Abstract
BACKGROUND
L-selectin ligands, localized to the luminal epithelium at the time of implantation, may support the early stages of blastocyst attachment. We have assessed the expression of two L-selectin ligands, defined by MECA-79 and HECA-452 monoclonal antibodies, and the sulfotransferase GlcNAc6ST-2, involved in generation of L-selectin ligand epitopes, in the secretory phase of the endometrium from fertile and infertile patients.
METHODS
Endometrial samples were obtained from 33 fertile, 26 PCOS, 25 endometriosis and 33 patients diagnosed with unexplained infertility. L-selectin ligands and GlcNAc6ST-2 expression was assessed by immunohistochemistry and immunoblotting.
RESULTS
Immunohistochemical staining of uterine epithelium, from fertile and infertile women, demonstrated differential expression of MECA-79 and HECA-452 epitopes. In fertile women in the secretory phase MECA-79 was more strongly expressed, particularly on the lumen, than in infertile women. HECA-452 staining was significantly stronger in the glands in PCOS and endometriosis patients than in fertile women. GlcNAc6ST-2 expression was reduced in infertile patients, correlating with MECA-79 expression.
CONCLUSIONS
This study demonstrated significant differences in expression of L-selectin ligands between fertile and infertile women in natural cycles, and could contribute to patient assessment prior to initiating fertility treatment.
Keywords: MECA-79, HECA-452, L-selectin ligands, endometrium, fertility
Introduction
Preparation of the endometrium for implantation is dependent on adequate hormonal stimulation and the presence of appropriate mediators at the endometrium–blastocyst interface. In humans, there is a distinct ‘window of implantation’ during the mid-luteal phase when the endometrium demonstrates maximal receptivity for embryo implantation (Carson et al., 2000). The implantation process of apposition, adhesion and invasion bears some similarity to leukocyte transmigration across the blood vessel wall. Numerous studies have shown that the initial interaction between leucocytes and the vascular endothelial cell surface is mediated by selectin adhesion systems (Alon and Feigelson, 2002; Rosen, 2004).
Selectins are a group of cell adhesion molecules that include P-selectin (CD62P), L-selectin (CD62L) and E-selectin (CD62E). All selectins bind with low affinity to glycans containing α2,3-linked sialic acid and α1,3-linked fucose residues such as the Sialyl Lewis x (sLex) determinant (NeuAcα2,3Galβ1,3[Fucα1,3]GlcNAcβ1-R). In addition, only P- and L-selectin bind more strongly to sulfated fucosylated glycoconjugates (Tangemann et al., 1999). Carbohydrate ligands that bind L-selectin are localized on human endometrial luminal epithelium at the time of implantation, whereas the trophoectoderm expresses L-selectin strongly after hatching (Genbacev et al., 2003). Cytotrophoblast progenitors, cytotrophoblasts in cell columns and invasive cytotrophoblast react strongly with L-selectin antibodies. Trophoblast lineages use L-selectin to bind to uterine epithelial oligosaccharide ligands; when L-selectin is blocked with specific antibodies, adhesion to the epithelium is impaired (Genbacev et al., 2003; Minas et al., 2005). This raises the possibility that selectin ligands expressed on the apical surface of the uterine epithelium support early stages of blastocyst attachment. Hatched blastocysts express L-selectin, and this molecule mediates its attachment to the luminal epithelial surface via carbohydrate ligands. The expression of L-selectin ligands has been described in the human endometrium during natural and oocyte donor cycles (Ben-Nun et al., 1992; Lai et al., 2005).
Lai and colleagues demonstrated significant differences in L-selectin ligand expression in luminal epithelium during the proliferative, early secretory and mid-secretory phase. The expression of L-selectin ligand was greatest from the periovulatory interval through mid-secretory phase. In the glandular epithelium the expression of L-selectin ligands was greatest in the mid-secretory phase (Lai et al., 2005). Immunolocalization studies on normal endometrium have demonstrated that the L-selectin carbohydrate ligand MECA-79 is up-regulated from the day of ovulation to day 6-post ovulation and is reduced throughout the follicular phase or in anovulatory cycles (Genbacev et al., 2003; Lai et al., 2005; Shamonki et al., 2006).
MECA-79 is an antibody that recognizes 6-sulfo sLex, a sulfation dependent determinant on L-selectin ligands that overlaps with the ligands’ recognition epitope (Pablos et al., 2005). The expression of GlcNAc6ST-2 (N-acetylglucosamine-6-O-sulfotransferase), a sulfotransferase localized in high endothelial venules, is essential for the elaboration of functional ligands within the lymph node as well as the generation of the MECA-79 epitope (Hiraoka et al., 1999). The role of GlcNAc6ST-2 in the generation of L-selectin ligands in lymphoid organ has been clearly established in mice genetically deficient in this enzyme (Hemmerich et al., 2001). The use of GlcNAc6ST-2 specific antibodies has demonstrated de novo induction of this enzyme in the high endothelial venules-like vessels and its correlation with the presence of MECA-79 epitope in several mouse models with chronic inflammation (Yeh et al., 2001; Bistrup et al., 2004).
HECA-452 is an anti-sLex mAb requiring both the presence of α2,3 sialylation and α1,3 fucosylation for epitope recognition (Toppila et al., 1999). Additional analyses have shown HECA-452 up-regulation, when the endometrium becomes receptive during the window of implantation (Genbacev et al., 2003).
