Abstract
BACKGROUND
Recently, we demonstrated that single blastomeres of a 4-cell stage human embryo are able to develop into blastocysts with inner cell mass and trophectoderm. To further investigate potency at the 4-cell stage, we aimed to derive pluripotent human embryonic stem cells (hESC) from single blastomeres.
METHODS
Four 4-cell stage embryos were split on Day 2 of preimplantation development and the 16 blastomeres were individually cultured in sequential medium. On Day 3 or 4, the blastomere-derived embryos were plated on inactivated mouse embryonic fibroblasts (MEFs).
RESULTS
Ten out of sixteen blastomere-derived morulae attached to the MEFs, and two produced an outgrowth. They were mechanically passaged onto fresh MEFs as described for blastocyst ICM-derived hESC, and shown to express the typical stemness markers by immunocytochemistry and/or RT–PCR. In vivo pluripotency was confirmed by the presence of all three germ layers in the teratoma obtained after injection in immunodeficient mice. The first hESC line displays a mosaic normal/abnormal 46, XX, dup(7)(q33qter), del(18)(q23qter) karyotype. The second hESC line displays a normal 46, XY karyotype.
CONCLUSION
We report the successful derivation and characterization of two hESC lines from single blastomeres of four split 4-cell stage human embryos. These two hESC lines were derived from distinct embryos, proving that at least one of the 4-cell stage blastomeres is pluripotent.
Keywords: human embryonic stem cell line, single blastomere, morula, 4-cell stage human embryo
Introduction
Totipotency is defined as the capacity of a single cell to produce fertile offspring (Tarkowski et al., 2001). Totipotency is lost when the cell becomes restricted in differentiation capacity. Subsequently, this cell becomes pluripotent, unipotent and finally differentiates into a specialized cell. The totipotent cell is -on its own- able to divide and produce the extra-embryonic tissues necessary for implantation and foetal development, and all the differentiated cells composing a new organism including germ cells. The ultimate totipotent cell is the zygote. During cleavage divisions, the zygote divides into blastomeres that appear morphologically identical. The first sign of developmental restriction becomes apparent with the segregation of the two distinct cell lineages during blastocyst formation: the trophectoderm (TE) and the inner cell mass (ICM). How and when blastomeres lose their totipotent competence during preimplantation development is still unknown. In animal models, the potency of single blastomere-derived embryos can be defined by transferring them into the uterus of foster animals. In mice offspring have been obtained from split 2-cell stage embryos. Split 4-cell stage murine embryos implant but do not develop to term, most likely because of lack in embryo mass. Proof that at least two cells of a 4-cell stage mouse embryo are totipotent was provided after aggregation of diploid 4-cell stage blastomeres with tetraploid carrier blastomeres resulting in the production of a fertile diploid twin (reviewed in Tarkowski et al., 2001). To our knowledge, the bovine is the only example in which all four blastomeres of a single embryo developed into live offspring (a monozygotic quadruplet) (Johnson et al. 1995).
Recently, we have shown that the sister blastomeres of a 4-cell stage human embryo are plastic and have the capacity to develop in vitro into blastocysts with ICM and TE (Van de Velde et al., 2008). Our hypothesis is that the 4-cell stage human blastomeres are equal and totipotent. In the human, totipotency of cleavage-stage blastomeres cannot be proven because cloning experiments are ethically unacceptable. Therefore, we consider the 4-cell stage blastomeres to be ‘potentially totipotent’. Evidence that at least one single blastomere of a 4-cell human embryo is totipotent was provided by the birth of a healthy baby after the transfer of a frozen-thawed embryo of which only one out of four blastomeres was intact (Veiga et al., 1987;Veiga, personal communication).
To further evaluate the potency of the blastomeres at the 4-cell stage, we aimed to derive human embryonic stem cell (hESC) lines from the four sister blastomeres. Human ICM cells and ICM-derived embryonic stem cell lines are considered to be pluripotent. HESC have the capacity to differentiate into cell types representing the three embryonic germ layers both in vitro (i.e. in embryoid bodies) and in vivo (i.e. in teratomas formed in immunodeficient mice) (Thomson et al., 1998; Reubinoff et al., 2000). They also have the capacity to differentiate in vitro into specialized cells, including germ cells, extra-embryonic tissue and trophoblast cells (Xu et al. 2002; Hyslop et al. 2005). Therefore, the capacity of a single blastomere to develop into a hESC line could be used to define the pluripotency of that cell. We report here the derivation of two hESC lines from single blastomeres of distinct 4-cell stage human embryos.
