Abstract
Background and Purpose
The role of interleukin (IL)-1β remains unknown in early brain injury (EBI) after subarachnoid hemorrhage (SAH), although IL-1β has been repeatedly reported to increase in the brain and cerebrospinal fluid. The aim of this study is to examine the effects of IL-1β inactivation on EBI after SAH in mice.
Methods
The endovascular perforation model of SAH was produced and 112 mice were assigned to sham, SAH+ vehicle, and SAH+ N-Ac-Tyr-Val-Ala-Asp-chloromethyl ketone (Ac-YVAD-CMK, 6 and 10 mg/kg) groups. Ac-YVAD-CMK, a selective inhibitor of IL-1β converting enzyme, or vehicle was administered intraperitoneally 1 hour post-SAH. EBI was assessed in terms of mortality within 24 hours, neurological scores, brain water content at 24 and 72 hours, Evans blue dye extravasation and Western blot for IL-1β, c-Jun N-Terminal kinase (JNK), matrix metalloproteinase (MMP)-9 and zonula occludens (ZO)-1 at 24 hours after SAH.
Results
High-dose (10mg/kg), but not low-dose (6mg/kg) treatment group significantly improved neurological scores, mortality, brain water content and Evans blue dye extravasation compared with the vehicle group. Although both dosages of Ac-YVAD-CMK attenuated the mature IL-1β induction, only high-dose treatment group significantly inhibited the phosphorylation of JNK, MMP-9 induction and ZO-1 degradation.
Conclusion
IL-1β activation may play an important role in the pathogenesis of EBI after SAH. The neurovascular protection of Ac-YVAD-CMK may be provided by the inhibition of JNK-mediated MMP-9 induction and the consequent preservation of tight junction protein ZO-1.
Keywords: Subarachnoid hemorrhage, Early brain injury, Interleukin-1β, Brain edema, Neurovascular protection
Introduction
Subarachnoid hemorrhage (SAH) is a deadly cerebrovascular disorder with a high mortality rate.1 The high morbidity and mortality observed in SAH have persisted in spite of recent therapeutic advances.1 Recent studies have emphasized the importance of the management for post-SAH early brain injury (EBI) to improve outcome.2
Various mechanisms including inflammation have been implicated in the pathogenesis of EBI after SAH.2–4 In a clinical setting, many signs associated with EBI such as pyrexia, neutrophilia and cerebral edema secondary to disruption of the blood-brain barrier (BBB) are believed to be caused by cytokine activity.5 Among many proinflammatory cytokines, interleukin (IL)-1β is considered as a key mediator of neural injury in acute central nervous system (CNS) injuries, such as ischemic stroke and brain trauma.6 IL-1β is also reported to increase in the cerebral cortex7 and cerebrospinal fluid (CSF)8 after SAH in both humans and animal models. However, whether increased IL-1β is merely an epiphenomenon or a causative factor for EBI remains unproved; or rather, there is no literature to investigate the effects of anti-proinflammatory agents on EBI after SAH. IL-1β is synthesized as precursor molecules that are then processed to mature forms by caspase-1, the IL-1β converting enzyme.9 We therefore examined the effects of N-Ac-Tyr-Val-Ala-Asp-chloromethyl ketone (Ac-YVAD-CMK), a caspase-1 inhibitor that selectively inhibits the cleavage of precursor IL-1β, on EBI in the established endovascular perforation model of SAH in mice.
Materials and Methods
The animal and ethics review committee at Loma Linda University evaluated and approved all protocols. One hundred and twelve CD-1 mice, weighing 35 to 40 g, were assigned to 4 groups: sham (n=22), vehicle (SAH+dimethyl sulfoxide [DMSO], n=39), low-dose (SAH+Ac-YVAD-CMK −6mg/kg, n=28), and high-dose (SAH+Ac-YVAD-CMK −10mg/kg, n=23) groups.
