Abstract
How melanoma acquire a metastatic phenotype is a key issue. One possible mechanism is that metastasis is driven by microenvironment-induced switching between non-invasive and invasive states. However, whether switching is a reversible or hierarchical process is not known and is difficult to assess by comparison of primary and metastatic tumours. We address this issue in a model of melanoma metastasis using a novel intravital imaging method for melanosomes combined with a reporter construct in which the Brn-2 promoter drives GFP expression. A sub-population of cells containing little or no pigment and high levels of Brn2::GFP expression are motile in the primary tumour and enter the vasculature. Significantly, the less differentiated state of motile and intravasated cells is not maintained at secondary sites, implying switching between states as melanoma cells metastasize. We show that melanoma cells can switch in both directions between high and low pigment states. However, switching from Brn2::GFP high to low was greatly favoured over the reverse direction. Microarray analysis of high and low pigment populations revealed that TGFβ2 was up-regulated in the poorly pigmented cells. Furthermore, TGFβ signalling induced hypo-pigmentation and increased cell motility. Thus, a subset of less differentiated cells exits the primary tumour but subsequently give rise to metastases that include a range of more differentiated and pigment producing cells. These data demonstrate reversible phenotype switching during melanoma metastasis
Introduction
Melanoma is a type of skin cancer arising from the aberrant proliferation of melanocytes. Once melanoma begins to spread the prognosis deteriorates(1). Melanocytes originate from a sub-population of neural crest cells called melanoblasts(2). During development, melanoblasts migrate through the embryo. Melanoblasts stop migrating when they reach the skin and reside in the lower levels of the epidermis or in the dermis, depending on species, and differentiate to melanocytes(3). The differentiation of melanoblasts to mature melanocytes is associated with down-regulation of the transcription factor Brn-2/POU3F, which represses the transcription factor MITF(4). MITF is a key regulator of melanocyte function and high levels of MITF activity promote melanogenesis(5-7). Melanogenesis occurs in organelles called melanosomes. Specifically, pigment is produced in stage 3 and 4 melanosomes prior to being exported. However, the level of pigment produced is not the same for all melanocytes and is controlled by a range of genetic and environmental factors(3). While MITF promotes pigment production it suppress invasion(8, 9). Conversely, Brn-2, which represses MITF, can promote invasion in vitro(4). Brn-2 expressing sub-populations of cells can be found within melanoma(4). However, the behaviour and stability of Brn-2 expressing cells in vivo is not known.
Two models to account for melanoma metastasis can be envisaged. Firstly, acquisition of pro-metastatic genetic changes, such as NEDD9 amplification(10), could lead to a more invasive phenotype. This model predicts that metastases would have a genetic signature different from the majority of cells in the primary tumour(1, 11). Alternatively, metastasis may be driven by epigenetic events that could be reversible. This is supported by the observations of Hoek and colleagues(12). These changes may be initiated by signals originating in the tumour microenvironment that need not necessarily be maintained at secondary sites. Definitive proof that reversible phenotype switching occurs during metastasis is difficult to obtain by comparison of primary and metastatic tumours. Therefore, we used live imaging of a melanoma model to directly analyze motile cells escaping from the primary tumour and those that have entered the blood. The results reveal that disseminating cells are poorly pigmented and express high levels of the Brn2-GFP reporter. However, this phenotype can be reversed at metastatic sites.
Materials and Methods
Cell culture
B16F2 and B-RafV600E melanoma cells (called 4599(13)) were cultured in DMEM with 10% foetal calf serum.
Pigmentation experiments
B16F2 or 4599 cells were stimulated or inhibited with 2μg/ml αMSH (Calbiochem 05-23-0751), 10μM H89 (Sigma), 1ng/ml TGFβ1 (Peprotech 100-21C) or 1ng/ml TGFβ2 (R&D Biosystems 302-B2) for 48 hours.
Imaging techniques
In vitro
A ZeissLSM510META confocal microscope with a femto-second pulsed Ti-Sapphire laser was used. Pigment was imaged using transmitted-light without phase-contrast or NIRVE signal was captured by exciting the cells with NIR wavelengths between 700-850nm and collecting visible light emission. To determine the emission spectrum between 385-680nm a ZeissMETA detector was used. Analysis of cell migration speed was carried out by tracking phase-contrast movies of B16F2 cells plated on collagen/matrigel gels and treated with α-MSH, TGFβ1 or TGFβ2 for 24h hours prior to imaging. Tracks were calculated using Metamorph (Molecular Devices) and Mathematica (Wolfram) software.