Materials and Methods
Derivation and culture
With the approval of the Belgian Federal Committee on medical and scientific research on embryos in vitro and the Local Ethical Committee, in vitro fertilized embryos were created. Oocytes of three consenting patients (age 26, 35 and 37, respectively) were denuded and injected with spermatozoa from a consenting donor on the day of oocyte pick up (OPU). Fertilization was assessed 16–22 h after injection. On Day 2 after OPU, four top quality 4-cell stage embryos with equally-sized blastomeres and less than 10% fragmentation (estimated as a proportion of the total embryo volume) were split. The 16 single blastomeres were cultured individually as described earlier, but in contrast to our earlier work, without zona pellucida (Van de Velde et al., 2008). On Day 3 or 4 (Table I) after OPU, the resulting 16 blastomere-derived embryos were washed, plated in individual wells of a 24-well dish coated with mitomycin C-inactivated CF1 mouse embryonic fibroblasts (MEFs) and cultured in hESC medium as described previously (Mateizel et al., 2006). Whenever an outgrowth was present, it was mechanically passaged and cultured as described for blastocyst ICM-derived hESC. A modified vitrification protocol was used for cryopreservation (Reubinoff et al., 2001; Mateizel et al., 2006).
Table I.
Overview of the embryos used in this study.
| Original embryo | Oocyte donor age | Embryo | Developmental stage before plating | Plated | Attached | Outgrowth | hESC line |
|---|---|---|---|---|---|---|---|
| 1 | 35 | 1.1 | compacted | + | − | − | − |
| 1.2 | compacted | lost | − | − | − | ||
| 1.3 | compacted | + | + | − | − | ||
| 1.4 | compacted | + | + | + | VUB26_QUATRO | ||
| 2 | 35 | 2.1 | compacting | + | + | − | − |
| 2.2 | compacting | + | + | − | − | ||
| 2.3 | 6 cells | + | + | − | − | ||
| 2.4 | compacting | + | − | − | − | ||
| 3 | 37 | 3.1 | compacting | + | + | − | − |
| 3.2 | compacted | + | − | − | − | ||
| 3.3 | compacted | + | + | − | − | ||
| 3.4 | compacted | + | + | − | − | ||
| 4 | 26 | 4.1 | 2 cells | + | + | − | − |
| 4.2 | 2 cells | + | + | + | VUB27_PATRU | ||
| 4.3 | 2 cells | + | − | − | − | ||
| 4.4 | 2 cells | + | − | − | − | ||
| Total number | 16 | 15 | 10 | 2 | 2 |
The first three embryos were used in one study in which the resulting morulae were plated on Day 4 after ovarian pick up (OPU), resulting in VUB26_QUATRO. For the fourth embryo, plating was performed on day 3 after OPU, resulting in VUB27_PATRU.
Characterization
The expression of SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81, cell surface markers that characterize undifferentiated hESC was assessed by immunocytochemistry (Mateizel et al., 2006). The primary antibodies used were SSEA-3 [MC-631, Developmental Studies Hybridoma Bank (DSHB), Iowa, USA; 1:20], SSEA-4 (MC-813, DSHB, 1:20), TRA-1-60 (MAB4360, 1:100), TRA-1-81 (MAB4381), (both Millipore, Brussels, Belgium; 1:100). For the detection of the primary antibodies, Alexa488-conjugated secondary antibodies (Invitrogen, Merelbeke, Belguim; 1:250) were used. Gene expression studies for stemness markers (POU5F1, NANOG, DNMT3B, GDF3, LIN 28, NPM 1, REX-1, SOX-2) and alpha-fetoprotein (AFP) as a marker for differentiated cells were carried out on predominantly undifferentiated stem cells by qualitative reverse transcriptase–PCR (RT–PCR) (Mateizel et al., 2006). RNA was isolated using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) and was reverse transcribed to cDNA using the First-strand cDNA Synthesis Kit (GE Healthcare, Diegem, Belgium) with the NotI-d(T)18 primer. PCR was performed using 2 µl of cDNA in a 25 µl total PCR volume containing 10 pmol of primers, 0.2 mmol dNTP and 2.5 U of Taq polymerase (GE Healthcare). For DNMT3B, PCR was performed using the Expand High Fidelity PCR System (Roche Diagnostics GmbH, Mannheim, Germany). The primer sequences and conditions of the PCRs (35 cycles) are described in Table II. PCR products were analyzed on a 2% agarose gel. For the detection of alkaline phosphatase activity, the Vector Blue substrate kit (Vector Laboratories, Burlingame CA, USA) was used. Teratomas were obtained after injection of 2 × 107 cells into the rear leg muscle of a SCID mouse. Karyotyping was done by array Comparative Genomic Hybridization (aCGH, Vermeesch et al., 2005) and G-banding (Gosden et al., 1992).
Table II.