Mouse SAH model
SAH model was produced as described previously.10 Briefly, animals were anesthetized with an intraperitoneal injection of ketamine/xylazine (100/10 mg/kg). A blunted 4-0 monofilament nylon suture was introduced into the external carotid artery and advanced through the internal carotid artery (ICA) to the left anterior cerebral artery (ACA) near the anterior communicating artery, where resistance was encountered. The filament was advanced 5 mm further to perforate the ACA, then immediately withdrawn. In the sham surgery the filaments were advanced without arterial perforation. Body temperature was kept constant (37.5±0.5 °C) during the operation.
Drug administration
Six- or 10-mg/kg of Ac-YVAD-CMK (Cayman Chemical Company, Ann Arbor, MI) was intraperitoneally injected 1 hour after the induction of SAH. The drug was dissolved in DMSO and was further diluted in phosphate-buffered saline (PBS; final concentration of DMSO < 1.67%). Vehicle group was treated with the same volume of DMSO diluted in PBS.
Severity of SAH
The severity of SAH was quantified by use of the previously published grading scale at the sacrifice.11 Briefly, the basal cistern was divided into six segments. Each segment was allotted a grade from 0 to 3 depending on the amount of subarachnoid blood in the segment: grade 0, no subarachnoid blood; grade 1, minimal subarachnoid blood; grade 2, moderate blood clot with recognizable arteries; grade 3, blood clot obliterating all arteries within the segment. The animals received a total score ranging from 0 to 18 after adding the scores from all six segments.
Mortality and neurological scores
The neurological scores were evaluated in a blinded fashion at 24 and 72 hours post-SAH, based on the scoring system of Garcia et al.12 with modifications.13 Animals were given a score of 3 to 21 in one number steps (higher scores indicate greater function). Mortality was calculated at 24 hours after SAH.
Brain water content (brain edema)
Brains were quickly separated into the left and right cerebral hemispheres, cerebellum, and brainstem, and weighed (wet weight) at 24 hours (n=6, 9, 9 and 6 in sham, vehicle, low- and high-dose groups, respectively) or 72 hours (n=4 per group [sham, vehicle and high-dose groups]) after operation. Next, brain specimens were dried in an oven at 105°C for 24 hours and weighted again (dry weight). The percentage of water content was calculated as ([wet weight - dry weight]/wet weight) ×100%.14
BBB disruption
At 24 hours after operation, Evans blue dye (50 mg/kg) was injected intraperitoneally and allowed to circulate for 3 hours.15 Under deep anesthesia, mice (n=5 per group) were killed by intracardial perfusion with PBS and brains were removed and divided into the same regions as in the water content study. Brain specimens were weighed, homogenized in PBS, and centrifuged at 15,000g for 30 min. Then, 0.7 ml of the resultant supernatant was added to an equal volume of trichloroacetic acid. After overnight incubation and centrifugation at 15,000g for 30 min at 4°C, the supernatant was taken for spectrophotometric quantification of extravasated Evans blue dye at 615 nm as described previously.16
Artery sampling and 2,3,5-triphenyl tetrazolium chloride (TTC) staining
Seven mice (n=1, 3 and 3 in sham, vehicle and low-dose groups, respectively) were sacrificed by transcardial perfusion with 50 ml of ice-cold PBS at physiological pressure 24 hours post-surgery. Cerebral arteries on the perforation side were examined under microscope for the presence of thrombus. Each brain was coronally sectioned into four 2-mm-thick slices starting from the frontal pole, stained with 2% TTC and assessed for cerebral infarction.
Western blotting
Western blot analysis was performed as described previously.13 Briefly, the whole left cerebral hemispheres (n=6 per group) were homogenized, and aliquots were used to determine the protein concentration of each sample using a detergent compatible assay (Bio-Rad, Philadelphia, PA). Protein samples (50µg) were loaded on a Tris glycine gel, electrophoresed, and transferred to a nitrocellulose membrane. Membranes were incubated overnight at 4°C with the primary antibodies: rabbit polyclonal anti-IL-1β antibody (Abcam, Cambridge, MA; BioVision, Mountain View, CA), rabbit polyclonal anti-zonula occludens (ZO)-1 antibody (Zymed, Carlsbad, CA), rat monoclonal anti-matrix metalloproteinase (MMP)-9, and rabbit polyclonal anti-c-Jun N-Terminal kinase (JNK) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were processed with secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 21°C, probed and then exposed to x-ray film. Blot bands were quantified using Image J software (NIH). β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA) was blotted on the same membrane as a loading control, because β-Actin levels are reported to be unchanged in the brain after SAH.10, 13
Statistics
Data were expressed as mean±SD. Statistical differences among groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey multiple comparison post-hoc analysis. Comparisons between groups regarding the mortality were analyzed using χ2 and z tests. P<0.05 was considered statistically significant.