In vivo
Mouse procedures were carried out in accordance with PPL70/6164. Depending on availability either C57/BL6 or Nude mice were injected sub-cutaneously with 106 B16-GFP, B16-GFP-CAAX or B16-mRFP-CAAX Brn2::GFP cells. Intravital imaging was carried out on anaethetised mice with tumours using a ZeissLSM510 confocal microscope. An incision was made in the skin to expose the tumour. The animal was placed on a heated stage. Tumour areas were imaged for 20 minutes at 30-60 sec intervals. Collagen fibres were visualised by second harmonic signals (410-450nm), pigment was imaged at 565-615nm and 635-680nm following excitation at 850nm (~20mW power at objective). GFP-CAAX was imaged at 500-550nm following excitation with either a 488nm laser or 850nm; excitation using the later wavelength also resulted in signal being detected from NIRVE. To obtain cells from the circulation, vessels draining from the tumour were cut and were allowed to drain onto a cover-slip for one minute. PBS was then added to dilute the sample before analysis for GFP positive melanoma cells. Details of quantification are given in supplementary information. For analysis of sorted Brn2::GFP high and low and pigment high and low populations 106 cells were injected into the tail vein of C57/BL6 mice. Lung colonies were imaged after 11-12 days.
Flow cytometry
Analysis was carried out on an FACS Aria cell sorter (Becton Dickinson). Details of FACS settings are provided in supplementary information.
Microarray
RNA was extracted from B16F2 cells sorted from tumours by FACS using RNAeasy mini kit (QIAGEN). Microarray analysis was carried out by the Paterson Institute using Affymetrix chips (MOE430 2.0).
Immune fluorescence
Frozen sections of B16 tumours were fixed in 4% PFA, followed by 0.2% TritonX-100 and blocking with 5%BSA. Anti-F4/80 (Abcam #6640) was used to detect macrophages. Pigment was imaged as described above.
Further details of imaging methods are available on request.
Results
Intravital imaging of a melanoma model
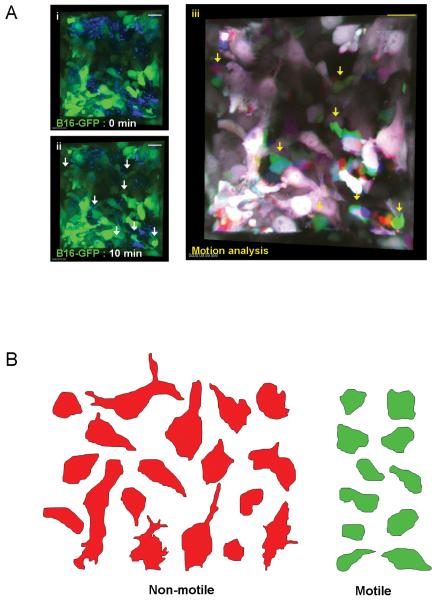
B16F2 melanoma cells can enter the vasculature and spread to the lymphatic system and lungs. To learn more about this process we performed intravital imaging of these tumours. B16F2 cells were engineered to express GFP before sub-cutaneous injection into mice. Tumour imaging was performed using single photon excitation at several locations with high resolution for 20 minute periods. Only a fraction of cells were motile in the primary tumour (Figure1A and Movie1 - Figure1Aiii shows overlaid images of three times points in red, green and blue. Non-motile cells appear white - the composite colour generated from red, green and blue. Motile cells are seen by the separation of the colours). Motile cells moved with speeds ranging from 0.2-5μm/min and had irregular, rounded and rapidly changing morphology (Figure1B and Movie1). Non-motile cells had a diverse array of shapes ranging from amorphous to some with extended ‘dendritic’ morphologies that were never observed in motile cells. We speculated that the motile sub-population of cells observed in the melanoma model might have an altered differentiation status compared to the non-motile population.
Figure 1. In vivo imaging of melanoma motility.
A) Panels (i)&(ii) show 3D reconstructions of a B16-GFP tumour at two times 10 minutes apart. GFP is shown in green and reflectance imaging is shown in blue, arrows highlight motile cells. Panel (iii) shows a merged image of GFP signal from the same tumour volume as in panels (i)&(ii) at three different times with 0min shown in red 5min shown in blue and 10min shown in green. White areas indicate static cells. Scale bar is 20μm. B) Representative tracings of the 3D morphology of non-motile (red) and motile (green) cells.