The primer sequences and conditions of the PCRs for the gene expression studies.
| gene | Forward primer | Reverse primer | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|---|
| NANOG | CAGAAGGCCTCAGCACCTAC | CTGTTCCAGGCCTGATTGTT | 55 | 216 |
| POU5F1 | GACAACAATGAGAACCTTCAGGAGA | TTCTGGCGCCGGTTACAGAACCA | 55 | 218 |
| HPRT | GCCGGCTCCGTTATGGCG | AGCCCCCCTTGAGCACACAGA | 55 | 226 |
| REX-1 | GCGTACGCAAATTAAAGTCCAGA | CAGCATCCTAAACAGCTCGCAGAAT | 58 | 306 |
| SOX-2 | CCCCCGGCGGCAATAGCA | TCGGCGCCGGGGAGATACAT | 58 | 448 |
| LIN 28 | AGTAAGCTGCACATGGAAGG | ATTGTGGCTCAATTCTGTGC | 58 | 420 |
| NPM1 | TGGTGCAAAGGATGAGTTGC | GTCATCATCTTCATCAGCAGC | 58 | 343 |
| GDF3 | AGACTTATGCTACGTAAAGGAGCT | CTTTGATGGCAGACAGGTTAAAGT | 58 | 150 |
| DNMT3B | TTGTAGCCATGAAGGTTGGC | TGTGTAGTGCACAGGAAAGC | 59 | 351 |
| AFP | GCTGGATTGTCTGCAGGATGGGGAA | TCCCCTGAAGAAAATTGGTTAAAAT | 55 | 216 |
Results
Four top quality 4-cell stage human embryos were split into single blastomeres that were used for hESC derivation. Two pluripotent hESC lines were derived from distinct 4-cell stage embryos.
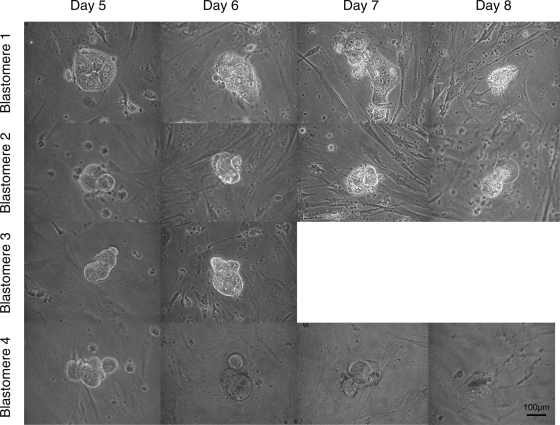
For the first line, three top quality 4-cell stage embryos were split into 12 single blastomeres that were cultured individually without zona pellucida. The 12 single blastomeres showed synchronous mitotic divisions and divided into two cells on Day 3. On Day 4, they all developed into small morulae (estimated 4–8 cells), some compacting (cell contours microscopically visible) and others fully compacted (no cell contours microscopically visible) (Table I). From the 12 blastomere-derived embryos, one was lost during the plating procedure and the remaining 11 were plated. On Day 5, 1 day after plating, 8 out of 11 blastomere-derived embryos attached to the MEFs. The ones that did not attach originated from three distinct embryos and degenerated without showing any sign of cavitation. We did not observe that all four cells of a single embryo led to attaching morulae, i.e. of every split embryo one blastomere-derived morula did not attach. Seven out of eight attached morulae subsequently degenerated without showing any sign of cavitation. The morphological features of four blastomere-derived morulae from the same embryo are shown in Fig. 1. Without showing any sign of cavitation, one blastomere-derived morula produced an outgrowth represented by a compact group of cells (Fig. 2b and c) (age mother 35, Table I). On Day 8, 4 days after plating, flattened TE-like cells were visible (Fig. 2d) but they did not survive the following days (Fig. 2e). No hESC-like cells were observed. However, by Day 16, hESC-like cells were visible for the first time (Fig. 2f). They subsequently developed into a hESC colony-like structure that was mechanically passaged on Day 22 and cultured further as described for blastocyst ICM-derived hESC (Fig. 2 g and h).
Figure 1.
The morphological features of four blastomeres-derived morulae from the same embryo (days after OPU).
Pictures were taken by 200× original magnification.
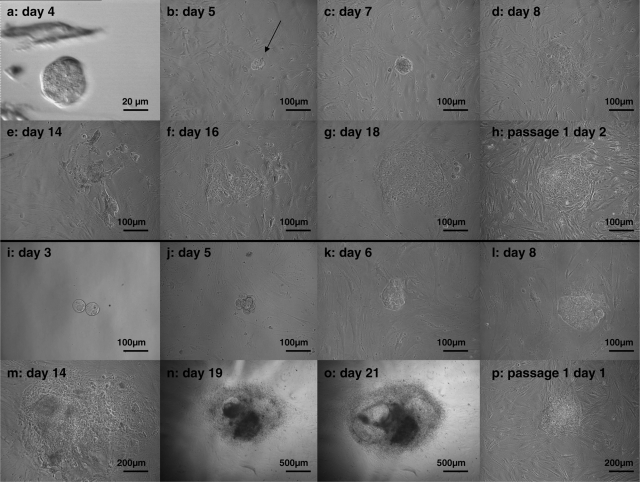
Figure 2.
