilibrate during measurement. Prior reports that T7-transcribed RNAs contain low levels of antisense transcripts that lead to dsRNA contaminants25,48 instigated a study in which TAR RNA was chemically synthesized to eliminate any such species, but this TAR was still found to be an activator.27 However, both of these reports can be reconciled with our model wherein TAR forms a self-complementary homodimer that activates PKR: such a homodimer does not need an antisense transcript to form and would be present even in chemically synthesized RNA.
Dimerization of TAR RNA regulates PKR binding. Native gel analysis and analytical ultracentrifugation demonstrate that the monomeric RNA hairpin forms a 1:1 complex with p20 or full length PKR (Fig. 5, Fig. 6, and Fig. 9), and that RNA dimerization leads to binding of a second PKR (Table 2). Thus, the ability of the RNA dimer to function as an activator is correlated with binding of two PKR monomers. We have previously reported a similar correlation of PKR activation and binding stoichiometry for a series of linear dsRNAs of increasing length.16 These data support an activation model where dimerization of the RNA hairpin facilitates sequential binding of two PKR monomers (Model 2), leading to protein dimerization and subsequent activation, as summarized in Figure 10. This model also explains the characteristic bell-shaped curve for PKR activation by both dsRNA 10,11 and TAR RNA hairpin,27 where low concentrations of dsRNA activate but higher concentrations are inhibitory due to dilution of the PKR monomers on to separate molecules of RNA. Our sedimentation velocity data are not consistent with an alternative model7,32 where PKR binding to a hairpin RNA enhances protein dimerization, leading to formation of an (RP)2 complex (Model 3, Table 2). Moreover, Model 3 also does not account for the PKR inhibition observed at high RNA concentrations, as seen in Figure 8 and reference [27].
Fig. 10.
Model for PKR activation by dimer RNA hairpin. Monomer RNA hairpin (~23 bp) binds PKR monomer and PKR is inhibited. In contrast, dimer RNA (~46 bp), which is double the number of monomer base pairs, binds PKR dimer and PKR is activated. Not shown are the two dsRBMs in the dsRBD, both of which contact TAR monomer.
The secondary structure defects in the RNA stem function as antideterminants to PKR binding and activation. The presence of three bulges in TARwt, A34U and U37A monomers decreases the binding affinity about 3–4 times when compared to the bulge-free scTAR monomer (Table 2). Thus, in contrast to the specific recognition of the trinucleotide bulge in TAR by the Tat protein,49 PKR prefers to bind to regular dsRNA lattices. This interpretation is consistent with previous studies demonstrating that the incorporation of G-I mismatches in the context of a poly(rI)-poly(rC) homopolymeric dsRNA blocks PKR activation,10 as does incorporation of GU wobbles.18 In contrast to our present findings, it was previously reported that the dsRBD of PKR binds to a TARwt monomer about 50-fold more strongly than to a fully complementary RNA hairpin monomer similar to the scTAR monomer used in this study.50
The effects of bulges on the interaction of PKR with RNA dimers are even more dramatic. Removal of the secondary structure defects present in TARwt and A34U:U37A dimers to produce the scTAR dimer allows a third PKR to bind and greatly enhances binding affinity (Fig. 9). The binding data correlate well with activation data. Both scTAR and pscTAR dimers are potent PKR activators, leading to autophosphorylation rates that approach those observed for a homogeneous 79 bp dsRNA (Fig. 8). The binding stoichiometries and affinities are also consistent with secondary structure probing results (Fig. 7). Both TARwt and A34U:U37A dimers consist of two 19 bp dsRNA regions interrupted by two bulges. We propose that the dsRNA regions function as isolated binding sites, each of which is long enough to bind one PKR. Native gel analysis and enzymatic structure mapping of TARwt and A34U:U37A dimers suggest TARwt is more asymmetric than A34U:U37A (Fig. 3 and Fig. 7). The TARwt dimer is also a poorer activator compared to A34U:U37A under the same assay conditions (Fig. 4). Presumably, the orientation of the two dsRNA regions in TARwt dimer is less favorable for productive PKR dimerization.