Visualisation of pigment in melanoma cells and melanocytes following near infra-red excitation
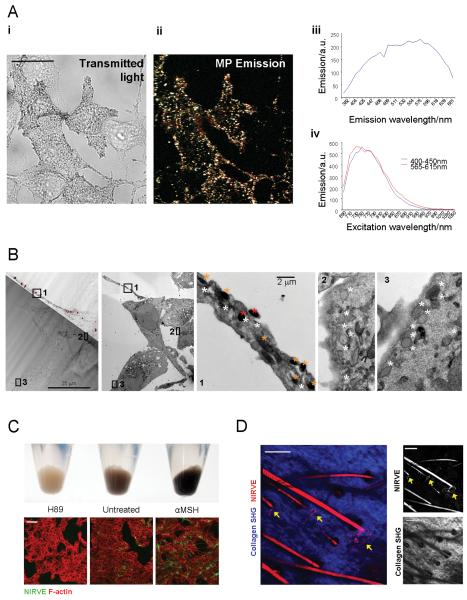
Pigment containing melanosomes can be identified by imaging cells with transmitted light (Figure 2A). However, this method can not be applied in live tumours. During our tumour imaging we noticed a vesicular pattern of light emission from pigment producing B16 tumours following illumination with NIR wavelengths. We speculated that this could originate from pigment containing vesicles. Figure 2A shows a remarkable correlation between these signals and transmitted light imaging of melanosomes. Visible light was emitted across a broad spectrum of visible wavelengths following NIR illumination between 700-900nm (Figure2A right hand panels). We will use the abbreviation NIRVE (Near Infra-Red Visible Emission) to describe this unexpected phenomenon. Correlative analysis of NIRVE signal with transmission electron microscopy of the same cells revealed that NIRVE signal originated from stage 3 and 4 melanosomes (orange and red*), which contain melanin, but not stage 2 melanosomes, which do not contain pigment (Figure2B). Treating B16F2 cells with either H89 or α-MSH, to inhibit PKA-dependent melanogenesis or stimulate melanin production, respectively, caused corresponding changes in NIRVE signal (Figure2C). NIRVE signal could also be detected in another melanoma culture, 4599, but not in breast cancer cells (Supplementary Figure1A). Imaging of mouse skin demonstrated that NIRVE signal is also detected in hair and around the base of some hair follicles, which is where melanocytes are located (Figure2D and Movie 2 for animation through z-sections)(14, 15). As further demonstration of the utility of this method, we found that NIRVE signal is also detected in formalin fixed paraffin embedded human melanoma samples (Supplementary Figure1B). Together these data show that NIRVE emission is a novel and specific means of imaging melanin pigment that can be widely applied to image tissues in situ.
Figure 2. Pigment containing melanosomes can be identified by visible light emission following near infra-red illumination.
A) B16F2 cells stimulated with α-MSH were imaged by transmitted light (i) and laser scanning microscopy following illumination at 800nm (ii) merged image of emission at 410-450nm (cyan), 500-550nm (green) and 565-615nm (red). Scale bar is 25μm. Emission and excitation spectra of near infrared stimulated visible emission are shown in panels (iii) and (iv), respectively. B) Transmitted light and NIRVE images (dark red) of B16 cells are shown overlaid (left-hand panel). A cell with a long process and high NIRVE signal is visible surrounded by cells with low NIRVE signal. Transmission electron microscopy images of the same area are shown. Mid-left panel shows a low magnification image of the NIRVE positive cell and surrounding cells. Inset panels 1-3 show high magnification images of the areas indicated on the low magnification image. White, orange and red asterisks are placed adjacent to stage 2, 3, and 4 melanosomes, respectively. C) Upper panel shows changes in pigment followingα-MSH or H89 treatment. Lower panels show NIRVE signal in green following α-MSH or H89 treatment. F-actin is in red. Scale bar is 50μm. D) Ear skin of mouse showing collagen (blue: collected between 420-490nm* following illumination at 800nm) and NIRVE signal (red: collected between 575-630nm following illumination at 800nm) are shown. See also Supplementary Movie2. Arrows indicate pigment producing melanocytes. * some NIRVE signal is also collected.
Motile cells in vivo lack pigment
Figure3A shows that pigment could also be imaged in B16F2 tumours in vivo and was usually in puncta near at the cell periphery, which was defined by GFP-CAAX expression. Timelapse analysis revealed that the NIRVE signal was dynamic (Movie3), possibly as result of melanosome movement. Immuno-histochemistry revealed that some NIRVE signal could also be found within melanoma associated macrophages, which are known to take up melanin(13, 14), but not breast tumour associated macrophages (Supplementary Figure1C).
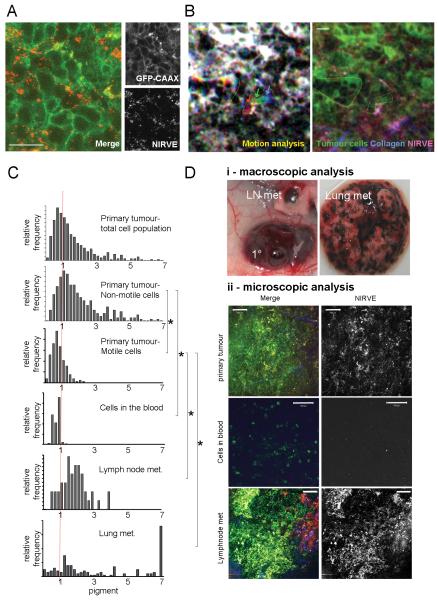
Figure 3. Monitoring pigment expression during melanoma dissemination.