In the statistical analysis, we calculated the power of the tests. The number of animals per group necessary to reach the desired power of 0.800 was in the range of four to six.
Results
Mortality
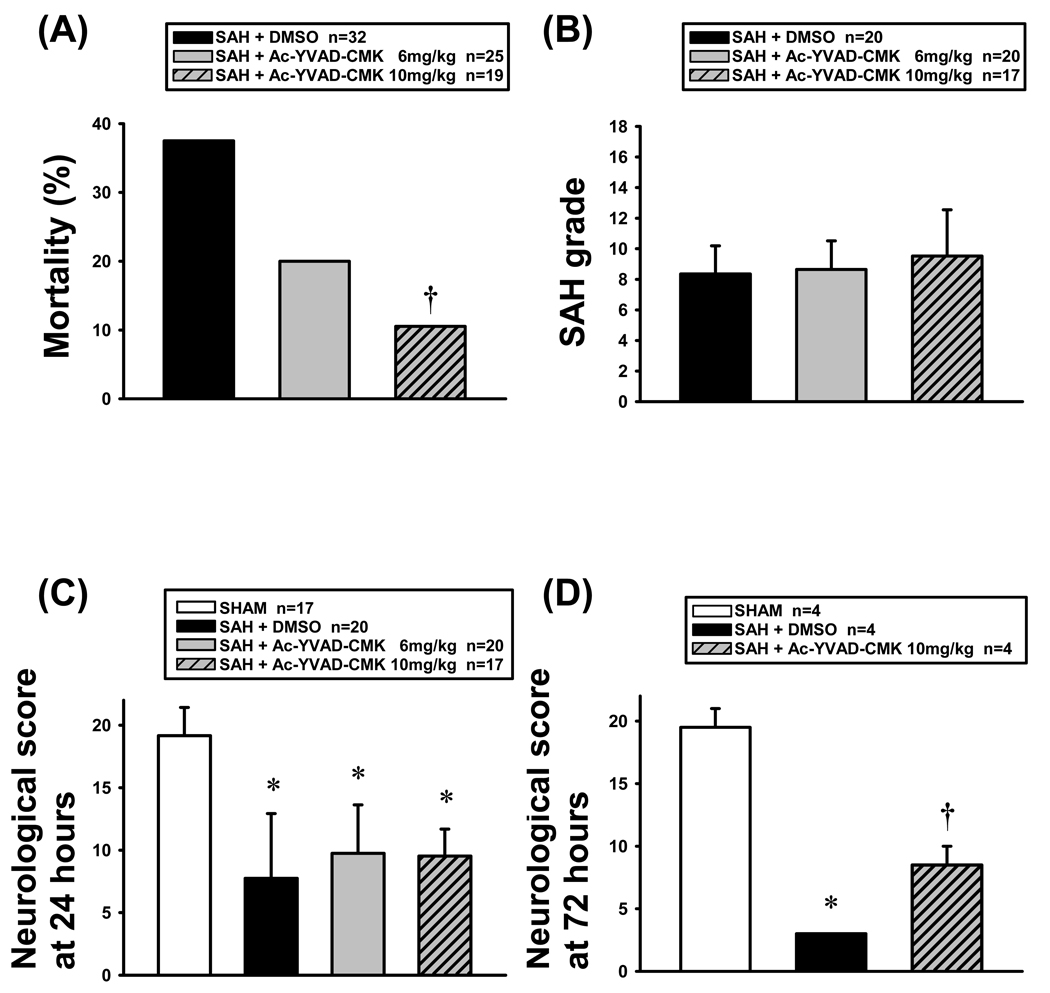
None of the sham-operated mice (n=22) died. In the vehicle group, 12 of 32 mice (37.5%) died within 24 hours after SAH. Ac-YVAD-CMK treatment reduced the mortality in a dose-dependent manner (20.0% [5 of 25 mice] and 10.5% [2 of 19 mice] in low- and high-dose groups, respectively), and the difference was significant between the high-dose and vehicle groups (Figure 1A).
