Abstract
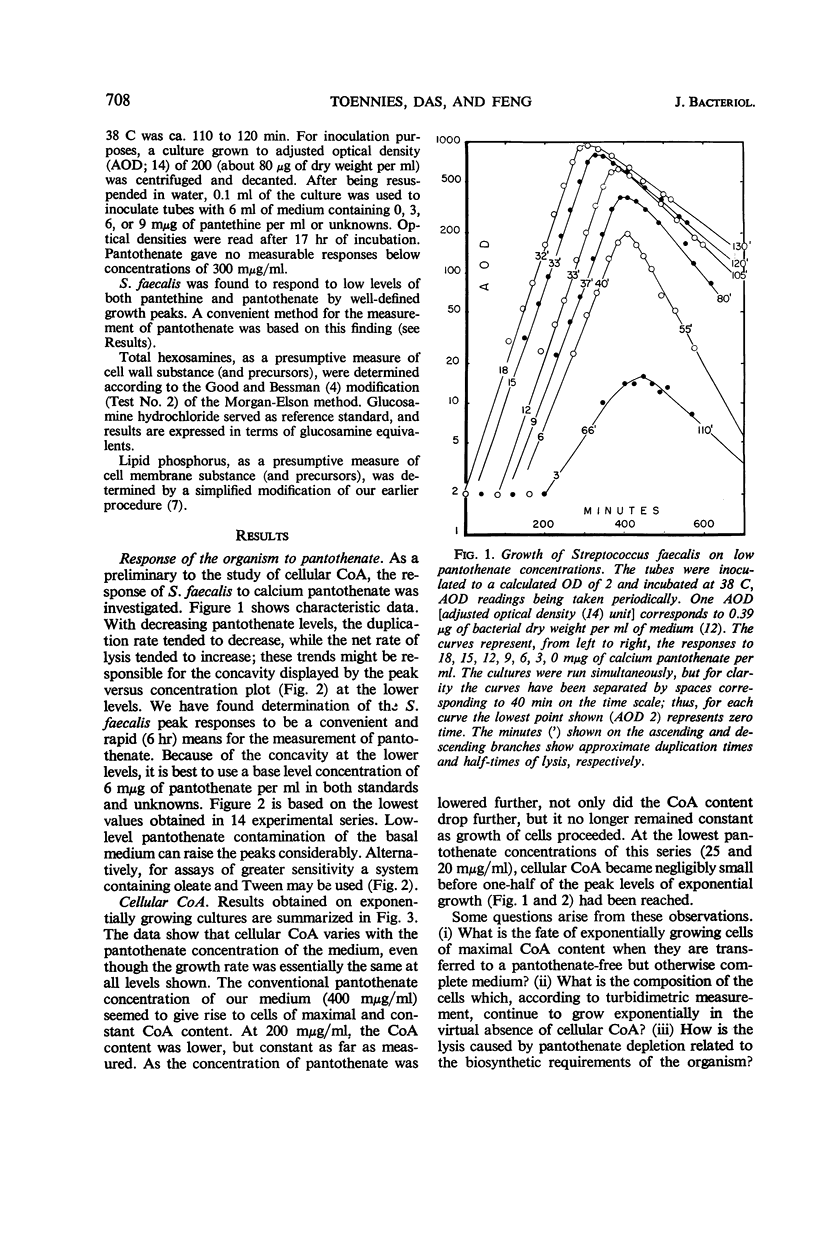
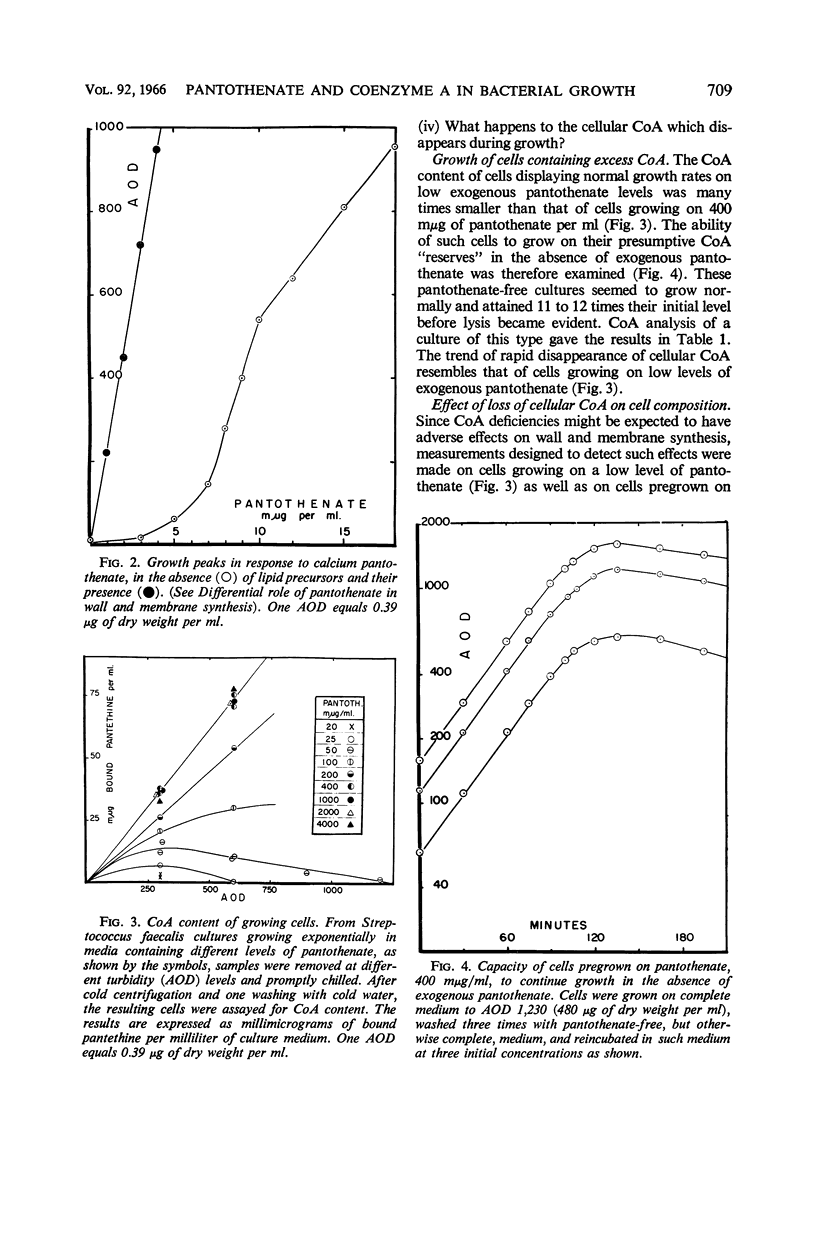
Toennies, G. (Temple University School of Medicine, Philadelphia, Pa.), D. N. Das, and F. Feng. Pantothenate and coenzyme A in bacterial growth. J. Bacteriol. 92:707–713. 1966.—The effect of environmental pantothenate levels on the growth of Streptococcus faecalis 9790 was studied in terms of growth rate, depletion phenomena, cellular coenzyme A (CoA) content, and differential rates of wall and membrane synthesis. Low concentrations of pantothenate yielded normal exponential growth curves up to peak turbidities which are a function of pantothenate concentration. Attainment of these peaks was followed by lysis. Under such conditions, bacterial CoA increased initially in proportion with cell substance, but attained a peak level much earlier than cell substance, and then gradually decreased down to vanishing amounts. With higher pantothenate concentrations, cellular CoA levels increased to a maximum, and, under these conditions, the CoA content remained constant during exponential growth. Four-fifths of the pantothenate requirement of growing cells was eliminated by environmental oleate and palmitate. When CoA disappeared during growth on low pantothenate levels, cell wall synthesis seemed to continue at nearly normal rates, but membrane synthesis was severely curtailed. The data suggest that in fermentative organisms pantothenate action might be confined to wall and membrane synthesis, that these two processes differ in their quantitative dependence on pantothenate, and that pantothenate might occur in the form of acyl carrier protein as well as CoA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN G. M. Assay and distribution of bound forms of pantothenic acid. J Biol Chem. 1959 Feb;234(2):379–382. [PubMed] [Google Scholar]
- BROWN G. M. The metabolism of pantothenic acid. J Biol Chem. 1959 Feb;234(2):370–378. [PubMed] [Google Scholar]