A) High resolution imaging of subcutaneous B16F2-GFP-CAAX tumour. GFP (green) and NIRVE signal (red - collected between 410-530nm following illumination at 790nm) are shown. Scale bar is 25μm. B) Left-hand panel shows motion analysis of intravital imaging of B16F2-GFP melanoma - white areas are non-motile, distinct areas of colour indicate motile cells (also highlighted with arrows). Right-hand panel shows NIRVE signal from the same region with inset panel showing the positions of motile cells relative to the NIRVE signal (see also Supplementary Movies 3&4). Scale bar is 25μm. C) Histograms showing the quantification of pigment content in both motile and non-motile tumour cells in the primary tumour, cells that had entered the vasculature, lymph node metastases and lung metastases. Values are normalised to 1 for the primary tumour. * indicates p<0.01 Kruskil-WallisANOVA test. D) i) Macroscopic image showing the highly pigmented nature of the primary tumour, a lymph node metastasis and lung metastases. ii) Images of B16F2-GFP-CAAX tumour cells in primary tumour, blood, and lymph node. Merge shows GFP in green, NIRVE signal in red, and collagen in blue - scale bar is 100microns.
B16F2 melanoma cells can metastasize. This process begins with cell motility in the primary tumour(11). Figure3B and Movie4 show examples of the motile behaviour of B16F2-GFP cells in vivo. Strikingly, motile cells contained very little pigment. The amount of pigment contained in motile and non-motile cells was quantified. The majority of the cells in the primary tumours are non-motile, therefore the distribution of pigment in the non-motile cells was similar to the distribution for all the cells. However, the motile cells contained lower levels of pigment than the non-motile cells (Figure3C compare second and third panels). Cells were also collected from the vasculature and imaged: these cells also had significantly less pigment than non-motile cells in the primary tumour.
Despite finding that the motile cells in the primary tumour contained a low level of pigment, spontaneous secondary metastatic tumours were highly pigmented (for example in the draining lymph nodes - Figure3Di). Furthermore, experimental lung metastases were also pigmented. This was confirmed by analysis of NIRVE signal at metastatic locations (Figure3C&Di), although some less pigmented cells were still present in metastases. These results suggested that the hypo-pigmentation of invasive melanoma cells could be a transient phenotype.
Brn2 promoter activity is upregulated in motile cells
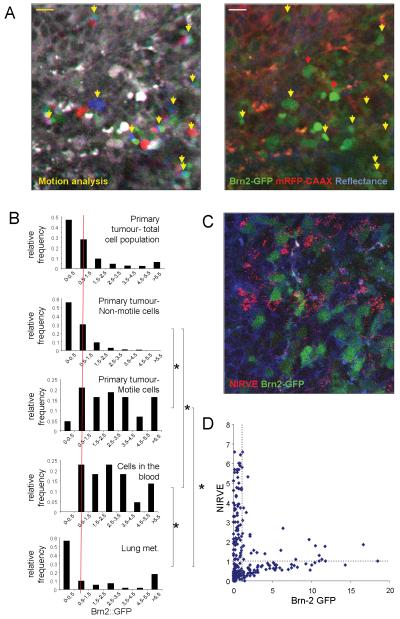
A failure to produce pigment does not necessarily indicate a change in differentiation status. We therefore sought another method to investigate the differentiation status of the motile melanoma cells. Brn-2/POU3F2 expression is high in migratory melanoblasts in culture and decreases as melanocytes differentiate(16, 17). To investigate the relationship between Brn-2 expression and melanoma dissemination we engineered B16 cells to contain the Brn-2 promoter driving the expression of GFP; these cells were also engineered to express mRFP-CAAX constitutively. Supplementary Figure 2 confirms that these cells down-regulate GFP expression in response to the pro-differentiation stimulus αMSH. Brn2::GFP was heterogeneously expressed in vivo (Figure4A). Furthermore, GFP levels were high in motile cells in vivo indicating elevated activity of the Brn-2 promoter (cells marked with yellow arrows). However, high levels of GFP expression were not sufficient to confer motility as not all GFP-positive cells were motile (marked with red arrows). We quantified the fluorescence intensity of GFP in numerous cells that were non-motile, motile, circulating or growing in the lungs. Figure4B shows a clear shift towards elevated Brn-2 promoter activity in the motile and circulating cells that is largely down-regulated in cells growing in the lungs. The data presented above suggest that Brn-2 promoter activity and pigment production should be inversely related. We investigated this by simultaneous imaging Brn-2 promoter driven GFP expression and NIRVE signal. Figures4C&D show that cells with high levels of pigment have low levels of Brn-2::GFP expression. Many cells had low levels of both indicating that there is not a simple inverse correlation between the two variables. Cells expressing high levels of Brn2::GFP generally had low levels of pigment although some cells expressed above average levels (upper right quadrant in Figure4D).