Derivation of the hESC lines VUB26_QUATRO (a–h) and VUB27_PATRU (i–p) by plating and outgrowth of a single blastomere-derived morula (days after OPU).
VUB26_QUATRO: The blastomere-derived morula (a) produced an outgrowth (arrow) represented by a compact group of cells (b, c). Four days after plating, flattened TE-like cells were visible (d) but they did not survive the following days (e). By Day 16, hESC-like cells were visible for the first time (f). They subsequently developed into a hESC colony-like structure (g) that was mechanically passaged and cultured further (h). VUB27_PATRU: the blastomere-derived morula attached to the fibroblast feeder layer at Day 5 (j). Starting from Day 6 (k), a colony-like structure developed and continued to expand (l–o). It was mechanically passaged on Day 23 and cultured further (p).
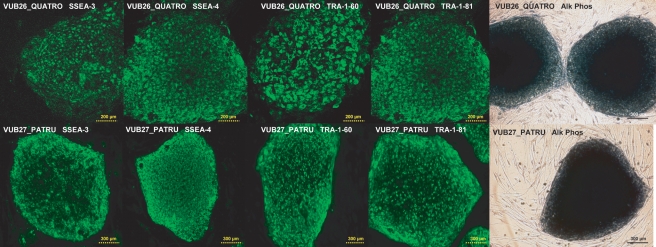
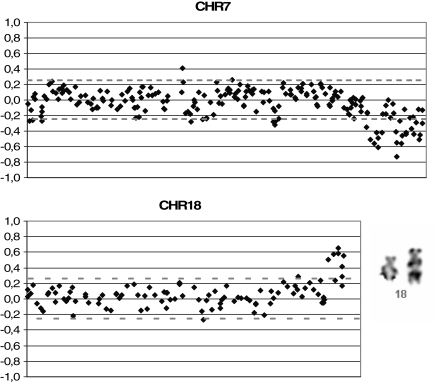
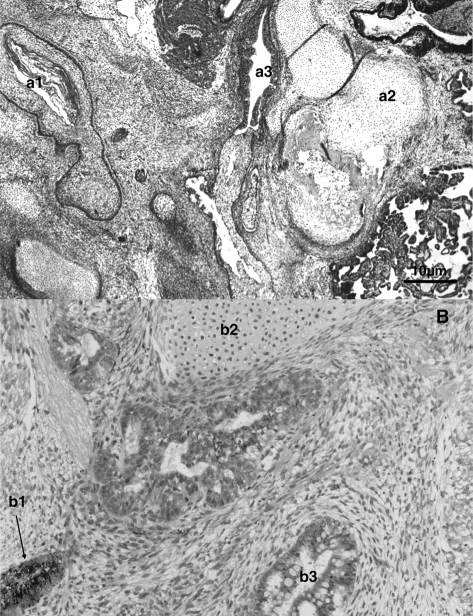
The putative hESC line VUB26_QUATRO expressed the typical markers of stemness (SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, POU5F1, NANOG, DNMT3B, GDF3, LIN 28, NPM 1, REX-1, SOX-2 and Alkaline Phosphatase) as detected by immunocytochemistry and/or RT–PCR performed at passage 18 and 13, respectively (Figs. 3 and 4a). After the first passage, the colony morphology and growth rates were similar to blastocyst ICM-derived hESC (data not shown). At passage 10, cells were collected for molecular karyotyping by aCGH and were shown to carry a deletion of chromosome 18q23qter and a duplication of chromosome 7q33qter (46, XX, dup(7)(q33qter), del(18)(q23qter)) (Fig. 5). This was confirmed at passage 35 when a derivative chromosome 18 was visualized by G-banding (Fig. 5), however, two out of six metaphases showed a normal karyotype. In vivo pluripotency was confirmed by the presence of derivatives of all three germ layers in the teratoma that was observed and collected 9 weeks after injection: squamous epithelium (ectoderm), cartilage (mesoderm) and gastro-intestinal epithelium (endoderm) (Fig. 6A). At present, VUB26_QUATRO is at passage 60 and it has been successfully frozen-thawed and reintroduced into culture.
Figure 3.
Gene expression studies on VUB26_QUATRO and VUB27_PATRU: immunocytochemical detection of SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81; Alkaline Phosphatase positive colonies.