The model in Figure 10 also explains the relative affinities for PKR binding to the bulged monomers and dimers. There is a modest decrease in Kd from 600–900 nM for the bulged monomers to 300–400 nM for the wt and A34U:U37A dimers, while there is also about a five-fold increase in Kd for the second PKR binding to the dimer relative to the first (Table 2). Assuming a simple model of a pair of noninteracting, identical sites in the dimer, these relative affinities agree with the expected statistical effects on macroscopic binding constants. The affinity for the first binding event of PKR to dimer RNA is predicted to be twice the affinity for binding to monomer RNA, while the second binding event of PKR to dimer RNA is expected to be 4-fold weaker than the first binding event to the dimer RNA.14 Larger statistical factors are predicted for PKR binding to regular dsRNAs because of the larger number of microstates accessible for nonspecific binding to a finite lattice.15,16,51 For example, PKR binds 10-fold more strongly to a 30 bp dsRNA than to a 20 bp dsRNA.16 The large number of bound configurations accessible for the 52 bp scTAR and pscTAR dimers is thus responsible for a third PKR binding, as well as the high affinity and potent PKR activation (Fig. 8 and Fig. 9, Table 2). As mentioned, PKR activation is decreased in presence of RNAs containing GU wobbles,18 which is supported by activation with pscTAR being 2-fold higher than with GU-containing scTAR. Roles for wobbles appear to be complex, however, as AG mismatches fully support PKR activation.19 Bulges may serve important roles in distinguishing self and non-self RNA52 and defining cellular and viral miRNAs, including TAR.53
Any role for TAR dimers in the life cycle of HIV is unclear at present. Early models for viral replication suggest that dimerization of genomic HIV RNA involves the stem adjacent to the TAR loop,34,54,55 but other studies, including recent probing of genomic RNA purified from virions,56 indicate that the TAR stem and other dimerization signals are not required for viral replication.57 The studies herein support the ability of TAR to activate PKR as a dilute dimer and to inhibit PKR as a concentrated dimer or a monomer of any concentration. Whether TAR functions as an activator or inhibitor in vivo will depend on the structure of TAR in the HIV RNA genome, as well as the structure of adjacent RNA regions and the binding of any proteins. The studies herein help establish fundamental behaviors of TAR RNA in its different folds and demonstrate that RNA refolding and multimerization can alter the innate immune response.
Materials and Methods
Protein expression and purification
Full length PKR, containing an N-terminal (His)6, was cloned into pET-28a (Novagen, Inc.) and transformed into E. coli BL21 (DE3) Rosetta cells (Novagen, Inc.), as previously described.14,20,58 Briefly, cells were sonicated and the protein was purified by a Ni2+-agarose column (Qiagen, Inc.). Isolated protein was either treated with λ-PPase (NEB) or a GST-λ-PPase fusion before PKR activation assays. For treatment with GST-λ-PPase, an additional glutathione column was run to isolate PKR from GST-λ-PPase. Then, PKR was dialyzed into storage buffer: 10 mM Tris (pH 7.6), 50 mM KCl, 2 mM MgOAc, 10% glycerol, and 7 mM βME. For the analytical ultracentrifugation experiments, an untagged, full-length PKR construct was expressed and purified as previously described.17
We also prepared p20, a truncated version of PKR that contains residues 1–184 and has an N–terminal (His)6 tag. The (His)6 tag has been shown to not interfere with binding to RNA.14 Wild-type and mutant p20s were cloned into the vector pET14b (Novagen, Inc.), transformed into E. coli BL21(DE3)pLysS cells, and overexpressed and purified as described.14
The fusion proteins p20-VP16 and VP16-p20 were cloned into pET-28a between Nde I and Bam HI, which resulted in an alanine-serine spacer between the two domains. Fusion proteins were generated by PCR amplification of the p20 and VP16 domains.36,59 (VP16 plasmid was a gift from B.F. Pugh, Penn State University)
RNA preparation and purification
Most RNAs were prepared by transcription from a hemiduplex by T7 RNA polymerase.60 A dangling G was added to the 5’-end of shTARs to aid T7 transcription. The scTAR variant was prepared by solid-phase synthesis (Dharmacon, Inc.). All RNAs were purified by denaturing 10% PAGE and the band corresponding to full-length transcript was cut out and soaked overnight at 4 °C in 1×TEN250 [10 mM Tris (pH 7.5), 1 mM EDTA, 250 mM NaCl]. Subsequently, RNAs were ethanol precipitated and resuspended in water or 1×TE [10 mM Tris (pH 7.5), 1 mM EDTA]. Concentrations were determined spectrophotometrically. 5’-end labeled RNAs were prepared by first treating with calf intestinal phosphatase (CIP), followed by polynucleotide kinase (PNK) treatment in the presence of [γ-32P]ATP.