Figure 4. Brn-2 promoter activity during melanoma dissemination.
A) Left-hand panel shows motion analysis of intravital imaging of B16F2 Brn-2:GFP/mRFP-CAAX melanoma - white areas are non-motile, distinct areas of colour indicate motile cells (highlighted with arrows - scale bar is 20μm). Right-hand panel shows GFP signal from the Brn-2 promoter (green) mRFP-CAAX (red) and reflectance (blue) in same region (motile cells are highlighted with arrows - see also Supplementary Movie5). B) Histograms showing the quantification of pigment content in both motile and non-motile tumour cells in the primary tumour, cells that had entered the vasculature, lymph node metastases and lung metastases. Values are normalised to 1 for the primary tumour. * indicates p<0.01 Kruskil-WallisANOVA test. C) Intravital image of B16F2 Brn2::GFP/mRFP-CAAX tumour. GFP is shown in green, mRFP-CAAX in blue and NIRVE signal in red. D) Scatter plot showing the relationship between NIRVE signal and GFP expression from the Brn-2 promoter. Each cross represents the values in individual cells imaged in vivo.
Switching between high and low pigment production and Brn2 expression states
The data outlined above indicate that melanoma cells in transit from primary to secondary sites have less differentiated characteristics that are only found in a subset of cells in primary tumours and metastases. This could be explained by reversible switching between states. Melanoma cells could reduce pigment production and elevate Brn2 expression as they exit primary tumours and reverse these changes when they have arrived at secondary locations. Another possibility is the switching between states is unidirectional. In this model high Brn2 low pigment cells can produce low Brn2 and high pigment progeny but not vice-versa. The heterogeneity at primary and metastatic sites would arise from the differentiation of the Brn2 high/pigment low cells. To test these contrasting possibilities we isolated either Brn2::GFP high and low or pigment high and low populations from primary tumours and re-introduced them into mice.
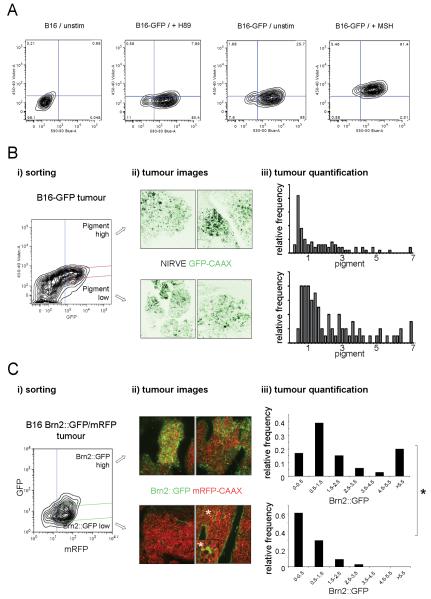
We developed a FACS method for sorting cells based on their level of pigment. We found a robust difference in light emitted around 450nm following excitation at 405nm between more pigmented α-MSH treated cells and less pigmented control cells or H89-treated cells, which contain almost no pigment (Figure5A, see also Figure1C). The most highly and least pigmented B16F2 cells were isolated from xenograft tumours based on 450-40 emission (Figure5Bi). These cells were then expanded in culture before injection into the tail vein of mice. After 11-12 days the lungs were analysed. Figure5B shows that a similar spectrum of pigment levels were observed in melanoma colonies derived from both pigment high and pigment low populations. These data demonstrate that switching can occur in both directions between high and low pigment production states.
Figure 5. Flow cytometry analysis of pigment content.