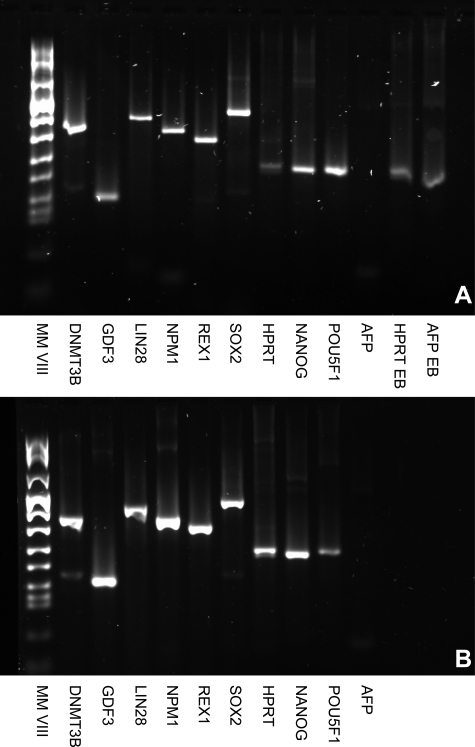
Figure 4.
Gene expression studies on VUB26_QUATRO (A) and VUB27_PATRU (B): RT–PCR results for stemness markers DNMT3B, GDF3, LIN 28, NPM 1, REX-1, SOX-2, POU5F1 and NANOG; housekeeping gene HPRT and alpha-fetoprotein (AFP) as a marker for differentiated cells.
In Fig. 4A, expression of HPRT and AFP was assessed in embryoid bodies from a fully characterized hESC line (VUB01) as a positive control for the differentiation marker AFP (HPRT EB, AFP EB).
Figure 5.
Karyotyping of VUB26_QUATRO: aCGH results showing a deletion of chromosome 18q23 and a duplication of chromosome 7q33 and G-banding showing the derivative chromosome 18.
Figure 6.
Teratomas obtained after injection of (A) VUB26_QUATRO and (B) VUB27_PATRU cells into the rear leg of a SCID mouse, showing the presence of cells from the three germ cell layers: ectoderm (a1: squamous epithelium, b1: retina progenitors), mesoderm (a2 and b2: cartilage) and endoderm (a3: gastro-intestinal epithelium, b3: glandular epithelium with Goblet cells).
For the second line, one top quality 4-cell stage embryo (age mother 26, Table I) was split into four single blastomeres and cultured individually without zona pellucida. As was described for the first hESC line, the four single blastomeres showed synchronous mitotic divisions and divided into two cells on Day 3. They were plated on Day 3 for practical reasons, on Day 4 the four blastomere-derived embryos showed signs of compaction. On Day 5, 2 days after plating, two out of four blastomere-derived embryos attached to the MEFs and one produced an outgrowth as described above for the first hESC line (Fig. 2). This putative hESC line VUB27_PATRU also expressed the typical markers of stemness as detected by immunocytochemistry and/or RT–PCR performed at passage 49 and 50, respectively (Figs. 3 and 4b). At passage 9, cells were collected for molecular karyotyping by aCGH and were shown to display a normal 46, XY karyotype (see Supplementary Figure S1). The presence of derivatives of all three germ layers in the teratoma that was observed and collected 10 weeks after injection, confirmed in vivo pluripotency: retina progenitors (ectoderm), cartilage (mesoderm) and glandular epithelium with Goblet cells (endoderm) (Fig. 6B). At present, VUB27_PATRU is at passage 67, it has been frozen and the survival capacity after thawing will be assessed.
Discussion
This report describes the successful derivation and characterization of hESC lines from 2 out of 16 single blastomeres isolated from four top quality 4-cell stage human embryos (success rate of 12.5%). These two blastomere-derived hESC lines were obtained from distinct embryos. Recently, the group of Feki et al. (2008) also reported the derivation of a hESC line from a single blastomere of a frozen-thawed 4-cell stage embryo in which only one blastomere survived thawing. We conclude that at least one blastomere is pluripotent at the 4-cell stage. However, to provide evidence that the four cells are equal and pluripotent, a hESC line should have been derived from each of the four sister blastomeres. Given the success rate of classic hESC derivation from blastocysts ICM (about 7% in our hands), a large number of embryos would have to be subjected to similar experiments which is practically almost impossible given the scarcity of the material.
Stable ESC lines have been derived from single blastomeres of 4- and 8-cell stage mouse embryos (Chung et al., 2005; Lorthongpanich et al., 2008). HESC lines derived from single blastomeres of 8-cell stage human embryos have also been reported (Klimanskaya et al., 2006, 2007); however, the success rate was very low (2%) and co-culture with an established hESC line in order to create a stem cell niche was necessary. Recently, the derivation rate was improved (20%) by co-culturing the blastomere with the parent embryo and by adding laminin to the medium (Chung et al., 2008). The protocol was further simplified by omitting co-culturing with hESC. We describe a simple method to derive hESC lines by plating blastomere-derived embryos on MEFs on which they attach spontaneously. No sign of cavitation was observed confirming that in order to derive ESC lines from cleavage-stage embryos (Tesar, 2005) or single blastomeres (Chung et al., 2008), blastulation is not necessary. Our derivation procedure does not include co-culture of the single blastomere with the parent embryo (Chung et al., 2008) or co-culture of the blastomere-derived so-called ‘vesicle’ with an existing hESC line (Klimanskaya et al., 2006, 2007). Moreover, presence of laminin in the medium is not essential in our protocol, although this might still improve the hESC derivation rate (Chung et al., 2008). Our protocol is similar to the one described by Feki et al. (2008) who obtained a karyotypically abnormal hESC line from a single blastomere of an arrested 4-cell stage embryo plated on human foreskin fibroblasts.