Figure 1.
Mortality (A), SAH grade (B) and neurological deficits (C and D). (A) Ac-YVAD-CMK treatment (10 mg/kg) significantly reduced the mortality rate vs vehicle group 24 hours post-SAH (†P<0.05, ANOVA). (B) The SAH grade was similar among groups. (C), (D) The modified Garcia scores were significantly lower in SAH groups vs sham group (*P<0.05, ANOVA; C, 24 hours; D, 72 hours post-SAH). Ac-YVAD-CMK treatment at 10 mg/kg significantly prevented neurological impairment 72 hours post-SAH (D; ANOVA, †P<0.05 vs vehicle group).
SAH grade
The average SAH grading score was 8.4, 8.7 and 9.5 in the vehicle (n=20), low-dose (n=20) and high-dose (n=17) groups at 24 hours (Figure 1B), and 7.5 and 7.8 in the vehicle (n=4) and high-dose (n=4) groups at 72 hours post-SAH, respectively. There was no significant difference among the groups.
Neurological deficits
The neurological scores in SAH animals (n=20, 20 and 17 in vehicle, low- and high-dose groups, respectively) were significantly lower than in sham-operated animals (n=17) at 24 hours post-SAH (P<0.05; Figure 1C). Although Ac-YVAD-CMK treatment did not improve neurological scores at 24 hours post-SAH, high-dose Ac-YVAD-CMK treatment (n=4) significantly prevented neurological impairment compared with vehicle group (n=4) at 72 hours post-SAH (P<0.05; Figure 1D).
Brain water content
Brain water content in the left (perforation-sided) cerebral hemisphere was significantly increased in vehicle group (n=9) compared with sham group (n=6) 24 hours post-SAH (P<0.05; Figure 2A). High-dose Ac-YVAD-CMK treatment (n=6 and 4 at 24 and 72 hours post-SAH, respectively) significantly reduced the water content in the left cerebral hemisphere at 24 hours (P<0.05 versus vehicle group, n=9; Figure 2A), and in the right cerebral hemisphere and cerebellum at 72 hours post-SAH (P<0.05 versus vehicle group, n=4; Figure 2B).
Figure 2.
Brain edema and BBB disruption. Lt, left; Rt, right. (A) Brain water content 24 hours post-SAH. ANOVA, *P<0.05 vs sham group; †P<0.05 vs vehicle group in the left cerebral hemisphere. (B) Brain water content 72 hours post-SAH. ANOVA, *P<0.05 vs sham group in the right and left cerebral hemispheres; †P<0.05 vs vehicle group in the right cerebral hemisphere and cerebellum, respectively. (C) Evans blue dye extravasation 24 hours post-SAH. ANOVA, *P<0.05 vs sham group in all brain regions; †P<0.05 vs vehicle group in the left cerebral hemisphere and brainstem, respectively.
BBB disruption
Vehicle-treated SAH mice (n=5) showed the marked extravasation of Evans blue dye into all brain regions 24 hours post-SAH (P<0.05 versus sham group, n=5; Figure 2C). High-dose treatment (n=5) significantly reduced the amount of Evans blue dye extravasation in the left cerebral hemisphere and brainstem (P<0.05 versus vehicle group [n=5], respectively; Figure 2C).
Pathological findings of brain and cerebral arteries
In the SAH mice (n=6), blood clots were seen especially around the bifurcation of the ACA and middle cerebral artery (MCA) in the basal cistern on the perforation side. Brain swelling was also seen predominantly on the perforation side (Figure 3A). There was no thrombus in the artery lumens (Figure 3B) and no infarction in TTC staining (Figure 3C).
Figure 3.
Pathological findings. (A) Mouse brain after SAH. Blood clots and brain swelling were seen predominantly on the perforation side. (B) Representative photograph of arterial lumen showing no thrombus. (C) TTC staining showing no infarction in the brain.