A) FACS profile of B16F2 cells with or without stable GFP expression (two left-hand panels). Effect of H89 or α-MSH on violet laser stimulated 450nm emission is shown by contour plots. B) (i) B16F2-GFP CAAX tumour cells isolated from xenograft tumours in nude mice. Contour plots show GFP (530-30nm) against Violet (450-40nm) emission. Blue line shows gate used to sort GFP expressing cells and magenta lines show the gates used to sort the top and bottom 10% pigmented cells. (ii) Pigment high and low populations were re-introduced into the tail vein of mice and the resulting lung colonies were imaged. Representative images are shown with NIRVE signal in black and GFP in green. (iii) Quantification of pigment levels assessed on a cell by cell basis in colonies generated from pigment high and low populations. Relative frequency of pigment levels is shown (data from 12 mice in three independent experiments). C) (i) B16F2 Brn2::GFP/mRFP-CAAX tumour cells isolated from xenograft tumours in nude mice. Contour plots show Red (620-40nm) against GFP (530-30nm) emission. Blue line shows gate used to sort mRFP expressing cells and green lines show the gates used to sort the top and bottom 10% Brn2::GFP cells. (ii) Brn2::GFP high and low populations were re-introduced into the tail vein of mice and the resulting lung colonies were imaged. Two representative images are shown with mRFP-CAAX signal in red and Brn2::GFP in green. * highlights three cells that have converted back to high levels of Brn2::GFP expression. (iii) Quantification of Brn2::GFP levels assessed on a cell by cell basis in colonies generated from Brn2::GFP high and low populations. Relative frequency of Brn2::GFP levels is shown (data from 10 mice in three independent experiments). *indicates p<0.01 Mann-Whitney test.
We performed a similar series of experiments with cells sorted for levels of Brn2::GFP expression. Figure 5C shows that Brn2::GFP high cells produced colonies with both GFP positive and negative cells. This result indicates that Brn2 high cells can produce Brn2 low progeny. In contrast, Brn2::GFP low cells produced colonies with predominantly Brn2::GFP low cells and only very few Brn2::GFP high cells (marked with asterisk and inset panel in Figure5C). These data indicate that switching from a Brn2 high state to a Brn2 low state is favoured over low to high switching. The number of melanoma colonies obtained with high and low pigment and Brn2::GFP high and low populations were equivalent (data not shown).
TGFβ2 is upregulated in cells lacking pigment
We next wished to investigate molecular differences between more and less pigmented cells that might explain the switching between high and low pigment states. We sorted pigment high and pigment low cells as described above and performed microarray analysis. Supplementary Table 1 lists the genes differentially regulated between the two cell populations. The green text indicates genes more highly expressed in the cells lacking pigment (this population contains the motile population observed in vivo). From the genes up-regulated in cells lacking pigment we chose to investigate the function of TGFβ2 further. TGFβ2 is extensively implicated in cancer progression and is also a target gene of TGFβ signalling (19). Therefore, high TGFβ2 expression may indicate a positive feedback loop of TGFβ signalling in less pigmented melanoma cells. Consistent with this, we observed an inverse pattern of pigmentation and activation of TGFβ signalling as judged by pSmad3 staining (data not shown).
TGFβ signalling induces hypo-pigmentation and cell motility
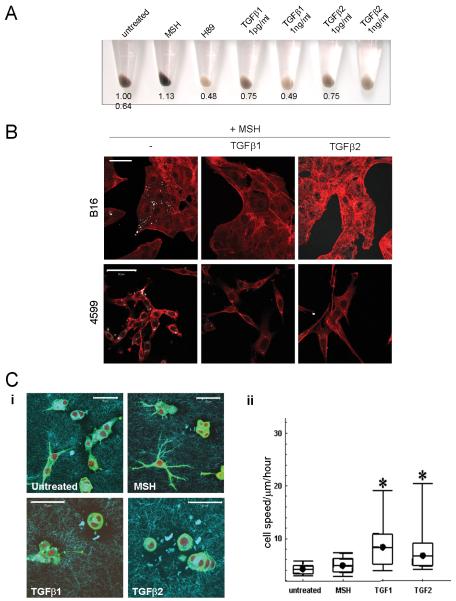
We next tested whether TGFβ signalling could affect pigment levels. We found that both TGFβ1 and TGFβ2 induced hypo-pigmentation of melanoma cells at relatively low doses (Figure6A). This was confirmed by reduction of NIRVE signal following stimulation with TGFβ in both α-MSH treated B16 and 4599 melanoma cells (Figure6B). We also investigated whether there was a connection between TGFβ signalling and melanoma motility. When plated on deformable collagen/matrigel matrix in vitro B16F2 appeared elongated with some dendritic protrusions (Figure6Ci). Treatment of cells with α-MSH enhanced the dendritic morphology that is characteristic of a differentiated melanocyte. However treatment of cells with either TGFβ1 or TGFβ2 inhibited dendrite formation and cells appeared more rounded (Figure6Ci). Furthermore, treatment with either TGFβ1 or TGFβ2, but not α-MSH, significantly increased cell motility (Figure6Cii). These data demonstrate that TGFβ signalling can reverse characteristics found in differentiated melanocytes including pigment production and dendritic morphology, and increase cell motility.
Figure 6. TGFβ induces hypopigmentation, inhibits dendrite formation and increases cell motility.