Native gel analysis
Native gels were used to analyze the monomeric and dimeric nature of the RNA and contained 10% of 29:1 crosslinking polyacrylamide. The buffer both in the gel and for electrophoresis was 0.5×TBE [50 mM Tris base, 40 mM boric acid, 0.5 mM EDTA]. Native gels were pre-run at 300 V, 16 °C for 45 min prior to loading samples. In general, samples were fractionated for ~2 h.
Prior to loading onto a native gel, trace amounts (~1 nM) of 5’-end labeled RNA was added to 0.2 to 50 µM unlabeled RNA and renatured in 1×TE, 1×TEK100 [10 mM Tris (pH 7.5), 1 mM EDTA, 100 mM KCl], or 1×TEK500 by incubating at 90 °C for 3 min. This sample was then treated in one of three ways: snap cooling on ice, incubation at room temperature for 10 min, or incubation at 55 °C for 10 min. In some instances, after incubating at 90 °C and room temperature, MgCl2 was added to a final concentration of 4 mM and the solution was heated to 55 °C for an additional 10 min, followed by another incubation at room temperature for 10 min. In the case of scTAR dimer renaturation, 20 µM RNA in 3.2 M NaCl was heated to 98 °C for 5 min and then slow cooled to ~40 °C over 1.5 h. Native gels were run in both analytical and preparative formats. Analytical gels were exposed to a storage screen for 16 h and analyzed on a PhosphorImager (Molecular Dynamics). Preparative gels were examined by UV shadowing, and bands of interest were isolated as described in the next subsection.
Purification of RNA from native gels
Native gels were used to purify monomeric and dimeric RNAs. In general, 50 or 100 µM RNA in 1×TEK100 was renatured in the presence or absence of radiolabeled RNA by incubating at 90 °C for 3 min, followed by room temperature for 10 min. Next, 5–8% glycerol was added to the RNA and it was fractionated on a native gel for 2 h. Monomer, dimer, and multimer RNA bands were visualized by UV shadowing and excised with a razor blade. Isolated variants were crushed and soaked overnight in 1×TEN250 at 4 °C. RNAs were then concentrated by ethanol precipitation and resuspended in 1×TE or water and stored at −20 °C. All radiolabeled samples were re-run on an analytical native gel to verify the integrity of monomer, dimer, and multimer.
Mobility–shift assays
Native gel mobility-shift analyses were performed using limiting concentrations of radiolabeled RNA, as described.14,61 Binding reactions were allowed to equilibrate at room temperature for at least 10 min, and then loaded on a 10% 79:1 crosslinking polyacrylamide native gel in 0.5×TBE. Longer incubation times did not change binding, indicating that equilibrium had been achieved. Electrophoresis was at 19 V/cm for 3–5 h, and temperature was maintained near 4 °C with a circulating system. Relative amounts of free and bound RNA were determined using a PhosphorImager (Molecular Dynamics). Each experiment was repeated 2 or more times and was reproducible.