There is limited evidence on the presence of L-selectin ligands in the endometrium from women with different pathological conditions that are associated with infertility. Our hypothesis is that L-selectin ligands play a significant role in the apposition and attachment of the embryo to the receptive endometrium during implantation. If expression of L-selectin ligands is an important factor in determining endometrial receptivity, then alteration in their expression could contribute to endometrium-related infertility. The objective of this study was therefore to evaluate the pattern of distribution and intensity of L-selectin ligands in the endometrium from both fertile and infertile women during the window of implantation.
Materials and Methods
Patients
Endometrial biopsy specimens were obtained from women during a natural menstrual cycle. All patients had menstrual cycles ranging in length from 28 to 35 days and had not received exogenous hormonal therapy for at least 2 months before the procedure. Women with systemic diseases or sexually transmitted infections were excluded from the study. The samples were examined by the same pathologists using the dating criteria described by Noyes et al. (1975). Samples with evidence of endometritis, endometrial hyperplasia and endometrial polyp were excluded from the study. The phase of the natural menstrual cycle was confirmed using ultrasound and histological criteria and by measuring plasma luteinizing hormone (LH) and progesterone. An LH urinary surge was also documented. Samples were taken around day 6 post ovulation (Cycle day 20–22). Endometrial biopsies were obtained by Pipelle endometrial sampling or by curettage (if the patients were having any surgical intervention in the form of laparoscopy or hysteroscopy). Ethical approval was obtained from the Local Research Ethics Committee at Abertawe Bro Morgannwg University Trust, Singleton Hospital. Formal written consent was obtained from all patients at the time of recruitment into the study.
The study groups consisted of infertile women with either endometriosis, PCOS or unexplained infertility (UI). Endometriosis was diagnosed at laparoscopy during investigation of pelvic pain or infertility. The diagnosis was confirmed by visual inspection of the pelvic organs or histological confirmation of endometriotic peritoneal lesions. PCOS was diagnosed based on the Rotterdam criteria of ultrasound features and clinical and biochemical features of hyperandrogenism (Welt et al., 2006). The UI group included women that were unable to conceive after 2 years with routine investigations of fertility showing no abnormality. The control group was formed by women with proven fertility and regular menstrual cycles (volunteers or patients having surgery for other than fertility investigations). The patients in the two groups were matched with regard to age, BMI and smoking habits.
Antibodies
Purified rat anti-mouse peripheral lymph node addressin (PNAd) carbohydrate epitope monoclonal antibody (MECA-79; BD Pharmingen) and purified rat anti-Human Cutaneous Lymphocyte Antigen (CLA) monoclonal antibody (HECA-452; BD Pharmingen) were used to determine the expression of L-selectin ligands. The MECA-79 antibody reacts with sulfate-dependent carbohydrate epitopes of PNAd (Hemmerich et al., 1994). The MECA-79 reactive antigen is closely associated with the carbohydrate ligands for L-selectin (e.g. CD34, GlyCAM-1 and MadCAM-1) (Toppila et al., 1999). The HECA-452 antibody reacts with CLA, a carbohydrate domain shared by sLex and sialyl Lewis a (sLea) antigens (Duijvestijn et al., 1988; Picker et al., 1991; Bos et al., 1993). A polyclonal antibody against human GlcNAc6ST-2 was kindly provided by Professor Steven D. Rosen (University of California, San Francisco, CA, USA).
Immunohistochemical detection
The samples were fixed in 10% buffered formaldehyde for 24 h, embedded in paraffin and 3–4 µm thick sections prepared on positively charged slides before immunohistochemical (IHC) studies. The sections were de-waxed with xylene and then descending grades of methanol and distilled water. The tissue sections were incubated with purified rat antihuman L-selectin ligand monoclonal antibodies at concentration 5 µg/ml (HECA-452; and MECA-79; BD Pharmingen). A positive control section of tonsil and a negative (no antibody) control section of endometrium tissue were used. For antigen retrieval, the slides were incubated in CC1 buffer (Ventana) for an hour on heated plates at 100°C on a Benchmark XT processor. Primary antibody incubation was for 32 min at dilution 1:30 at 37°C. Positive immunostaining was detected through interaction of avidin–biotin peroxidase complex with biotin conjugated secondary antibody using a Ventana I View DAB detection kit (Ventana BioTek Solutions, Tucson, AZ, USA). The slides were subsequently counter stained with haematoxylin, dehydrated, cleared and mounted in DPX mountant to be examined under light microscopy. We used an IHC scoring system, in which the same observers examined all the IHC sections using a multi headed microscope (Lai et al., 2005). The observers were blinded to the patients’ diagnosis, demographics and timing in the cycle of endometrial biopsy.
The endometrial epithelium was assessed separately for the lumen and glands and scored for intensity and distribution of staining. The intensity of staining was scored from (0)-absent to (4)-strong. The distribution of staining was defined as follows: (0) = absent, (1) = <30%, (2) = 30 to 60%, (3) = >60% and (4) = 100% of the tissue surface stained.
