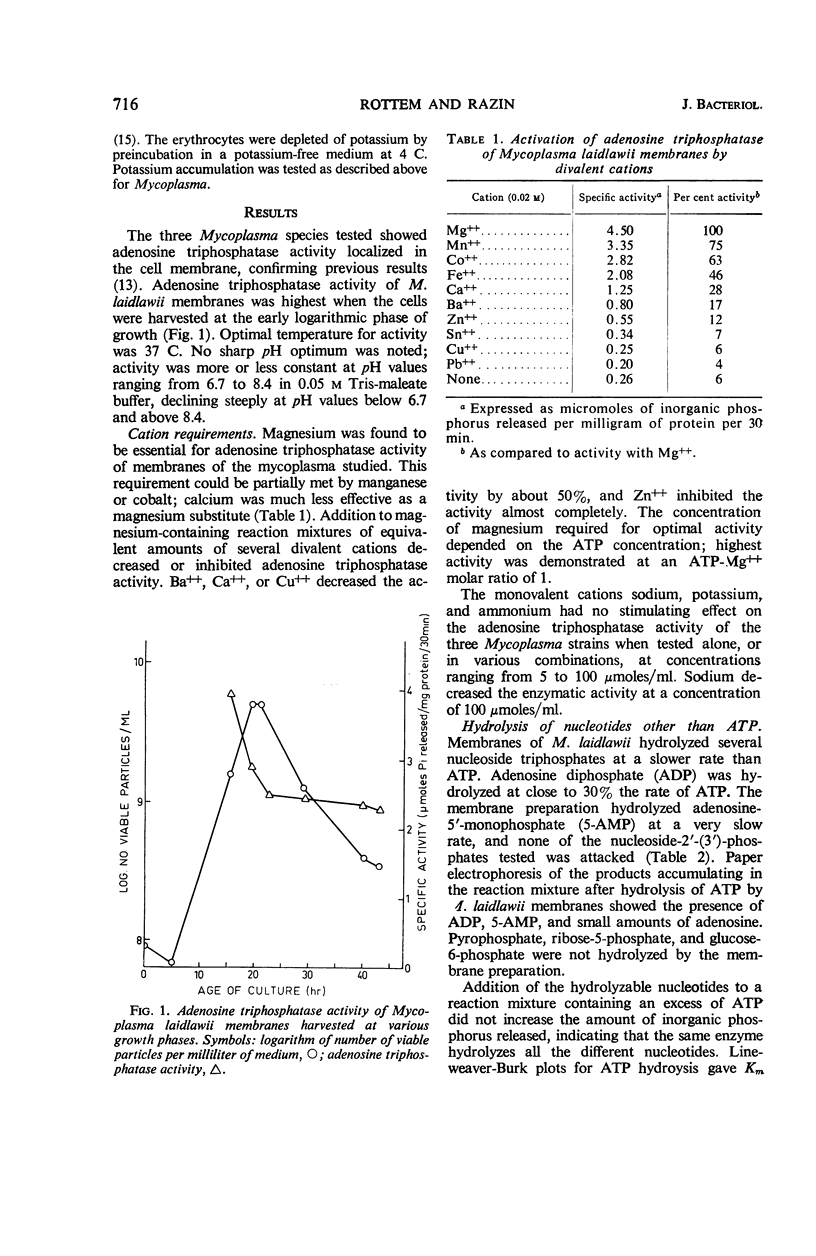
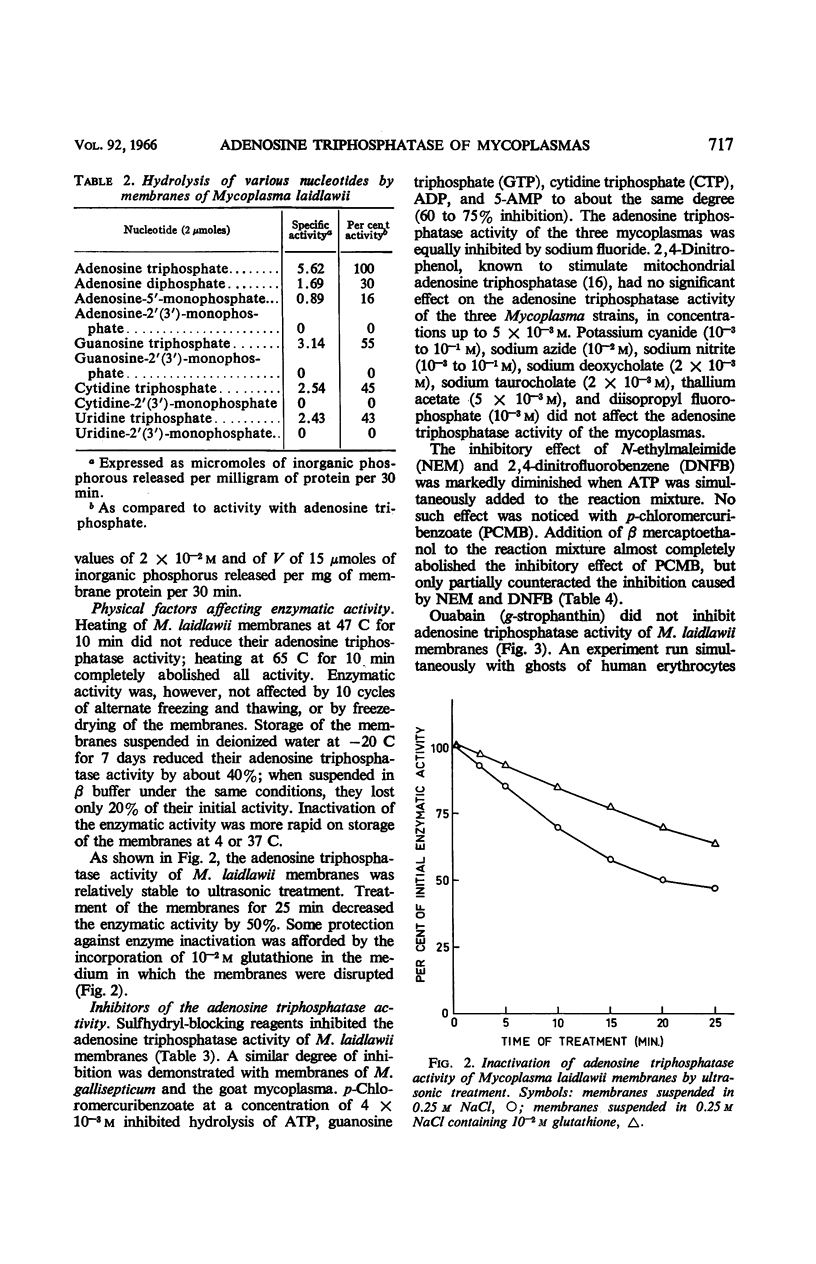
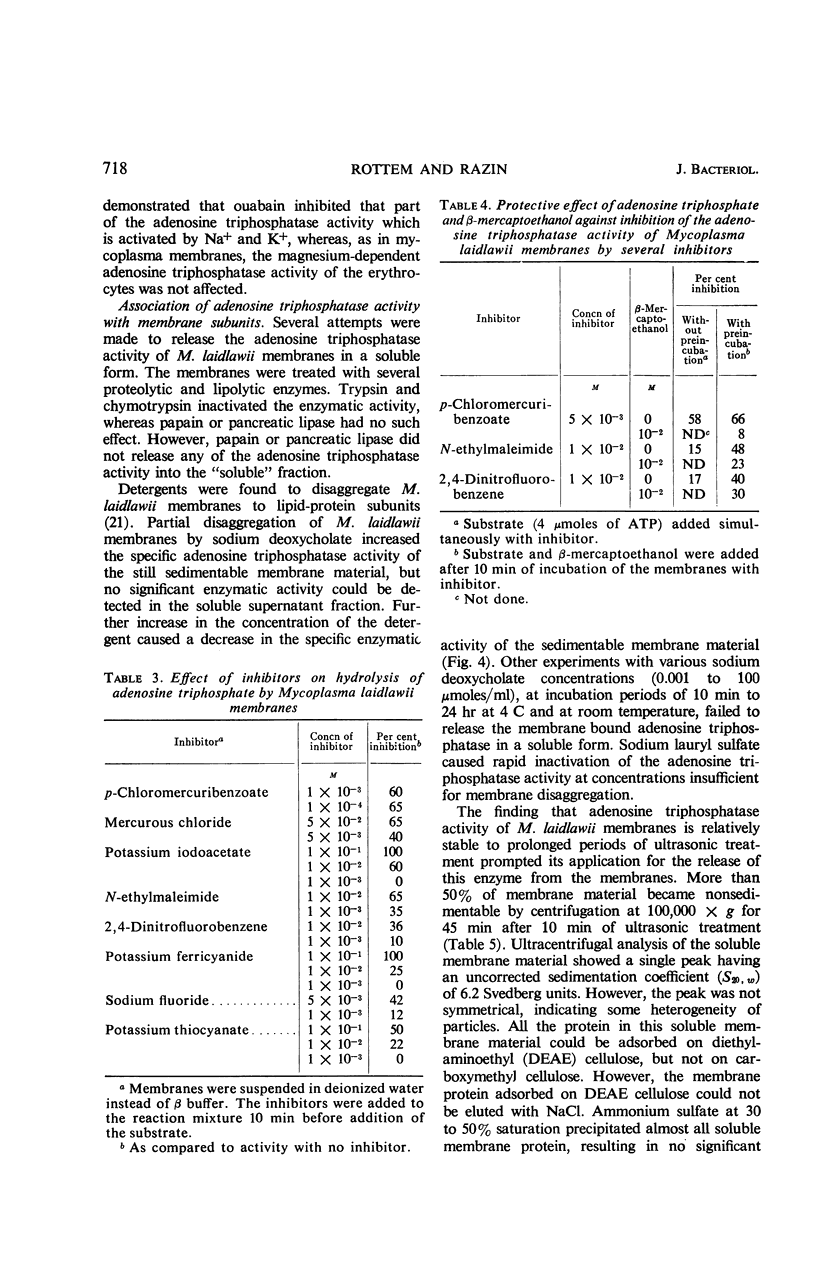
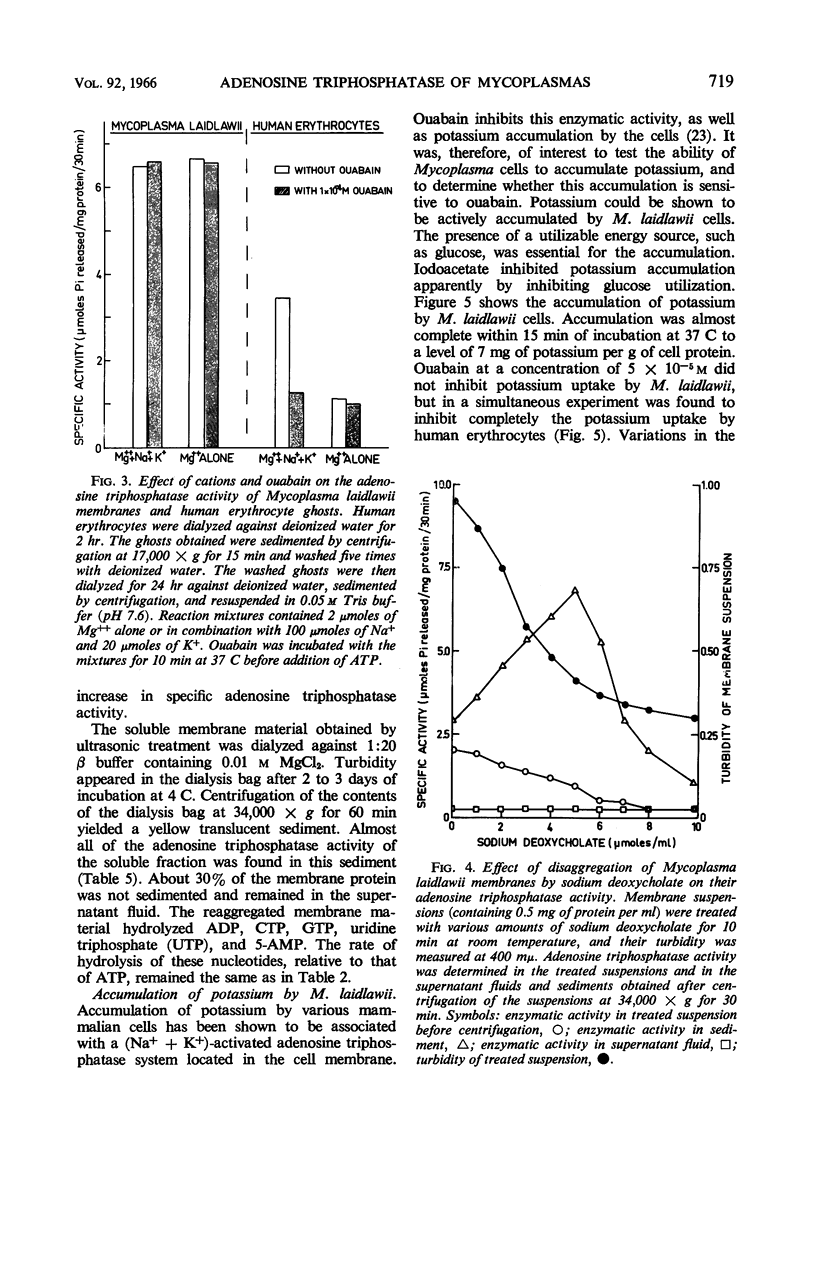
Abstract
Rottem, Shlomo (Hebrew University, Jerusalem, Israel), and Shmuel Razin. Adenosine triphosphatase activity of mycoplasma membranes. J. Bacteriol. 92:714–722. 1966.—Adenosine triphosphatase activity of Mycoplasma laidlawii, M. gallisepticum, and Mycoplasma sp. strain 14 was confined to the cell membrane. The enzymatic activity was dependent on magnesium, but was not activated by sodium and potassium. Ouabain did not inhibit the adenosine triphosphatase activity of the mycoplasmas, and did not interfere with the active accumulation of potassium by M. laidlawii cells. Sulfhydryl-blocking reagents and fluoride inhibited the enzymatic activity, whereas 2,4-dinitrophenol was without any effect. Membranes of M. laidlawii hydrolyzed other nucleotide triphosphates and adenosine diphosphate (ADP), but at a lower rate than adenosine triphosphate (ATP). Nucleoside-2′-(3′)-phosphates, ribose-5-phosphate, glucose-6-phosphate, and pyrophosphate were not hydrolyzed by the membrane preparations. It seems that the enzyme(s) involved in ATP hydrolysis by M. laidlawii membranes is strongly bound to the membrane subunits, which would account for the failure to purify the enzyme by protein fractionation techniques. The adenosine triphosphatase activity of mycoplasma membranes resembles in its properties that of similar enzymes studied in bacteria. The mycoplasma enzyme(s) seems to differ from the adenosine triphosphatase associated with ion transport in mammalian cell membranes and from mitochondrial adenosine triphosphatase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- CSAKY T. Z. TRANSPORT THROUGH BIOLOGICAL MEMBRANES. Annu Rev Physiol. 1965;27:415–450. doi: 10.1146/annurev.ph.27.030165.002215. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L. Water uptake and extrusion by mitochondria in relation to oxidative phosphorylation. Physiol Rev. 1962 Jul;42:467–517. doi: 10.1152/physrev.1962.42.3.467. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILITZER W., TUTTLE L. C. Thermal enzymes. IV. Partial separation of an adenosinetriphosphatase from an apyrase fraction. Arch Biochem Biophys. 1952 Aug;39(2):379–386. doi: 10.1016/0003-9861(52)90347-0. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Pollack M. E., Cleverdon R. C. Fractionation of mycoplasma cells for enzyme localization. Life Sci. 1965 May;4(9):973–977. doi: 10.1016/0024-3205(65)90200-6. [DOI] [PubMed] [Google Scholar]
- RAZIN S., COHEN A. Nutritional requirements and metabolism of Mycoplasma laidlawii. J Gen Microbiol. 1963 Jan;30:141–154. doi: 10.1099/00221287-30-1-141. [DOI] [PubMed] [Google Scholar]