PKR activation assays
Most RNAs were tested for their ability to activate PKR. Prior to activation assays, PKR was dephosphorylated by λ-PPase for 1 h at 30 °C, and then inhibited with 2 mM sodium orthovanadate. In some cases, PKR was pre-treated with GST-λ-PPase. The λ-PPase-treated PKR (0.1 to 5 µM) was incubated with various concentrations of RNA, 20 mM HEPES (pH 7.5), 4 mM MgCl2, 50 mM KCl, 1.5 mM DTT, 100 µM ATP (Ambion), and 15 µCi [γ-32P]-ATP. RNA monomers were renatured at 90 °C for 3 min to help remove any dimer, and final RNA concentrations did not exceed 1.2 µM due to the increased likelihood of introducing small amounts of dimer into renatured monomer samples. Reaction mixtures were incubated at 30 °C for 10 min, quenched with 1×SDS loading buffer, and loaded on 10% SDS-PAGE (Pierce). This timepoint was chosen because it is in the approach of kinetics to the plateau region.58 Gels were exposed to a storage PhosphorImager screen and intensities of PKR bands quantified using a PhosphorImager (Molecular Dynamics). In activation assay gels, a no-RNA lane is provided and phosphorylation activities are normalized to 0.01 µM 79 bp RNA.
Enzymatic structure mapping of RNA
Prior to all structure mapping, 5’-end labeled RNA was renatured with 1000-fold excess unlabeled RNA (50 µM), and isolated and purified from a native gel. This was done so that the same amount of ribonuclease could be used for both monomeric and dimeric RNAs. RNA monomers were renatured at 3 µM in water by incubating at 90 °C for 3 min to help remove any dimer, while dimer samples were not renatured. Final concentrations of monomer and dimer for native and denaturing reactions were 0.5 and 0.25 µM, respectively, to give the same total concentration of nucleotides. Ribonuclease concentrations were 1 ng/mL RNase A, 0.001 U/µL RNase Tl, 5×10−5 U/µL RNase T2, and 0.001 U/µL RNase V1. Higher concentrations of ribonucleases were tested, but resulted in non-specific cleavage. Ribonucleases were diluted into 10 mM Tris (pH 8.0), 10% glycerol, 1 mM DTT, as needed. Native RNA cleavage reactions were performed in 10 mM Hepes (pH 6.5), 25 mM KCl, and 5 mM MgCl2 at 37 °C for 15–30 min. Denaturing conditions were 0.1 U/µL T1, 4.7 M urea, 14 mM Sodium Citrate (pH 3.5), 0.7 mM EDTA at 50 °C for 30 min, and hydrolysis conditions were 50 mM Na2CO3/NaHCO3 (pH 9.0), 1 mM EDTA at 90 °C for 4 min. Fractionated samples were loaded onto 12% PAGE/1×TBE/8.3 M urea denaturing gel.
Analytical Ultracentrifugation
Sedimentation velocity analysis of protein-RNA binding was carried out at 20 °C and 40 000 RPM in a buffer containing 20 mM HEPES pH 7.5, 200 mM NaCl, 0.1 mM EDTA and 0.1 mM TCEP (AU200) as previously described.16 It was not possible to perform AUC analysis of PKR-TAR interactions in the lower-salt buffer used for the activity assays due to the formation of aggregates under these conditions. Initial analysis was performed using the time derivative method38 with DCDT+62 to obtain g(s*) distributions. Distributions were normalized by area (absorbance) for presentation. Multiple datasets were globally fit to association models using SEDANAL.63 Joint confidence intervals were obtained using the F-statistic to define a statistically significant increase in the variance upon adjusting each parameter from its best-fit value.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grants R01- 58709 (P.C.B.) and AI-53615 (J.L.C).
Abbreviations
- A34U:U37A
TAR double variant containing both the A34U and U37A changes
- [A34U+U37A]
an equal concentration mixture of the A34U and U37A single variants
- AUC
analytical ultracentrifugation
- bp
base pair
- dsRBD
dsRNA binding domain
- dsRNA
double-stranded RNA
- eIF2α
eukaryotic initiation factor 2α
- HIV-1
human immunodeficiency virus, type 1
- NC
nucleocapsid protein
- p20
20 kDa double-stranded RNA binding domain of PKR
- PAGE
polyacrylamide gel electrophoresis
- PKR
protein kinase R
- pscTAR
perfectly self-complementary TAR
- scTAR
self-complementary TAR with two GU wobbles in the base of the stem
- shTAR
short hairpin TAR
- TARwt
HIV-1 transactivation-response region
- VP16
activation domain from herpes simplex virus, type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Our studies were conducted in the absence of NC protein because when dimerization of TAR was conducted in the presence of NC, as per Andersen and co-workers (2004), TAR dimerization was less complete. Also, activation assays conducted on NC-dimerized RNA did not give detectable activation. Phenol-chloroform extractions of this RNA preparation to remove NC from RNA did not improve activation either, perhaps because NC forms very tight complexes with the RNA and is present in very large quantities: the amount of NC protein required for inducing RNA dimerization is ~17-fold excess over RNA (Muriaux et al., 1996; Andersen et al., 2004).