Epithelial cells isolation and treatment
For isolation of epithelial cells, biopsy samples were collected in Dulbecco's modified eagle media (DMEM) containing antibiotic–antimycotic solution. The tissues were washed twice in DMEM, finely minced, and enzymatically digested with collagenase (134 U/ml) and deoxyribonuclease type I (156 U/ml) for 1 h at 37°C. After centrifugation at 400 × g for 4 min, the pellet was resuspended in maintenance medium of DMEM, 10% dextran-coated charcoal-treated FBS (DCC-FBS), 2 mM L-glutamine and 1% antibiotic–antimycotic solution (Invitrogen, UK). Epithelial cells were separated from stromal cells on the basis of their differential attachment to the culture plate. Total protein was isolated from confluent monolayers to analyze expression of MECA-79 and sulfotransferase GlcNAc6ST-2.
Western blot analysis
Protein quantification was by Bradford assay (Sigma, UK) and 20 µg of total protein was loaded and electrophoresed on a 10% sodium dodecyl sulphate–polyacrylamide gel. Separated proteins were electrotransferred onto activated polyvinylidene fluoride membrane (Bio-Rad, UK) with transfer buffer (50 mM Tris–HCl, 95 mM glycine and ethanol) for 1 h at 100 V. Membranes were blocked overnight with 5% bovine serum albumin (BSA) diluted in 0.1% Tween-20-Tris buffered saline (TTBS, blocking buffer) for MECA-79 detection or in 10% skimmed milk, in 0.1% TTBS for GlcNAc6ST-2 expression. Membranes were subsequently incubated 1 h at 4°C with rat anti-MECA-79 monoclonal antibody diluted 1/1000 in 5% BSA-TTBS or with rabbit anti-GlcNAc-GST2 antibody diluted 1/1000 in 5% BSA-TTBS buffer. After washing five times with TTBS, blots were incubated for 1 h at room temperature with the appropriated secondary antibody coupled to horseradish peroxidase. Between the various incubation steps, the membranes were washed several times with TTBS. The above samples were processed in parallel using a rabbit anti-GAPDH antibody (Santa Cruz Biotech) and the appropriated secondary antibody in order to normalize the protein load on each well.
Protein bands were visualized using a ChemiDoc System Bio-Rad Imager (Bio-Rad) and quantified by Quantity One® Imaging software (Bio-Rad) as a function of volume data (intensity/mm2). The Volume Rectangle Tool was used to measure the total signal intensity inside a boundary drawn around the bands without overlapping adjacent bands. Background was subtracted from each band volume by using local background subtraction. Intensities of bands acquired from each protein extract were normalized against corresponding values for bands of the house-keeping protein GAPDH and for comparative purposes expressed as normalized volume. Results were expressed as percentage of normalized volume with respect to the value of the fertile untreated group (100%).
Statistical analysis
Data distributions were assessed for normality using the Ryan Joiner and Kolmogorov Smirnov tests. A non-parametric Kruskal–Wallis test was applied to determine significant differences in the median between groups (having data that was not normally distributed) for immunohistochemistry scores and the percentage of normalized volume (immunoblot). A Mann–Whitney U-test was then applied in a post hoc manner to determine significant differences between specific group pairs for median scores (immunohistochemistry) and the percentage of normalized volume (immunoblot) between, mainly fertile versus study groups. For data with a normal distribution, an ANOVA test followed by an unpaired t-test was used (Table I) to determine significant differences between groups. The test statistic and corresponding P-value were reported. All data analysis was performed using Statistical Package for the Social Sciences version 10.0 (SPSS, Chicago, IL, USA).
Table I.
Comparison of patients' demographics
| Fertile | Endometriosis | PCOS | UI | |
|---|---|---|---|---|
| AGE | 28.40 ± 4.76 | 27.02 ± 3.03 | 28.91 ± 3.02 | 28.10 ± 3.78 |
| P = 0.78 | P = 0.85 | P = 0.905 | ||
| BMI (kg/m2) | 25.83 ± 6.75 | 24.30 ± 3.4 | 27.36 ± 4.87 | 25.63 ± 4.3 |
| P = 0.9 | P = 0.7 | P = 0.96 |
Note: Values are mean ± standard deviation. UI, unexplained infertility.
Results
Clinical data and patients demographics
One hundred and thirty-one (131) patients were enrolled into this study and endometrial samples were obtained from 117 patients in the secretory phase of the cycle at LH + 6. Samples were classified into four groups: fertile (n = 33), ovulatory PCOS (n = 26), endometriosis (n = 25), UI (n = 33). There were no statistically significant differences in the mean age (P = 0.934) or BMI (P = 0.669) in the samples or between the fertile and infertile patients (Table I).
IHC localization of MECA-79 in fertile and infertile patients
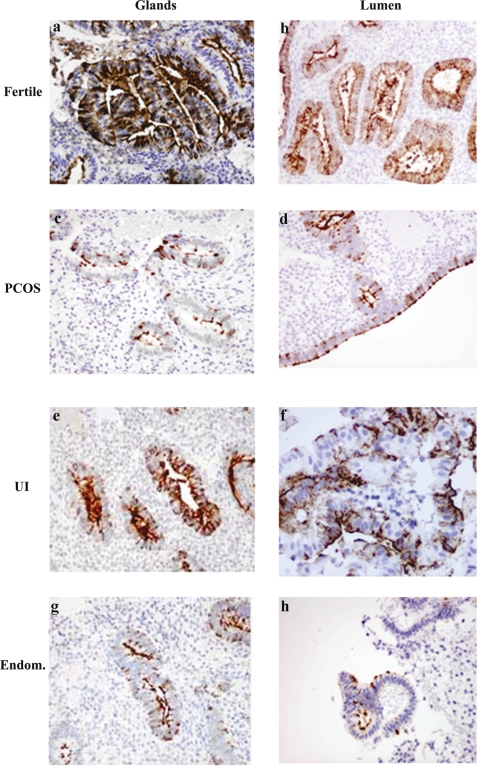
Antibody reactivity localized at the luminal and glandular epithelium was assessed by the intensity and distribution of the staining. MECA-79 was localized to the epithelium, with no visible expression in the stroma, and was observed predominantly at the cell membrane with little cytoplasmic staining (Fig. 1).