An important question concerns the chromosomal abnormality that was observed in the VUB26_QUATRO hESC line. Array-CGH showed that the hESC line carried a duplication 7q33qter and a deletion 18q23qter. G-banding showed that the abnormality was a derivative chromosome 18 and that the line was mosaic 46, XX/46, XX, der(18). Our second hESC line VUB27_PATRU displays a normal (46, XY) karyotype at passage 9 as shown by array-CGH. Comparing the genetic constitution of the two blastomere-derived VUB hESC lines, we do not believe the genetic abnormalities observed in VUB26_QUATRO were caused by the age of the mother or by the slightly different hESC derivation methodology. The fact that the line was mosaic for the derivative 18 strongly indicates that the abnormality was acquired in culture. Further characterization of the abnormality and discussion on the mechanism of origin and the role of the derivative 18 in cell survival can be found in Spits et al. (2008). Feki et al. (2008) also reported that their hESC line was chromosomally abnormal with triploidies and several deletions and duplications. Coming from a thawed 4-cell stage embryo of which only one blastomere survived, the multiple chromosomal abnormalities in their line probably originated from the embryo rather than from the in vitro culture conditions.
HESC lines have been derived from blastocyst ICM cells (Thomson et al., 1998; Mateizel et al., 2006), morulae (Strelchenko et al., 2004; Strelchenko and Verlinsky, 2006), chromosomally abnormal embryos (Munné et al., 2005), abnormally developing or arrested embryos (Zhang et al., 2006) and single blastomeres of 8-cell stage embryos (Klimanskaya et al., 2006, 2007; Chung et al., 2008) and 4-cell stage embryos (Feki et al. (2008) and the present report). Marked differences in expression and differentiation potential between these distinct hESC lines are to be expected especially because variation between blastocyst ICM-derived hESC lines has already been described (Osafune et al., 2008). Differences could be due to the stage of embryonic development at derivation, variation in techniques of derivation, culturing and passaging methods, chromosomal or other genetic abnormalities and epigenetic variability. In particular, epigenetic mechanisms such as transient histone modifications at the level of key developmental genes seem to play a major role in ESC pluripotency and cell lineage determination (Gan et al., 2007). Due to asynchronous epigenetic mechanisms, heterogeneity may be created in morphologically homogeneous ICM cells as well as in the blastomeres at earlier stages of development. Thus epigenetic plasticity may play a key role in maintaining totipotency/pluripotency.
Through the establishment of a hESC from a cell obtained from a blastomere at a 4-cell stage embryo, we provide evidence of pluripotency at that particular stage of development. In addition, this finding may have important ethical consequences, since the embryo, in contrast to prior methods to derive embryonic stem cells, is not destroyed. Hence, the hESC obtained from the embryo could be used as savior cells for tissue regeneration in several models of human disease, e.g. hematopoietic stem cells (HSC), kidney cells and pancreatic beta-cells. Currently, preimplantation genetic diagnosis (PGD) is used to obtain a human leukocyte antigen (HLA)-matched child as a donor for cord blood HSC to transplant and save a sibling with bone marrow failure syndromes or other hematological diseases (Bielorai et al., 2004; Grewal et al., 2004). This so-called PGD/HLA testing type 1 (de Wert et al., 2007) is morally accepted in most countries on the condition that the parents intend to care for the donor child to the same extend as they do for their other children and because the newborn is not harmed when collecting the cord blood. However, this approach is limited by several factors including implantation rate and the duration of the pregnancy for those cases requiring urgent transplantation. An alternative option for the parents would be not to transfer the HLA-matched embryo but to derive a hESC line for the in vitro generation of HSC for cell therapy (PGD/HLA testing type 2) (de Wert et al., 2007). Using the conventional hESC derivation methodology starting from blastocyst ICM cells, the embryo would be destroyed. By deriving a hESC line from a 4-cell stage embryo, the attempt to conceive an HLA-matched healthy baby could be theoretically combined with the derivation of a hESC line thereby increasing the possibilities to cure the sick child after HSC transplantation. Likewise, the hESC could be used to generate cell types for other, more experimental cellular therapy.