- RAZIN S. Nucleic acid precursor requirements of Mycoplasma laidlawii. J Gen Microbiol. 1962 Jun;28:243–250. doi: 10.1099/00221287-28-2-243. [DOI] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Hilberg C. The effect of sulphydryl-blocking reagents and of urea on the (Na+ + K+)-activated enzyme system. Biochim Biophys Acta. 1965 Nov 22;110(2):359–369. doi: 10.1016/s0926-6593(65)80043-1. [DOI] [PubMed] [Google Scholar]
- Sokawa Y. Liberation of inorganic phosphate from ATP and GTP by a protoplast membrane fraction of Streptococcus pyogenes. J Biochem. 1965 May;57(5):706–708. [PubMed] [Google Scholar]
- Tanaka R., Strickland K. P. Role of phospholipid in the activation of Na+, Ka+-activated adenosine triphosphatase of beef brain. Arch Biochem Biophys. 1965 Sep;111(3):583–592. doi: 10.1016/0003-9861(65)90239-0. [DOI] [PubMed] [Google Scholar]
- Yayashi M., Uchida R. A cation activated adenosinetriphosphatase in cell membranes of halophilic Vibrio parahaemolyticus. Biochim Biophys Acta. 1965 Oct 25;110(1):207–209. doi: 10.1016/s0926-6593(65)80113-8. [DOI] [PubMed] [Google Scholar]