Figure 1.
Expression of MECA-79 in glands and lumen in the secretory phase endometrium.
Secretory phase endometrium from fertile (33 patients), endometriosis (endom) (25 patients), PCOS (26 patients) and unexplained infertility (UI) patients (33 patients) was analyzed for the expression of MECA-79 as described in Materials and Methods. In fertile women MECA-79 was strongly expressed on the luminal epithelium and the glands compared with endometriosis (endom.), PCOS and UI . Magnifications ×400 (a, d, e and f), ×200 (b, c, g and h). Statistical analysis of the immunohistochemistry scores is showed in Fig. 3A.
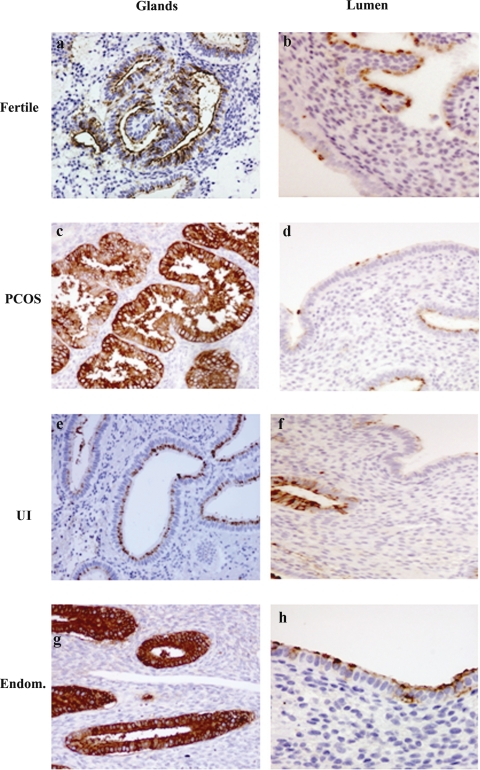
Figure 2.
Expression of HECA-452 in glands and lumen in the secretory phase endometrium.
Secretory phase endometrium from fertile (33 patients), endometriosis (endom) (25 patients), PCOS (26 patients) and unexplained infertility (UI) patients (33 patients) was analyzed for the expression of HECA 452 as described in Materials and Methods. In endometriosis (endom.) and PCOS the staining in the glands was significantly stronger that in the fertile group. The UI group did not present any significant difference in staining of the glands compared with the fertile group (see Fig. 3B). Magnifications ×400 (b, h), ×200 (a, c, d, e, f and g). Statistical analysis of the immunohistochemistry scores is showed in Fig. 3B.
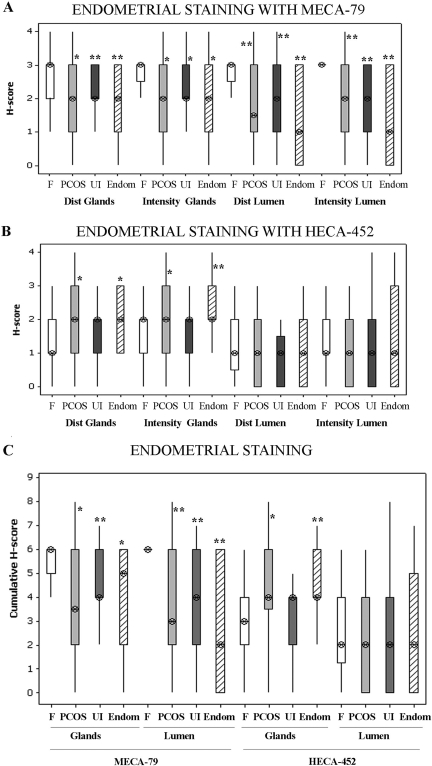
MECA-79 distribution of staining in both the glandular and luminal epithelium was significantly different between groups (H = 10.84, P = 0.013 glands; H = 19.60, P = 0.000 lumen). In fact MECA-79 distribution of staining was significantly greater in fertile patients than in patients with PCOS (P = 0.012 glands, and P = 0.002 lumen), UI (P = 0.007 glands, and P = 0.002 lumen) and endometriosis (P = 0.0007 glands, and P = 0.000 lumen) (Figs 1 and 3A). Similarly MECA-79 intensity of staining in the glandular and luminal epithelium was significantly different between groups (H = 8.34, P = 0.040 glands; H = 24.75, P = 0.000 lumen). When comparing the fertile versus the infertile groups, MECA-79 staining was significantly higher in fertile patients than in patients with PCOS (P = 0.0192 glands, and P = 0.001 lumen), UI (P = 0.010 glands, and P = 0.0002 lumen) and endometriosis (P = 0.010 glands, and P = 0.000 lumen) (Figs 1 and 3A). MECA-79 intensity of staining in the fertile luminal epithelium was preponderantly strong with a minimum variation from the median, resulting in an inter-quartile range value of zero for individual and the cumulative H-scores (Fig. 3A and C). There was no statistically significant difference in MECA-79 expression between PCOS, UI and endometriosis study groups (Figs 1 and 3A).