Expression of IL-1β after SAH
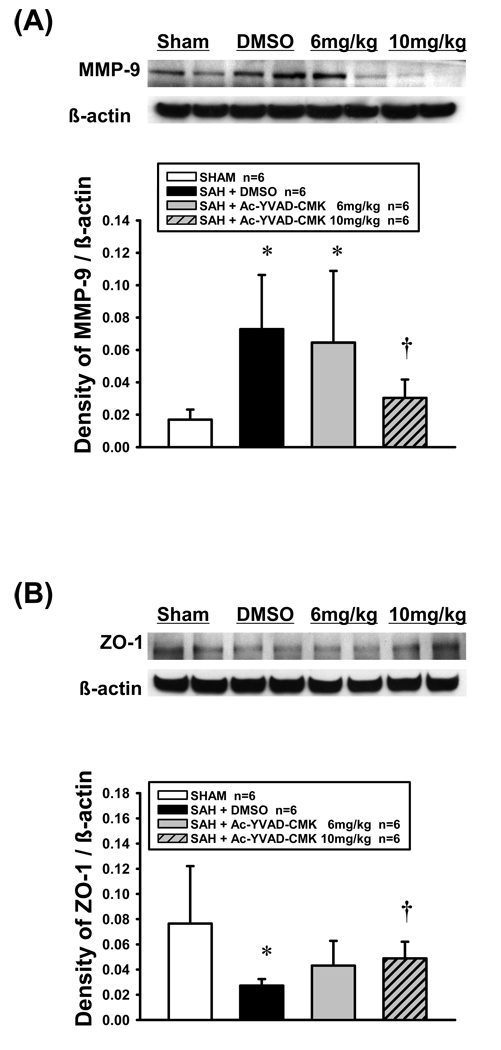
Western blot analysis showed that the levels of precursor IL-1β were significantly higher in all SAH groups than in sham group in the left cerebral hemisphere at 24 hours post-SAH (P<0.05, n=6 per group; Figure 4A). Both doses of Ac-YVAD-CMK treatment significantly reduced the levels of mature IL-1β, an active form of IL-1β, compared with vehicle group (P<0.05, n=6 per group; Figure 4B).
Figure 4.
Western blot analysis of precursor IL-1β (A) and mature IL-1β (B) in the left cerebral hemisphere 24 hours post-SAH. ANOVA, *P<0.05 vs sham group; †P<0.05 vs vehicle group.
Effect of Ac-YVAD-CMK on MMP-9 induction and ZO-1 degradation
A significant increase in MMP-9 was observed in vehicle group, and high-dose Ac-YVAD-CMK treatment significantly inhibited the MMP-9 induction compared with vehicle group (P<0.05, n=6 per group; Figure 5A). ZO-1 was significantly degraded after SAH in vehicle group compared with sham group (P<0.05), and high-dose Ac-YVAD-CMK treatment significantly reduced the degradation of ZO-1 compared with vehicle group (P<0.05, n=6 per group; Figure 5B). The MMP-9 immunoreactivities had an inverse relation with the ZO-1 immunoreactivities, and the administration of Ac-YVAD-CMK seemed to reverse these trends, with the conservation of ZO-1 immunoreactivities and marked reduction of MMP-9 immunoreactivities in the left cerebral hemisphere 24 hours post-SAH.
Figure 5.
Western blot analysis of MMP-9 (A) and ZO-1 (B) in the left cerebral hemisphere 24 hours post-SAH. ANOVA, *P<0.05 vs sham group; †P<0.05 vs vehicle group.
Activation of JNK pathway
The ratio of phosphorylated JNK to total JNK increased significantly in vehicle group compared with sham group (P<0.05) and decreased significantly in high-dose group compared with vehicle group in the left cerebral hemisphere 24 hours post-SAH (P<0.05, n=6 per group; Figure 6).
Figure 6.
Western blot analysis of phosphorylated and total JNK in the left cerebral hemisphere 24 hours post-SAH. ANOVA, *P<0.05 vs sham group; †P<0.05 vs vehicle group.