At present, the hESC derivation rate starting from one blastomere of cleavage-stage embryos is too low for clinical application. Also, before clinical application, hESC derivation and differentiation in clinical grade conditions need to be proven efficient and safe. Moreover, the developmental and implantation potential of biopsied 4-cell embryos has not been thoroughly investigated so far. Since stable hESC lines can be derived from single blastomeres at the 4- and 8-cell stage, we need to determine which stage will combine optimal embryonic development and an efficient hESC derivation rate. The removal of one or two cells at the 8-cell stage (12.5 and 25% of the total mass, respectively) is generally used for PGD. A recent report showed that the loss of two cells at the 8-cell stage interferes significantly with blastocyst formation and live birth rate (De Vos et al., 2009). Safety of the biopsy at the 4-cell stage has also been questioned (Hardy and Handyside, 1993). Nevertheless, recent studies on cryodamaged 4-cell stage embryos showed that the loss of one blastomere (25%) does not affect the embryo's implantation capacity (Edgar et al., 2007). Since blastomeres are taken at random for PGD, plasticity/totipotency of all blastomeres is a prerequisite for both embryo development and hESC derivation. We recently showed that single blastomeres of a 4-cell stage human embryo are individually able to develop into blastocysts with ICM and TE and therefore show characteristics of plasticity/totipotency (Van de Velde et al., 2008). However, evidence that all cells at the 8-cell stage show the same characteristics has not yet been provided. If the cells become allocated to ICM or TE after the embryonic genome activation, which in the human occurs between the 4- and the 8-cell stage (Braude et al., 1988), the random removal of one blastomere may affect both embryo development and hESC derivation more at the 8-cell stage than at the 4-cell stage. More basic research is needed to understand potency, plasticity and allocation during human preimplantation development.
In conclusion, we have shown for the first time that a karyotypically normal hESC line can be derived from a blastomere of a 4-cell stage human embryo. We derived two hESC lines from distinct split 4-cell stage embryos, proving that at least one of the 4-cell stage blastomeres is pluripotent. Our results may have far-reaching repercussions on ethical dilemmas surrounding the destruction of preimplantation embryos for hESC derivation.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Funding
Our research is supported by grants from the Scientific Research Foundation—Flanders (FWO-Vlaanderen) and the Research Council (OZR) of the VUB.
Acknowledgements
We are grateful to the patients donating oocytes for research and to the colleagues of the Centre for Reproductive Medicine and the Centre for Medical Genetics of the UZ Brussel and the Department of Embryology and Genetics of the Vrije Universiteit Brussel. We are also grateful to Mr Whitburn of the Language Education Centre (Vrije Universiteit Brussel) for proofreading the manuscript. Mieke Geens and Greet Cauffman are both Research Fellow at the Scientific Research Foundation—Flanders (FWO-Vlaanderen). Claudia Spits is a Postdoctoral Fellow at the FWO Vlaanderen. Ileana Mateizel is supported by the OZR of the VUB.
References
- Bielorai B, Hughes M, Auerbach AD, Nagler A, Loewenthal R, Rechavi G, Toren A. Successful umbilical cord blood transplantation for Fanconi anaemia using preimplantation genetic diagnosis for HLA-matched donor. Am J Hematol. 2004;77:397–399. doi: 10.1002/ajh.20201. [DOI] [PubMed] [Google Scholar]
- Braude PR, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- Chung Y, Klimanskaya I, Becker S, Marh J, Lu SJ, Johnson J, Meisner L, Lanza R. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature. 2005;439:216–219. doi: 10.1038/nature04277. [DOI] [PubMed] [Google Scholar]
- Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–117. doi: 10.1016/j.stem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- De Vos A, Staessens C, De Rycke M, Verpoest W, Haentjens P, Devroey P, Liebaers I, Van de Velde H. Impact of cleavage-stage embryo biopsy in view of PGD on human blastocyst implantation: a prospective cohort of single embryo transfers. Hum Reprod. 2009 doi: 10.1093/humrep/dep251. (in press) [DOI] [PubMed] [Google Scholar]
- de Wert G, Liebaers I, Van de Velde H. The future (re)evolution of preimplantation genetic diagnosis/human leukocyte testing: ethical reflections. Stem Cells. 2007;25:2167–2172. doi: 10.1634/stemcells.2006-0625. [DOI] [PubMed] [Google Scholar]