Figure 3.
MECA-79 and HECA-452 expression, intensity and distribution of staining in lumen and glands.
(A) and (B) Intensity and distribution scores for staining of HECA and MECA antibodies in endometrium of fertile and study groups are plotted. (C) Cumulative scores for each antibody and groups are plotted. Values are median (⊗) and inter-quartile range (box and whisker). The Kruskal–Wallis test followed by a Mann–Whitney test was performed to determine first statistical significance between groups and secondly to compare specific group pairs. *P ≤ 0.05 and **P ≤ 0.01 are considered significant and are compared with fertile patients. Fertile (white box, n= 33 patients), PCOS (grey box, n = 26 patients), UI (Dark grey box, n = 33 patients), Endometriosis (Endom, hatched box, n = 25 patients).
IHC localization of HECA-452 in fertile and infertile patients
A different pattern of staining was observed using HECA-452 antibody compared with MECA-79. HECA-452 staining was found to be stronger at the apical membrane of the cells than at the cytoplasmic level (Fig. 2). In the glandular epithelium, HECA-452 intensity and distribution of staining was significantly different (H = 11.84, P = 0.008 intensity; H = 10.43, P = 0.015 distribution). There were significant increases in distribution (P = 0.010) and intensity (P = 0.007) of staining in the glands in the endometriosis compared with fertile patients (Figs. 2 and 3B). Similarly, in the glandular epithelium, there was a significant increase in HECA-452 distribution (P = 0.010) and intensity (P = 0.040) of staining in women with PCOS compared with fertile patients (Figs. 2 and 3B). There was no significant difference in the intensity or distribution scores in the glands in women with UI compared with fertiles (Figs. 2 and 3B).
In the luminal epithelium, no significant differences were observed in the intensity or distribution scores between the samples (H = 0.71, P = 0.872 intensity; H = 1.47, P = 0.688 distribution).
Moreover, the scores for HECA-452 staining and distribution of staining in the luminal epithelium were not significantly different when specific pair groups were compared (Figs. 2 and 3B). In endometriosis and PCOS, but not in UI patients, significantly higher scores for distribution (P = 0.013 and P = 0.004, respectively) and intensity (P = 0.02 and P = 0.010, respectively) in the glands compared with the lumen were observed.
Comparison between luminal and glandular expression of L-selectin ligands
Comparative analysis of the cumulative scores (distribution plus intensity scores) for MECA-79 indicated significant differences between groups (H = 9.78, P = 0.021 glands; H = 21.58, P = 0.000 lumen) (Fig. 3C). However, no significant differences were observed between glands and lumen in each group. The cumulative scores showed that MECA-79 was more strongly represented in the glands and lumen of fertile patients than in PCOS (P = 0.020 glands, P = 0.002 lumen), endometriosis (P = 0.0123 glands, P = 0.000 lumen) and UI patients (P = 0.005 glands, P = 0.001 lumen) (Fig. 3C).
The cumulative scores indicated that HECA-452 was significantly different in the glands (H = 12.73; P = 0.005) between groups. HECA-452 staining was more strongly represented in the glands in PCOS (P = 0.020) and endometriosis (P = 0.002) than in fertile patients (Fig. 3C). On the contrary, no significant differences in the cumulative scores were found between UI and fertile patients in the glandular epithelium stained with HECA-452 antibody. There were no statistically significant differences between the cumulative scores in the lumen (H = 1.08, P = 0.782) for HECA staining between groups (Fig. 3C).
In the UI group, the cumulative score of staining for HECA-452 in the glands was not significantly higher compared with the lumen. In the fertile group, there was no significant difference in the cumulative score between the glands and the lumen (Fig. 3C).
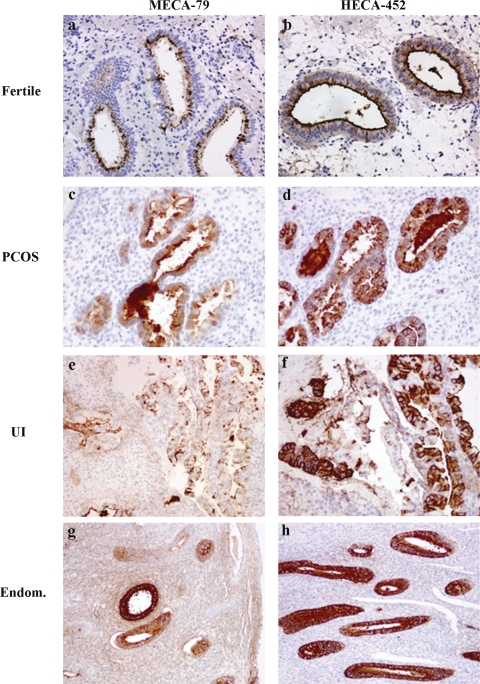
Reciprocal relation between expression of MECA-79 and HECA-452
In individual cases an apparent reciprocal relationship between MECA-79 and HECA-452 staining was observed for the fertile and study groups (Fig. 4). When analyzing the fertile group versus any of the study groups, a stronger staining for MECA-79 and lower staining of HECA-452 was observed in fertile samples. The endometrium of PCOS, UI and endometriosis groups showed a strong staining for HECA-452 and a low staining for MECA-79 when compared with the staining of fertile samples.