- CROOM J. A., MCNEILL J. J., TOVE S. B. BIOTIN DEFICIENCY AND THE FATTY ACIDS OF CERTAIN BIOTIN-REQUIRING BACTERIA. J Bacteriol. 1964 Aug;88:389–394. doi: 10.1128/jb.88.2.389-394.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD T. A., BESSMAN S. P. DETERMINATION OF GLUCOSAMINE AND GALACTOSAMINE USING BORATE BUFFERS FOR MODIFICATION OF THE ELSON-MORGAN AND MORGAN-ELSON REACTIONS. Anal Biochem. 1964 Nov;9:253–262. doi: 10.1016/0003-2697(64)90183-6. [DOI] [PubMed] [Google Scholar]
- HOFMANN K., HENIS D. B., PANOS C. Fatty acid interconversions in lactobacilli. J Biol Chem. 1957 Sep;228(1):349–355. [PubMed] [Google Scholar]
- KOLB J. J., WEIDNER M. A., TOENNIES G. Microdetermination of lipid phosphorus as a measure of bacterial membrane substance. Anal Biochem. 1963 Jan;5:78–82. doi: 10.1016/0003-2697(63)90061-7. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Alberts A. W., Vagelos P. R. Acyl carrier protein. VII. The primary structure of the substrate-binding site. J Biol Chem. 1965 Dec;240(12):4723–4726. [PubMed] [Google Scholar]
- Pugh E. L., Wakil S. J. Studies on the mechanism of fatty acid synthesis. XIV. The prosthetic group of acyl carrier protein and the mode of its attachment to the protein. J Biol Chem. 1965 Dec;240(12):4727–4733. [PubMed] [Google Scholar]
- SALTON M. R. CHEMISTRY AND FUNCTION OF AMINO SUGARS AND DERIVATIVES. Annu Rev Biochem. 1965;34:143–174. doi: 10.1146/annurev.bi.34.070165.001043. [DOI] [PubMed] [Google Scholar]
- TOENNIES G. ROLE OF AMINO ACIDS IN POSTEXPONENTIAL GROWTH. J Bacteriol. 1965 Aug;90:438–442. doi: 10.1128/jb.90.2.438-442.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D., KOLB J. J. Differential effects of amino acid deficiencies on bacterial cytochemistry. Biochemistry. 1963 Mar-Apr;2:294–296. doi: 10.1021/bi00902a017. [DOI] [PubMed] [Google Scholar]
- Toennies G., Feng F., Kolb J. J., Luttner P. M. Bacterial nucleate and phosphorus partition. Anal Biochem. 1965 Jun;11(3):473–496. doi: 10.1016/0003-2697(65)90067-9. [DOI] [PubMed] [Google Scholar]