For some RNAs, especially those with extensive tertiary structure, divalent ions are important for formation of certain native gel bands, but were found to be unnecessary for dimerization of TAR-related RNAs, perhaps because the dimers contain primarily secondary structure.
There is a small amount of dimer observed for 3 µM A34U:U37A in Fig. 2a, not seen in Fig. 2b. This is likely due to the presence of small amounts of salt in the loading buffer in Fig. 2a that was not used in subsequent experiments.
Later studies revealed that identical PKR activation and AUC results for monomer species could be obtained from renaturation of dilute RNA solutions that were not native gel-purified. Therefore, the native gel purification of monomer was omitted in some experiments.
Mobility-shift experiments were conducted with p20 rather than full-length PKR because the latter gave only poor gel shifts (data not shown).
An N-terminal VP16 fusion to p20 was also prepared but did not give gel shifts to RNA and so was not pursued (data not shown).
The electrophoretic mobility of the TAR-p20 complex and the free TAR-A30 were similar (Fig. 5b, Lanes 2 and 5). However, this should not interfere with the mixture of p20 and both TAR RNAs because sufficient p20 was added to shift all of the free TAR-A30 (Fig. 5b, Lane 4).
We note that a few cleavages by RNaseA occurred in the stem of TARwt monomer and dimer (Fig. 8a). These occurred either at AU-rich stretches near a bulge, or at a GU wobble, consistent with breathing of these weaker regions. In addition, the intensity of these cleavages was weak relative to those that occurred in the single-stranded hairpin loop.
In contrast to aforementioned PKR activation assays that contained 50 mM KCl, this experiment was conducted in 70 mM NaCl because of carry over from the 3.2 M NaCl renaturation conditions. Experiments on 79 bp control were also done in 70 mM NaCl and gave similar results to 50 mM KCl showing that effect of this salt change is negligible (Fig. 9b)
References
- 1.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 2.Langland JO, Cameron JM, Heck MC, Jancovich JK, Jacobs BL. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119:100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Rothenburg S, Seo EJ, Gibbs JS, Dever TE, Dittmar K. Rapid evolution of protein kinase PKR alters sensitivity to viral inhibitors. Nat. Struct. Mol. Biol. 2009;16:63–70. doi: 10.1038/nsmb.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanduri S, Carpick BW, Yang Y, Williams BR, Qin J. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 1998;17:5458–5465. doi: 10.1093/emboj/17.18.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 7.McKenna SA, Lindhout DA, Kim I, Liu CW, Gelev VM, Wagner G, Puglisi JD. Molecular framework for the activation of RNA-dependent protein kinase. J. Biol. Chem. 2007;282:11474–11486. doi: 10.1074/jbc.M700301200. [DOI] [PubMed] [Google Scholar]
- 8.Vanoudenhove J, Anderson E, Kreuger S, Cole JL. Analysis of PKR structure by small angle scattering. J. Mol. Biol. 2009;387:910–920. doi: 10.1016/j.jmb.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole JL. Activation of PKR: an open and shut case? Trends Biochem. Sci. 2007;32:57–62. doi: 10.1016/j.tibs.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minks MA, West DK, Benvin S, Baglioni C. Structural requirements of double-stranded RNA for the activation of 2',5'-oligo(A) polymerase and protein kinase of interferon-treated HeLa cells. J. Biol. Chem. 1979;254:10180–10183. [PubMed] [Google Scholar]