Figure 4.
Relation between the staining with HECA-452 and MECA-79 in endometrium of fertile and infertile women.
Same regions were compared for staining with HECA-452 and MECA-79 for all groups. In all cases a reciprocal relation between staining with MECA-79 and HECA-452 was observed regardless of the fertility status of the patients. Fertile (n = 33 patients), PCOS (n = 26 patients), UI (n = 33 patients), Endometriosis (Endom, n = 25 patients). Magnifications ×200 (a, b, c, d and f) ×100 (e, g and h).
Analysis of MECA-79 and GlcNAc6ST-2 expression in endometrial epithelial cells isolated from biopsies
The results for MECA-79 above suggested that sulfation of sLex decorated L-selectin ligands may be impaired in infertile patients. To explore this possibility MECA-79 epitopes were investigate in endometrial cells in vitro and compared with expression of the sulfotransferase GlcNAc6ST-2 involved in MECA-79 epitope formation.
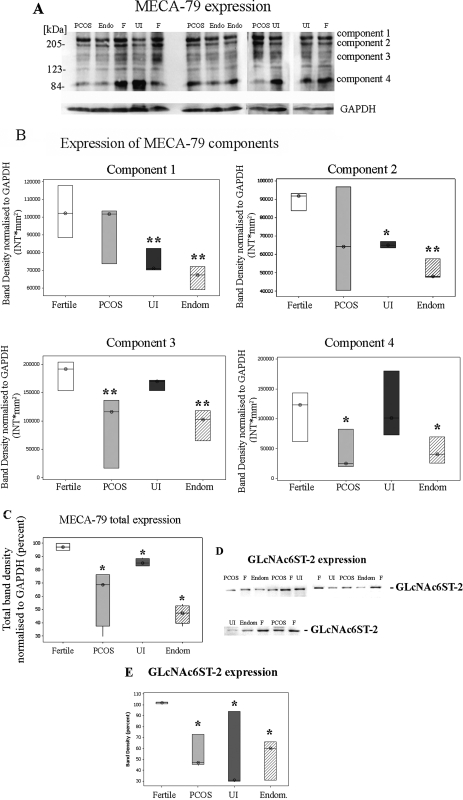
Total protein was isolated from confluent epithelial cells, isolated from endometrial biopsies of fertile and infertile patients, and MECA-79 expression detected by western blotting. Immunoreactivity of MECA-79 was not restricted to a single band but was detected in protein species of multiple molecular weights (Fig. 5A). This pattern can be explained due to the expression of MECA-79 epitope in several proteins in the cells such as mucins and CD34 (Genbacev et al., 2003; Wang et al., 2008). A strong immunoreactivity was found in four areas of the membrane previously described by Wang and colleagues as component 1 (more than 200 kDa), 2 (∼200 kDa), 3 (∼120–170 kDa) and 4 (∼85 kDa). Therefore, when analyzing MECA-79 expression, all bands represented in these specific areas were quantified using a rectangle tool (Fig. 5B) as well as the total band densities (sum of all components) (Fig. 5C) between fertile and study groups.
Figure 5.
Expression of MECA-79 and sulfotransferase (GlcNAc-GST2) in epithelial cells isolated from endometrial biopsies.
(A) and (D) Protein extracts of epithelial cells isolated from biopsies obtained from fertile (F, n = 6), endometriosis (Endom or Endo, n = 6), PCOS (n = 6) and UI (n = 6) patients were analyzed for the expression of MECA-79 (A) and GlcNAc-GST2 (D) as described in Materials and Methods. Protein levels were normalized to GAPDH protein levels (A). In fertile (F) women MECA-79 as well as GlcNAc-GST2 was strongly expressed compared with endometriosis (endom.), PCOS and UI. Representative blots are showed. (B) Densitometric analysis of each MECA-79 component was performed using the Quantity One software (Bio-Rad). Values are band density normalized to GAPDH and are expressed as intensity per square milimiters (INT*mm2). Values are expressed as median (⊗) and inter-quartile range (box and whisker). Fertile (white box), PCOS (grey box), UI (Dark grey box), Endometriosis (Endom, hatched box). (C) Densitometric analysis of the area covering all MECA-79 components was perfomed using the Quantity One software (Bio-Rad). Values are band density normalized to GAPDH and expressed as Intensity per square milimiters (INT*mm2). Values as expressed as median (⊗) and inter-quartile range (box and whisker). Fertile (white box), PCOS (grey box), UI (Dark grey box), Endometriosis (Endom, hatched box). (E) Densitometry analysis of the immunoblots GlcNAc-GST2 levels was performed using the Quantity One software (Bio-Rad). Values obtained are expressed as band density compared with fertile patients and are expressed as percent. Values are median (⊗) and inter-quartile range (box and whisker). Fertile (white box), PCOS (grey box), UI (Dark grey box), Endometriosis (Endom, hatched box). Statistical analysis of the samples (B, C and E) was performed using the Kruskal–Wallis test to determine significance between groups followed by a Mann–Whitney test to compare specific group pairs. *P ≤ 0.05 and **P ≤ 0.01 are considered significant.