A) Cell pellets of B16F2 cells treated with H89 αMSH or TGFβ for 48 hours are shown. Numbers indicate digital analysis of pellet darkness. B) NIRVE (white - collected between 410-530nm following illumination at 790nm) and F-actin (red) merged images of B16F2 and 4599 mouse melanoma cells treated with αMSH for 24hrs and, where indicated TGFβ1 or TGFβ2 for 24 hours prior to α-MSH treatment are shown. Scale bar is 50μm. C) i) B16F2 cells plated on deformable collagen/matrigel matrix (cyan) and stained for F-actin (green) and DNA (red). Scale bar is 50μm. ii) Box plots of cell speeds in microns/hour taken from phase contrast movies of B16F2 cells plated on deformable collagen/matrigel matrix over 16 hour period.*indicates p<0.01 Mann-Whitney test.
Discussion
Heterogeneity between cancer cells in the same tumour is a feature of many cancers. In the case of melanoma, distinct Brn2 and MITF expressing populations can be found in the same tumour(4). It has been suggested that reversible changes in between cell states underlie metastatic behaviour. Melanoma cells can switch reversibly between more and less pigmented states(18) and inter-conversion between proliferative and invasive states has been reported in primary tumours(12). However, it is unclear how changes in these states may relate to the various stages of melanoma dissemination, if changes in cell state are truly reversible and what factors may drive these changes. In this study we use intravital imaging to show transient changes in pigment production and differentiation status during the metastatic process.
Both primary tumours and metastases contain Brn2-high and -low cells and pigment-high and -low cells. Combined analysis of Brn2::GFP levels and NIRVE signal demonstrated that Brn2-high cells generally had lower levels of pigment than Brn2-low cells. Nonetheless, some Brn2-high cells had above average levels of pigment and many cells had low levels of both pigment and Brn2 expression (Figure4D). Our intravital imaging revealed that only Brn2-high/pigment-low cells were motile. Therefore it is the coincidence of elevated Brn2 with very low pigment levels that most closely correlates with motility. These traits are characteristics of less differentiated more melanoblast-like cells. The less differentiated nature of the disseminating cells could result from cell extrinsic factors transiently promoting de-differentiation. Alternatively, there may be unidirectional switching between states and only cells that are intrinsically less differentiated would be competent to metastasize. We find that switching of pigment and Brn2 expression can occur in both directions. Although, Brn2-low cells rarely converted to melanoblast-like Brn2-high cells (Figure5C). The conversion to high Brn2 expression may result from a rare combination of cell extrinsic factors or may simply be stochastic. These data support a transient and reversible switch to a less differentiated phenotype during melanoma dissemination (Supplementary Figure3).
For melanoma cells to become motile and begin disseminating a combination of conditions need to occur. Firstly, the cell must be in a Brn2-high state. However, Brn2 expression is not sufficient to completely suppress pigment production or induce motility in vivo. Therefore additional cues are required to further suppress levels of pigment and induce motility. Our microarray data and subsequent analysis demonstrate that TGFβ signalling is a prime candidate for this additional signal. Elevated levels of TGFβ ligands in the primary tumour could suppress pigment production and promote motility. However, these changes may not be maintained if TGFβ levels are low at secondary sites. TGFβ2 has previously been reported to be increased in metastatic melanoma(19) and is associated with a more invasive phenotype(9). The inverse correlation between pigment and TGFβ signalling that we observe in tumours is consistent with previous reports that TGFβ antagonises MITF function and melanosome maturation(20, 21). TGFβ signalling in the less differentiated disseminating melanoma cells may also promote mesenchymal characteristics and even stem cell traits(22). Our microarray analysis shows that less pigmented cells have elevated levels of a mesoderm specific transcript. However, we did not find evidence for stem cell behaviours in our system. All the populations we describe have similar clonigenic potential both in vitro and in vivo (data not shown). This is consistent with a recent report that melanoma lacks a stem cell compartment(23). TGFβ did not promote Brn-2::GFP expression (data not shown). The factors that drive Brn2 expression in a subset of cells are unclear, but they could include B-Raf or β-catenin signalling(24, 25).
Several features of this model have parallels in the normal physiology of melanoblasts and melanocytes. Firstly, less differentiated melanoblasts that are migratory during development. Differentiation is essentially an irreversible process and this may explain why we observe Brn2-low cells converting to Brn2-high cells with low frequency. Secondly, reversible transitions between high and low levels pigment production frequently occur in melanocytes: either to co-ordinate with the hair growth or to respond to UV exposure of the skin. In both cases TGFβ signalling is implicated in reducing pigment levels(21, 26). However, in these cases TGFβ signalling does not cause cell motility, probably because the cells are well differentiated.