- Edgar DH, Archer J, Mcbain J, Bourne H. Embryonic factors affecting outcome from single cryopreserved embryo transfer. Hum Reprod. 2007;14:718–723. doi: 10.1016/s1472-6483(10)60674-8. [DOI] [PubMed] [Google Scholar]
- Feki A, Hovatta O, Jaconi M. Letter to the Editor: Derivation of human embryonic stem cell lines from single cells of 4-cell stage embryos: be aware of the risks. Hum Reprod. 2008;23:2874. doi: 10.1093/humrep/den369. [DOI] [PubMed] [Google Scholar]
- Gan Q, Yoshida T, McDonald OG, Owens GK. Concise review: epigenetic mechanisms contribute to pluripotency and cell lineage determination of embryonic stem cells. Stem Cells. 2007;25:2–9. doi: 10.1634/stemcells.2006-0383. [DOI] [PubMed] [Google Scholar]
- Gosden C, Davdson C, Robertson M. Lymphocyte culture. In: Rooney DE, Czepulkowsky BH, editors. Human Cytogenetics. Oxford, UK: Oxford University Press; 1992. pp. 37–47. [Google Scholar]
- Grewal SS, Kahn JP, MacMillan ML, Ramsay NK, Wagner JE. Successful haematopoietic stem cell transplantation for fanconi anaemia from an unaffected HLA-genotype-identical sibling selected using preimplantation genetic diagnosis. Blood. 2004;103:1147–1151. doi: 10.1182/blood-2003-02-0587. [DOI] [PubMed] [Google Scholar]
- Hardy K, Handyside AH. Cell allocation in twin half embryos bisected at the 8-cell stage: implications for preimplantation genetic diagnosis. Mol Reprod Dev. 1993;36:16–22. doi: 10.1002/mrd.1080360104. [DOI] [PubMed] [Google Scholar]
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- Johnson WH, Loskutoff NM, Plante Y, Betteridge KJ. Production of four identical calves by separation of blastomeres from a in vitro derived four-cell embryo. Vet Rec. 1995;137:15–16. doi: 10.1136/vr.137.1.15. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cells derived from single blastomeres. Nature. 2006;444:481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Derivation of human embryonic stem cells from single blastomeres. Nat Protoc. 2007;2:1963–1972. doi: 10.1038/nprot.2007.274. [DOI] [PubMed] [Google Scholar]
- Lorthongpanich C, Yang S-H, Piotrowska-Nitsche K, Pampai R, Chan AWS. Development of single mouse blastomeres into blastocysts, outgrowth and the establishment of embryonic stem cells. Reproduction. 2008;135:805–813. doi: 10.1530/REP-07-0478. [DOI] [PubMed] [Google Scholar]
- Mateizel I, De Temmerman N, Ullmann U, Cauffman G, Sermon K, Van de Velde H, De Rycke M, Degreef E, Devroey P, Liebaers I, et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]
- Munné S, Vellila E, Colls P, Garcia Bermudez M, Vemuri MC, Steuerwald N, Garrisi J, Cohen J. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil Steril. 2005;84:1328–1334. doi: 10.1016/j.fertnstert.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton D. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol. 2008;26:1361–1363. doi: 10.1038/nbt.1510. [DOI] [PubMed] [Google Scholar]
- Strelchenko N, Verlinsky Y. Embryonic stem cells from morula. Methods Enzymol. 2006;148:93–108. doi: 10.1016/S0076-6879(06)18006-4. [DOI] [PubMed] [Google Scholar]
- Strelchenko N, Verlinsky O, Kukharenko V, Verlisnky Y. Morula-derived embryonic stem cells. Reprod Biomed Online. 2004;9:623–629. doi: 10.1016/s1472-6483(10)61772-5. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Ozenski W, Czolowska R. Mouse singletons and twins developed from isolated diploid blastomeres supported with tetraploid blastomeres. Int J Dev Biol. 2001;45:591–596. [PubMed] [Google Scholar]
- Tesar PJ. Derivation of germ-line competent embryonic stem cells from preblastocyst mouse embryos. PNAS. 2005;102:8239–8244. doi: 10.1073/pnas.0503231102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Van de Velde H, Cauffman G, Tournaye H, Devroey P, Liebaers I. The four blastomeres of a 4-cell stage human embryo are able to develop individually into blastocysts with inner cell mass and trophectoderm. Hum Reprod. 2008;23:1742–1747. doi: 10.1093/humrep/den190. [DOI] [PubMed] [Google Scholar]
- Veiga A, Calderon G, Barri PN, Coroleu B. Pregnancy after the replacement of a frozen-thawed embryo with less than 50% intact blastomeres. Hum Reprod. 1987;2:321–323. doi: 10.1093/oxfordjournals.humrep.a136542. [DOI] [PubMed] [Google Scholar]
- Vermeesch JR, Melotte C, Froyen G, Van Vooren S, Dutta B, Maas N, Vermeulen S, Menten B, Speleman F, De Moor B, et al. Molecular karyotyping: array CGH quality criteria for constitutional genetic diagnosis. J Histochem Cytochem. 2005;53:413–422. doi: 10.1369/jhc.4A6436.2005. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–2676. doi: 10.1634/stemcells.2006-0377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.