Statistically significant differences were observed after applying a Kruskall–Wallis test to the data for each MECA-79 component (H = 16.89, P = 0.001 component 1; H = 11.85, P = 0.008 component 2; H = 18.07, P = 0.000 component 3; H = 8.05, P = 0.045 component 4). As shown in Fig. 5A and B, a statistically significant increase in component 1 and 2 of MECA-79 was observed in fertile patients compared with endometriosis (P = 0.0046 component 1; P = 0.004 component 2) and UI (P = 0.0046 component 1; P = 0.041 component 2) patients. However, no significant differences were detected in components 1 and 2 between fertile and PCOS (P = 0.3734 component 1; P = 0.3197 component 2) patients (Fig. 5B). A significant increase in component 3 and 4 was detected in fertile patients compared with endometriosis (P = 0.0051 component 3; P = 0.0289 component 4) and PCOS (P = 0.0014 component 3; P = 0.0289 component 4) patients (Fig. 5B). No significant differences in components 3 and 4 were observed between fertile and UI (P = 0.1242 component 3; P = 0.8082 component 4) patients (Fig. 5B).
Furthermore statistically significant differences were observed when the total band density corresponding to MECA-79 expression was compared between groups (H = 13.03, P = 0.005). Distinctively MECA-79 total band density was significantly lower in PCOS (P = 0.0304), endometriosis (P = 0.030) and UI patients (P = 0.050) than in fertile patients (Fig. 5C). These results confirmed the immunohistochemistry findings of a reduction on MECA-79 expression in the infertile groups compared with the fertile group (Figs. 1 and 5A).
The expression of GlcNAc6ST-2 was also significantly different between groups (H = 10.11, P = 0.018). Specifically expression of GlcNAc6ST-2 was significantly lower in samples from PCOS (P = 0.0112), UI (P = 0.0347) and endometriosis (P = 0.0369) cultures compared with the fertile group (Fig. 5D and E).
Discussion
During the secretory phase of the menstrual cycle, the endometrium completes its differentiation in preparation for implantation. Several candidate molecules have been suggested to be involved in this process and included those located in the luminal epithelium suggested to be involved in embryo-endometrium apposition, adhesion and attachment in the early stages of establishment of pregnancy (McEver, 2002). Previous studies have investigated the role of L-selectin ligands during the menstrual cycle and in early implantation (Horne et al., 2002; Lai et al., 2005). L-selectin binds with low affinity sLex decorated ligands and sulfation of sLex -terminated glycans on the 6-position of GlcNAc activates the epitope as a high affinity ligand for L-selectin (Hemmerich et al., 1995; McEver, 2002). We have observed an increase in HECA-452 epitopes in glandular epithelium of infertile patients diagnosed with endometriosis and PCOS. Implantation in these groups may be impaired because the abundance of low affinity ligands predominates over the high affinity (sulfated) ligands.
Also reciprocal expression between MECA-79 and HECA-452 was observed in some of the patients from all groups: fertile and infertile women. Previous studies showed that in fertile patients abundance of HECA-452 and MECA-79 epitopes increases from proliferative to secretory phase (Lai et al., 2005). The results described here indicate the expression of MECA-79 in fertile patients increases the most from proliferative to secretory phase endometrium supporting the role of sulfated carbohydrate ligands as the major ligands for L-selectin in the uterus (Margarit and White, unpublished data and Fig. 3C).
Accordingly MECA-79 epitopes were expressed at a higher level in fertile than in infertile patients diagnosed with PCOS, endometriosis or UI, supporting a role of high affinity interactions between L-selectins and its ligands in implantation. Moreover, we have shown that the expression pattern of sulfated O-glycosylated proteins recognized by MECA-79 antibody also differs between these groups. Interestingly, patients with endometriosis expressed a significantly lower level of all major components of MECA-79, whereas PCOS and UI patients differ from fertile patients in lower (components 3 and 4) or higher (components 1 and 2) molecular weight components, respectively. A previous study demonstrated significant reduction in the expression of L-selectin ligands in a group of patients with repeat implantation failure (subgroup of couples that suffer from UI) during estradiol/progesterone stimulated cycles (Foulk et al., 2007). Demonstration of reduction in MECA-79 in patients with UI during normal, non-stimulated cycles eliminates any potential effects of the ovulation induction regimen.
Recent studies confirmed that sLea, sLex and 6-sulfo sLex are expressed in the epithelial cells but not the endothelial cells of human endometrium (Eggens et al., 1989; Simon and Valbuena, 1999; Foulk et al., 2007). Optimal binding to L-selectin requires sulfation, sialylation and fucosylation of ligands. Analysis of GlyCAM-1 has revealed two sulfation modifications (galactose [Gal]-6-sulfate and N-acetylglucosamine [GlcNAc]-6-sulfate) of sLex (Tangemann et al., 1999; Yamaguchi et al., 2006). In this study, we have shown that expression of GlcNAc6ST-2 is decreased in infertile patients when compared with fertile, which correlates with the decrease in MECA-79 expression. This data is in agreement with that of Kao and colleagues, who described down-regulation of GlcNAc-6ST gene expression in endometriosis patients (Kao et al., 2003). Further studies to investigate potential correlation between other enzymes which modify L-selectin ligands will reveal if GlcNAc6ST-2 is uniquely down-regulated in association with endometrial infertility.