- 11.Manche L, Green SR, Schmedt C, Mathews MB. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol. 1992;12:5238–5248. doi: 10.1128/mcb.12.11.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostura M, Mathews MB. Purification and Activation of the Double-Stranded RNA-Dependent eIF-2 Kinase DAI. Mol. Cell. Biol. 1989;9:1576–1586. doi: 10.1128/mcb.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmedt C, Green SR, Manche L, Taylor DR, Ma Y, Mathews MB. Functional Characterization of the RNA-binding Domain and Motif of the Double-stranded RNA-dependent Protain Kinase DAI (PKR) J. Mol. Biol. 1995;249:29–44. doi: 10.1006/jmbi.1995.0278. [DOI] [PubMed] [Google Scholar]
- 14.Bevilacqua PC, Cech TR. Minor-Groove Recognition of Double-Stranded RNA by the Double-Stranded RNA-Binding Domain from the RNA-Activated Protein Kinase PKR. Biochemistry. 1996;35:9983–9994. doi: 10.1021/bi9607259. [DOI] [PubMed] [Google Scholar]
- 15.Ucci JW, Kobayashi Y, Choi G, Alexandrescu AT, Cole JL. Mechanism of interaction of the double-stranded RNA (dsRNA) binding domain of protein kinase R with short dsRNA sequences. Biochemistry. 2007;46:55–65. doi: 10.1021/bi061531o. [DOI] [PubMed] [Google Scholar]
- 16.Lemaire PA, Anderson E, Lary J, Cole JL. Mechanism of PKR Activation by dsRNA. J. Mol. Biol. 2008;381:351–360. doi: 10.1016/j.jmb.2008.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemaire PA, Lary J, Cole JL. Mechanism of PKR activation: dimerization and kinase activation in the absence of double-stranded RNA. J. Mol. Biol. 2005;345:81–90. doi: 10.1016/j.jmb.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Nallagatla SR, Bevilacqua PC. Nucleoside modifications modulate activation of the protein kinase PKR in an RNA structure-specific manner. RNA. 2008;14:1201–1213. doi: 10.1261/rna.1007408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevilacqua PC, George CX, Samuel CE, Cech TR. Binding of the protein kinase PKR to RNAs with secondary structure defects: role of the tandem A-G mismatch and noncontiguous helixes. Biochemistry. 1998;37:6303–6316. doi: 10.1021/bi980113j. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Bevilacqua PC. Activation of the protein kinase PKR by short double-stranded RNAs with single-stranded tails. RNA. 2004;10:1934–1945. doi: 10.1261/rna.7150804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell. 2002;108:221–232. doi: 10.1016/s0092-8674(02)00616-5. [DOI] [PubMed] [Google Scholar]
- 22.Edery I, Petryshyn R, Sonenberg N. Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell. 1989;56:303–312. doi: 10.1016/0092-8674(89)90904-5. [DOI] [PubMed] [Google Scholar]
- 23.SenGupta DN, Silverman RH. Activation of interferon-regulated, dsRNA-dependent enzymes by human immunodeficiency virus-1 leader RNA. Nucleic Acids Res. 1989;17:969–978. doi: 10.1093/nar/17.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SenGupta DN, Berkhout B, Gatignol A, Zhou AM, Silverman RH. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U S A. 1990;87:7492–7496. doi: 10.1073/pnas.87.19.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunnery S, Rice AP, Robertson HD, Mathews MB. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. U S A. 1990;87:8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunnery S, Green SR, Mathews MB. Tat-responsive region RNA of human immunodeficiency virus type 1 stimulates protein synthesis in vivo and in vitro: Relationship between structure and function. Proc. Natl. Acad. Sci. U S A. 1992;89:11557–11561. doi: 10.1073/pnas.89.23.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maitra RK, McMillan NAJ, Desai S, McSwiggen J, Hovanessian AG, Sen G, Williams BRG, Silverman RH. HIV-1 TAR RNA Has an Intrinsic Ability to Activate Interferon-Inducible Enzymes. Virology. 1994;204:823–827. doi: 10.1006/viro.1994.1601. [DOI] [PubMed] [Google Scholar]
- 28.Nekhai S, Kuman A, Bottaro DP, Petryshyn R. Peptides Derived from the Interferon-Induced PKR Prevent Activation by HIV-1 TAR RNA. Virology. 1996;222:193–200. doi: 10.1006/viro.1996.0410. [DOI] [PubMed] [Google Scholar]