To conclude, both primary and metastatic melanoma contain a heterogeneous mix of more and less differentiated cells. Strikingly, actively disseminating cells are more uniform with low levels of pigment and high levels of Brn2 expression. However, neither of these traits is stable. Changes in pigment production are reversible and may be transiently induced by TGFβ signalling in the primary tumour. While, Brn2-high cells give rise to cells expressing low levels of Brn2 and high levels of pigment. Thus, although only a subset of less differentiated cells leave the primary tumour they re-differentiate at secondary sites.
Supplementary Material
Acknowledgements
We thank Cancer Research UK for funding, the Paterson Institute Micro-array facility, and LRI colleagues for comments and advice.
Abrreviations
- αMSH
Alpha Melanocyte Stimulating Hormone
- NIR
Near Infra-Red
- MITF
MIcrophthalmia-associated Transcription Factor
- TGFβ
Transforming Growth Factor β
References
- 1.Gaggioli C, Sahai E. Melanoma invasion - current knowledge and future directions. Pigment Cell Res. 2007;20(3):161–72. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 2.Bronner-Fraser M. Neural crest cell migration in the developing embryo. Trends Cell Biol. 1993;3(11):392–7. doi: 10.1016/0962-8924(93)90089-j. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282(38):27557–61. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- 4.Goodall J, Carreira S, Denat L, et al. Brn-2 represses microphthalmia-associated transcription factor expression and marks a distinct subpopulation of microphthalmia-associated transcription factor-negative melanoma cells. Cancer Res. 2008;68(19):7788–94. doi: 10.1158/0008-5472.CAN-08-1053. [DOI] [PubMed] [Google Scholar]
- 5.Gaggioli C, Busca R, Abbe P, Ortonne JP, Ballotti R. Microphthalmia-associated transcription factor (MITF) is required but is not sufficient to induce the expression of melanogenic genes. Pigment Cell Res. 2003;16(4):374–82. doi: 10.1034/j.1600-0749.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 6.Wellbrock C, Marais R. Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation. J Cell Biol. 2005;170(5):703–8. doi: 10.1083/jcb.200505059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 8.Carreira S, Goodall J, Denat L, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20(24):3426–39. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoek KS, Schlegel NC, Brafford P, et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006;19(4):290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Gans JD, Nogueira C, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125(7):1269–81. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7(10):737–49. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 12.Hoek KS, Eichhoff OM, Schlegel NC, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68(3):650–6. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 13.Dhomen N, Reis-Filho JS, da Rocha Dias S, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15(4):294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Tobin DJ, Slominski A, Botchkarev V, Paus R. The fate of hair follicle melanocytes during the hair growth cycle. J Investig Dermatol Symp Proc. 1999;4(3):323–32. doi: 10.1038/sj.jidsp.5640239. [DOI] [PubMed] [Google Scholar]
- 15.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 16.Eisen T, Easty DJ, Bennett DC, Goding CR. The POU domain transcription factor Brn-2: elevated expression in malignant melanoma and regulation of melanocyte-specific gene expression. Oncogene. 1995;11(10):2157–64. [PubMed] [Google Scholar]
- 17.Vance KW, Goding CR. The transcription network regulating melanocyte development and melanoma. Pigment Cell Res. 2004;17(4):318–25. doi: 10.1111/j.1600-0749.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DC. Differentiation in mouse melanoma cells: initial reversibility and an on-off stochastic model. Cell. 1983;34(2):445–53. doi: 10.1016/0092-8674(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 19.Van Belle P, Rodeck U, Nuamah I, Halpern AC, Elder DE. Melanoma-associated expression of transforming growth factor-beta isoforms. Am J Pathol. 1996;148(6):1887–94. [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Esparza M, Jimenez-Cervantes C, Beermann F, Aparicio P, Lozano JA, Garcia-Borron JC. Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1. J Biol Chem. 1997;272(7):3967–72. doi: 10.1074/jbc.272.7.3967. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Li Y, Nishimura EK, et al. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell. 2008;32(4):554–63. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 23.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456(7222):593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodall J, Martinozzi S, Dexter TJ, et al. Brn-2 expression controls melanoma proliferation and is directly regulated by beta-catenin. Mol Cell Biol. 2004;24(7):2915–22. doi: 10.1128/MCB.24.7.2915-2922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodall J, Wellbrock C, Dexter TJ, Roberts K, Marais R, Goding CR. The Brn-2 transcription factor links activated BRAF to melanoma proliferation. Mol Cell Biol. 2004;24(7):2923–31. doi: 10.1128/MCB.24.7.2923-2931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hibino T, Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004;35(1):9–18. doi: 10.1016/j.jdermsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.