Discussion
Our study had three major findings. First, the production and cleavage of precursor IL-1β were activated in the left cerebral hemisphere 24 hours after SAH. Second, a selective caspase-1 inhibitor, Ac-YVAD-CMK, inhibited the cleavage on precursor IL-1β and attenuated the mortality, neurological impairment, BBB disruption and brain edema. Third, the protective effects of Ac-YVAD-CMK were associated with the inhibition of JNK phosphorylation, MMP-9 induction and the consequent preservation of tight junction protein ZO-1. To the best of our knowledge, this is the first report to describe the protective effects of an intraperitoneal administration of Ac-YVAD-CMK on EBI after SAH.
IL-1β, one of proinflammatory cytokines, has been reported to be a key mediator in acute CNS injuries.6 In addition, precursor IL-1β has been reported to be induced in the CSF in an acute stage of SAH in animal models17 and in a clinical setting.8 However, it has not been studied if the biologically active form, mature IL-1β that caspase-1 produces via the direct cleavage of inactive precursors of IL-1β, is induced after SAH, and if IL-1β plays a role in the pathogenesis of EBI after SAH. This study showed that mature IL-1β was up-regulated and could play a crucial role in EBI after SAH by using a selective caspase-1 inhibitor.
In this study, we examined the possible mechanisms involved in the pathogenesis of EBI by focusing on the relationship between IL-1β and MMP-9. IL-1β plays a pivotal role in the induction of MMP-9 in a variety of acute and chronic inflammatory states and conditions.18, 19 MMPs comprise a family of zinc endopeptidases that can modify several components of the extracellular matrix.20 In particular, the gelatinases MMP-2 and MMP-9 can degrade neurovascular matrix integrity. A recent study provides the direct evidence that MMPs open the BBB by degrading tight junction proteins.21 The BBB is formed by specialized brain endothelial cells that are interconnected by tight junctions. Tight junctions in the BBB are essential for maintaining the microenvironment.22 ZO-1 is a peripheral tight junction protein that is found on the epithelial and endothelial cells membrane. Loss of ZO-1 from endothelial tight junction facilitates capillary leakage and hence increases BBB permeability.23 We observed that mature IL-1β and MMP-9 were induced, and a caspase-1 inhibitor prevented an increase in MMP-9 and suppressed the degradation of ZO-1 in the mouse brain after SAH. These findings support the hypothesis that IL-1β may be a key regulator of brain MMP-9 and cause BBB disruption after SAH, and suggest that IL-1β can be a therapeutic target on post-SAH EBI treatment.
Another finding in this study is that the protective effect of Ac-YVAD-CMK was associated with the inhibition of JNK phosphorylation. It is reported that IL-1β can activate three types of mitogen activated protein kinase (MAPK), extracellular signal-regulated kinase, p38, and JNK, and that MMP-9 may be upregulated by IL-1β via the MAPK signaling pathways.24 Taken together, it is possible that IL-1β activation causes MMP-9 induction and therefore BBB disruption after SAH, at least partly via the JNK pathway. On the other hand, it is notable that both doses of Ac-YVAD-CMK suppressed the IL-1β activation, but only high-dose treatment inhibited the phosphorylation of JNK, MMP-9 induction and ZO-1 degradation, leading to the improvement of outcome. One possible explanation is that Ac-YVAD-CMK could be effective independent of its caspase-1 inhibitor activity.25, 26 Further studies are needed in this regard.
The endovascular perforation model has some weaknesses, although it mimics clinical mechanism of artery rupture and shows a high mortality and acute metabolic changes similar to clinical findings, and thus being the most attractive model for studies of EBI after SAH.3, 10, 11, 13, 27 The well-known weakness is the poorly control of bleeding volume and variable severity of SAH, which has been reported to be correlated with the severity of brain injuries.11, 27 In this study, the average SAH grading score was similar among the groups; this indicates similar injury among compared groups, and suggests that differences in outcomes are the result of different treatments. Nevertheless, the discrepancy observed in this study may be due to the poor control of severity of SAH among animals that the cerebellum and brainstem showed a significant increase in Evans blue dye extravasation but little to no edema 24 hours post-SAH (Figure 2A and 2C). The limitation of methodology of in vitro measurement of edema and BBB reported in this study requires two groups of different animals. Future in vivo MRI monitoring of brain edema and BBB function should be considered. Another weakness is a potential risk of SAH-unrelated ischemia. Because surgical procedure itself may cause the arterial obstruction, we excluded the arterial obstruction, intra-arterial thrombus, and cerebral infarction in this study.