This is, to our knowledge, the first report of significant differences between the expression of L-selectin ligands between fertile and infertile women diagnosed with PCOS, endometriosis and UI in natural cycles. This study highlights the potential importance of the relative abundance of L-selectin ligands and in particular the specific MECA-79 component, present in endometrial tissue. During the secretory phase, expression of MECA-79 and HECA-452 in the endometrium was related to the fertility status. Hence the pattern of expression of L-selectin ligands may be a factor that contributes to endometrium-related infertility through control of high and low affinity interactions with embryo trophoblast.
Funding
We wish to acknowledge the Welsh Assembly Government (JOW) and Mr Peter Bowen-Simpkins via the Fertility Research and Development Fund (LM, LJ) for their financial support.
Acknowledgements
We thank Steven Rosen for donating the GlcNAc6ST-2 antibody for the study, the nursing staff at ABM Trust for help with sample collection and the members of the Reproductive Biology group for helpful discussions.
References
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Ben-Nun I, Jaffe R, Fejgin MD, Beyth Y. Therapeutic maturation of endometrium in in vitro fertilization and embryo transfer. Fertil Steril. 1992;57:953–962. doi: 10.1016/s0015-0282(16)55008-0. [DOI] [PubMed] [Google Scholar]
- Bistrup A, Tsay D, Shenoy P, Singer MS, Bangia N, Luther SA, Cyster JG, Ruddle NH, Rosen SD. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 Epitope. Am J Pathol. 2004;164:1635–1644. doi: 10.1016/S0002-9440(10)63722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J, de Boer O, Tibosch E, Das P, Pals S. Skin-homing T lymphocytes: detection of cutaneous lymphocyte-associated antigen (CLA) by HECA-452 in normal human skin. Arch Dermatol Res. 1993;285:179–183. doi: 10.1007/BF00372006. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A, Horst E, Pals S, Rouse B, Steere A, Picker L, Meijer C, Butcher E. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- Eggens I, Fenderson B, Toyokuni T, Dean B, Stroud M, Hakomori S. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem. 1989;264:9476–9484. [PubMed] [Google Scholar]
- Foulk R, Zdravkovic T, Genbacev O, Prakobphol A. Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. J Assist Reprod Genet. 2007;24:316–321. doi: 10.1007/s10815-007-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang Z-Q, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-Selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Butcher E, Rosen S. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–22126. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in glycam-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Bistrup A, Singer MS, van Zante A, Lee JK, Tsay D, Peters M, Carminati JL, Brennan TJ, Carver-Moore K, et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Suzuki M, Yeh J-C, Izawa D, Tanaka T, Miyasaka M, Lowe JB, et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo Sialyl Lewisx, an L-selectin ligand displayed by CD34. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- Horne AW, White JO, Lalani e-N. Adhesion molecules and the normal endometrium. BJOG. 2002;109:610–617. doi: 10.1111/j.1471-0528.2002.t01-1-01017.x. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Lai T-H, Shih I-M, Vlahos N, Ho C-L, Wallach E, Zhao Y. Differential expression of L-selectin ligand in the endometrium during the menstrual cycle. Fertil Steril. 2005;83:1297–1302. doi: 10.1016/j.fertnstert.2004.11.040. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- Minas V, Loutradis D, Makrigiannakis A. Factors controlling blastocyst implantation. Reprod Biomed Online. 2005;10:205–216. doi: 10.1016/s1472-6483(10)60942-x. [DOI] [PubMed] [Google Scholar]
- Noyes R, Hertig A, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Pablos J, Santiago B, Tsay D, Singer M, Palao G, Galindo M, Rosen A. HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells. BMC Immunol. 2005;6:6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Warnock RA, Burns AR, Doerschuk CM, Berg EL, Butchert EC. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-Selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Shamonki MI, Kligman I, Shamonki JM, Schattman GL, Hyjek E, Spandorfer SD, Zaninovic N, Rosenwaks Z. Immunohistochemical expression of endometrial L-selectin ligand is higher in donor oocyte recipients with embryonic implantation. Fertil Steril. 2006;86:1365–1375. doi: 10.1016/j.fertnstert.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Simon C, Valbuena D. Embryonic implantation. Ann Endocrinol (Paris) 1999;60:134–136. [PubMed] [Google Scholar]
- Tangemann K, Bistrup A, Hemmerich S, Rosen SD. Sulfation of a high endothelial venule–expressed ligand for L-selectin: effects on tethering and rolling of lymphocytes. J Exp Med. 1999;190:935–942. doi: 10.1084/jem.190.7.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppila S, Paavonen T, Nieminen MS, Häyry P, Renkonen R. Endothelial L-selectin ligands are likely to recruit lymphocytes into rejecting human heart transplants. Am J Pathol. 1999;155:1303–1310. doi: 10.1016/S0002-9440(10)65232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF. High expression of L-selectin ligands in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol. 2008;60:127–134. doi: 10.1111/j.1600-0897.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, Ingadottir G, Crowley WF. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam Criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91:4842–4848. doi: 10.1210/jc.2006-1327. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kitaya K, Daikoku N, Yasuo T, Fushiki S, Honjo H. Potential selectin L ligands involved in selective recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. Biol Reprod. 2006;74:35–40. doi: 10.1095/biolreprod.105.045971. [DOI] [PubMed] [Google Scholar]
- Yeh J, Hiraoka N, Petryniak B, Nakayama J, Ellies L, Rabuka D, Hindsgaul O, Marth J, Lowe J, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]