- 29.Carpick BW, Graziano V, Schneider D, Maitra RK, Lee X, Williams BRG. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J. Biol. Chem. 1997;272:9510–9516. doi: 10.1074/jbc.272.14.9510. [DOI] [PubMed] [Google Scholar]
- 30.Spanggord RJ, Vuyisich M, Beal PA. Identification of Binding Sites for Both dsRBMs of PKR on Kinase-Activating and Kinase-Inhibiting RNA Ligands. Biochemistry. 2002;41:4511–4520. doi: 10.1021/bi0120594. [DOI] [PubMed] [Google Scholar]
- 31.McKenna SA, Kim I, Liu CW, Puglisi JD. Uncoupling of RNA binding and PKR kinase activation by viral inhibitor RNAs. J. Mol. Biol. 2006;358:1270–1285. doi: 10.1016/j.jmb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 32.McKenna SA, Lindhout DA, Shimoike T, Aitken CE, Puglisi JD. Viral dsRNA inhibitors prevent self-association and autophosphorylation of PKR. J. Mol. Biol. 2007;372:103–113. doi: 10.1016/j.jmb.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puglisi JD, Tan R, Calnan BJ, Frankel AD, Williamson JR. Conformation of the TAR RNA-Arginine Complex by NMR Spectroscopy. Science. 1992;257:76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- 34.Andersen ES, Contera SA, Knudsen B, Damgaard CK, Besenbacher F, Kjems J. Role of the trans-activation response element in dimerization of HIV-1 RNA. J. Biol. Chem. 2004;279:22243–22249. doi: 10.1074/jbc.M314326200. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Sheng S, Shao Z, Guo P. A dimer as a building block in assembling RNA. A hexamer that gears bacterial virus phi29 DNA-translocating machinery. J. Biol. Chem. 2000;275:17510–17516. doi: 10.1074/jbc.M909662199. [DOI] [PubMed] [Google Scholar]
- 36.Fang G, Cech TR. Oxytricha telomere-binding protein: DNA-dependent dimerization of the alpha and beta subunits. Proc. Natl. Acad. Sci. U S A. 1993;90:6056–6060. doi: 10.1073/pnas.90.13.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stafford WF., 3rd Boundary analysis in sedimentation transport experiments: a procedure for obtaining sedimentation coefficient distributions using the time derivative of the concentration profile. Anal. Biochem. 1992;203:295–301. doi: 10.1016/0003-2697(92)90316-y. [DOI] [PubMed] [Google Scholar]
- 39.McGhee JD, von Hippel PH. Theoretical Aspects of DNA-Protein Interactions: Co-operative and Non-co-operative Binding of Large Ligands to a One-dimensional Homogeneous Lattice. J. Mol. Biol. 1974;86:469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- 40.Riordan FA, Bhattacharyya A, McAteer S, Lilley DM. Kinking of RNA helices by bulged bases, and the structure of the human immunodeficiency virus transactivator response element. J. Mol. Biol. 1992;226:305–310. doi: 10.1016/0022-2836(92)90947-i. [DOI] [PubMed] [Google Scholar]
- 41.Lockard RE, Kumar A. Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. Nucleic Acids Res. 1981;9:5125–5140. doi: 10.1093/nar/9.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowman HB, Draper DE. On the recognition of helical RNA by cobra venom V1 nuclease. J. Biol. Chem. 1986;261:5396–5403. [PubMed] [Google Scholar]
- 43.Ehresmann C, Baudin F, Mougel M, Romby P, Ebel J-P, Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987;15:9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muesing MA, Smith DH, Capon DJ. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987;48:691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- 45.Berkhout B, Silverman RH, Jeang KT. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 46.Kierzek R, Burkard ME, Turner DH. Thermodynamics of single mismatches in RNA duplexes. Biochemistry. 1999;38:14214–14223. doi: 10.1021/bi991186l. [DOI] [PubMed] [Google Scholar]
- 47.Markham NR, Zuker M. UNAFold: software for nucleic acid folding and hybridization. Methods Mol. Biol. 2008;453:3–31. doi: 10.1007/978-1-60327-429-6_1. [DOI] [PubMed] [Google Scholar]