In conclusion, preventing cleavage of precursor IL-1β to its active form by a caspase-1 inhibitor could be a new strategy to prevent or attenuate EBI after SAH via neurovascular protection. The findings in this study warrant more research.
Acknowledgement
This study is supported in part by a grant from NIH NS53407 to JHZ.
References
- 1.Schievink WI, Riedinger M, Jhutty TK, Simon P. Racial disparities in subarachnoid hemorrhage mortality: Los Angeles County, California, 1985–1998. Neuroepidemiology. 2004;23:299–305. doi: 10.1159/000080096. [DOI] [PubMed] [Google Scholar]
- 2.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 3.Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 4.Sercombe R, Dinh YR, Gomis P. Cerebrovascular inflammation following subarachnoid hemorrhage. Jpn J Pharmacol. 2002;88:227–249. doi: 10.1254/jjp.88.227. [DOI] [PubMed] [Google Scholar]
- 5.McKeating EG, Andrews PJ. Cytokines and adhesion molecules in acute brain injury. Br J Anaesth. 1998;80:77–84. doi: 10.1093/bja/80.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 7.Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–1092. [PubMed] [Google Scholar]
- 8.Kwon KY, Jeon BC. Cytokine levels in cerebrospinal fluid and delayed ischemic deficits in patients with aneurysmal subarachnoid hemorrhage. J Korean Med Sci. 2001;16:774–780. doi: 10.3346/jkms.2001.16.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1beta, interleukin-18, and the interleukin-1beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 14.Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32:2932–2938. doi: 10.1161/hs1201.099820. [DOI] [PubMed] [Google Scholar]
- 15.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rössner W, Tempel K. [Quantitative determination of the permeability of the so-called blood-brain barrier of Evans blue (T 1824)] Med Pharmacol Exp Int J Exp Med. 1966;14:169–182. [PubMed] [Google Scholar]
- 17.Iseda K, Ono S, Onoda K, Satoh M, Manabe H, Nishiguchi M, Takahashi K, Tokunaga K, Sugiu K, Date I. Antivasospastic and antiinflammatory effects of caspase inhibitor in experimental subarachnoid hemorrhage. J Neurosurg. 2007;107:128–135. doi: 10.3171/JNS-07/07/0128. [DOI] [PubMed] [Google Scholar]
- 18.Ruhul Amin AR, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: a role for the dual signaling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 19.Vecil GG, Larsen PH, Corley SM, Herx LM, Besson A, Goodyer CG, Yong VW. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J Neurosci Res. 2000;61:212–224. doi: 10.1002/1097-4547(20000715)61:2<212::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 22.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 23.Kondo T, Hafezi-Moghadam A, Thomas K, Wagner DD, Kahn CR. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood-brain barrier. Biochem Biophys Res Commun. 2004;317:315–320. doi: 10.1016/j.bbrc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 24.Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90:1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Rosenberg PA. Caspase-1 and poly (ADP-ribose) polymerase inhibitors may protect against peroxynitrite-induced neurotoxicity independent of their enzyme inhibitor activity. Eur J Neurosci. 2004;20:1727–1736. doi: 10.1111/j.1460-9568.2004.03651.x. [DOI] [PubMed] [Google Scholar]
- 26.Gray J, Haran MM, Schneider K, Vesce S, Ray AM, Owen D, White IR, Cutler P, Davis JB. Evidence that inhibition of cathepsin-B contributes to the neuroprotective properties of caspase inhibitor Tyr-Val-Ala-Asp-chloromethyl ketone. J Biol Chem. 2001;276:32750–32755. doi: 10.1074/jbc.M103150200. [DOI] [PubMed] [Google Scholar]
- 27.Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH. Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res. 2008;44:197–204. doi: 10.1111/j.1600-079X.2007.00508.x. [DOI] [PubMed] [Google Scholar]