- 48.Mellits KH, Pe'ery T, Manche L, Robertson HD, Mathews MB. Removal of double-stranded contaminants from RNA transcripts: synthesis of adenovirus VA RNAI from a T7 vector. Nucleic Acids Res. 1990;18:5401–5406. doi: 10.1093/nar/18.18.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puglisi JD, Chen L, Blanchard S, Frankel AD. Solution structure of a bovine immunodeficiency virus Tat-TAR peptide- RNA complex. Science. 1995;270:1200–1203. doi: 10.1126/science.270.5239.1200. [DOI] [PubMed] [Google Scholar]
- 50.Kim I, Liu CW, Puglisi JD. Specific recognition of HIV TAR RNA by the dsRNA binding domains (dsRBD1-dsRBD2) of PKR. J. Mol. Biol. 2006;358:430–442. doi: 10.1016/j.jmb.2006.01.099. [DOI] [PubMed] [Google Scholar]
- 51.Ucci JW, Cole JL. Global analysis of non-specific protein-nucleic interactions by sedimentation equilibrium. Biophys. Chem. 2004;108:127–140. doi: 10.1016/j.bpc.2003.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nallagatla SR, Toroney R, Bevilacqua PC. A brilliant disguise for self RNA: 5'-end and internal modifications of primary transcripts suppress elements of innate immunity. RNA Biol. 2008;5:25–29. doi: 10.4161/rna.5.3.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouellet DL, Plante I, Landry P, Barat C, Janelle ME, Flamand L, Tremblay MJ, Provost P. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic Acids Res. 2008;36:2353–2365. doi: 10.1093/nar/gkn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersen ES, Jeeninga RE, Damgaard CK, Berkhout B, Kjems J. Dimerization and template switching in the 5' untranslated region between various subtypes of human immunodeficiency virus type 1. J. Virol. 2003;77:3020–3030. doi: 10.1128/JVI.77.5.3020-3030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paillart JC, Shehu-Xhilaga M, Marquet R, Mak J. Dimerization of retroviral RNA genomes: an inseparable pair. Nat. Rev. Microbiol. 2004;2:461–472. doi: 10.1038/nrmicro903. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson KA, Gorelick RJ, Vasa SM, Guex N, Rein A, Mathews DH, Giddings MC, Weeks KM. High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states. PLoS Biol. 2008;6:e96. doi: 10.1371/journal.pbio.0060096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das AT, Harwig A, Vrolijk MM, Berkhout B. The TAR hairpin of human immunodeficiency virus type 1 can be deleted when not required for Tat-mediated activation of transcription. J. Virol. 2007;81:7742–7748. doi: 10.1128/JVI.00392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE, Bevilacqua PC. 5’-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science. 2007;318:1455–1458. doi: 10.1126/science.1147347. [DOI] [PubMed] [Google Scholar]
- 59.Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 60.Milligan JF, Uhlenbeck OC. Synthesis of Small RNAs Using T7 RNA Polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- 61.Zheng X, Bevilacqua PC. Straightening of bulged RNA by the double-stranded RNA-binding domain from the protein kinase PKR. Proc. Natl. Acad. Sci. U S A. 2000;97:14162–14167. doi: 10.1073/pnas.011355798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philo JS. Improved methods for fitting sedimentation coefficient distributions derived by time-derivative techniques. Anal. Biochem. 2006;354:238–246. doi: 10.1016/j.ab.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 63.Stafford WF, Sherwood PJ. Analysis of heterologous interacting systems by sedimentation velocity: curve fitting algorithms for estimation of sedimentation coefficients, equilibrium and kinetic constants. Biophys. Chem. 2004;108:231–243. doi: 10.1016/j.bpc.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Muriaux D, Fossé P, Paoletti J. A Kissing Complex Together with a Stable Dimer Is Involved in the HIV-1Lai RNA Dimerization Process in Vitro. Biochemistry. 1996;35:5075–5082. doi: 10.1021/bi952822s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.