Abstract
The purpose of this study was to demonstrate the usefulness of the Teager-Kaiser Energy (TKE) operator to assess surface electromyographic (sEMG) activity from the hip and trunk muscles during pediatric gait in children with and without cerebral palsy (CP). Muscle activity was recorded from the trapezius, erector spinae, rectus abdominus, external oblique, gluteus maximus and medius, rectus femoris, and semitendinosus bilaterally in ten children with typical development (TD) and five children with CP ages 44.4 ± 18.6 months. Duration of muscle activity was calculated as a percentage of the gait cycle, and compared to two common onset detection methods, a standard deviation (SD) amplitude threshold method, and the visual inspection from two raters (R1, R2). Relative and absolute agreement was determined using intraclass correlation coefficients (ICCs) and Bland-Altman plots. Of the two automated methods, the TKE method demonstrated better agreement with visual inspection (0.45–0.89) than the SD (0.11–0.76) method. The Bland-Altman plots indicated a smaller bias and 95% confidence interval for the TKE method in comparison to the raters (TKE to R1: −5, 113%; TKE to R2: 4, 95%; SD to R1: −24, 170%; SD to R2: −15, 151%). The use of the TKE operator may better detect sEMG activity in children than the standard amplitude method.
Keywords: Electromyography, Gait analysis, Pediatrics
INTRODUCTION
Poor control of the postural muscles is considered a primary impairment in cerebral palsy (CP).25 Poor postural control contributes to compensation by other muscles to assist in maintaining an upright posture, and limiting those muscles from functioning effectively as primary movers of the extremities.4,21,22 Core trunk and hip muscle activation patterns have not been systematically investigated in individuals with CP. One method by which hip and trunk muscle function can be assessed is through the use of surface electromyography (sEMG).
There are several variables that can be extracted from the sEMG signal that can provide insight into muscle activity in children with and without CP, including the use of time-frequency analyses,12–14,30 examination of the amplitude of the sEMG signal in response to force generation and task demands,27 and the occurrence of muscle onsets and offsets.3 While the combined examination of all components of the sEMG would provide the greatest understanding of muscle function,24 the method most commonly employed clinically is the determination of the onset and offset of muscle activity during the movement. Determining the duration of muscle activity is clinically useful in order to investigate differences between individuals with and without neuromuscular impairments, and to assess coactivation of the agonist and antagonist muscles.
The determination of the onset and offset of muscle activity is fairly straightforward when clear bursts of phasic activity are recorded. It is complicated when the amplitude of the activation bursts are low, the baseline activity is high, the muscles are tonically active or quiet throughout the movement, and when the quality of the signal is compromised by internal or external noise. These scenarios are likely to occur in children with CP because their movement is characterized by muscle weakness, prolonged activation times, spasticity, and poor neuromuscular activation.25 In addition, cardiac electrical activity is commonly recorded with the sEMG signals of the trunk musculature, making the analysis of the signal difficult.1
Several automated methods have been proposed in the literature to facilitate the detection of muscle activity, such as the use of a standard deviation (SD) threshold with respect to a resting baseline,5,9 pattern recognition and template matching,18,31 or probabilistic criterion matching.2,19,24,28 These methods, however, rely primarily on changes in the amplitude component of the sEMG signal to determine the onset and offset of activity, and may fail to detect distinct bursts of activity when there is a poor signal to noise ratio.6 Thus, visual inspection of the raw sEMG trace remains a popular method even though errors with this method and disagreement between experienced raters are known limitations.9
Recently the Teager-Kaiser Energy (TKE) operator was proposed as another method to detect muscle activity using both amplitude and frequency information from the sEMG. This method improves the signal to noise ratio16 by taking into consideration the modulations that occur both in amplitude and frequency within the sEMG signal during movement by calculation of the energy of the signal.12,14 When applied to sEMG signals from able-bodied adults during isometric contractions, the TKE was more accurate in detecting the onset of EMG activity than a standard amplitude method.26 The method has also been successfully applied to sEMG signals during walking in older adults.10 Use of the TKE method has not been examined with sEMG signals from children or from individuals with spasticity. Thus, the purpose of this study was to apply the TKE operator to the detection of sEMG activity from the hip and trunk muscles recorded during gait in children with and without CP to determine the usefulness of this method.
METHODS
Subjects
Data were analyzed from ten children with typical development (TD), and five children with CP (Table 1). All of the children were part of a larger study examining hip and trunk muscle activity patterns in early walkers. The parents of all the children signed a university institutional review board approved consent form, and the children gave verbal assent. Inclusion criteria for the pilot study were: (1) 0.5–60 months of walking experience, (2) the ability to ambulate barefoot at least 15 ft with supervision, children with CP could use any hand held assistive device (i.e., canes, crutches, and walkers) that did not stabilize or restrict movement of the trunk or pelvis; and (3) the ability to follow 1-step verbal directions. Children were excluded if they: (1) had surgery to or fracture of the lower extremities in the past 12 months; (2) had botulinum toxin injection in the lower extremities in the past 6 months; (3) had a history of dorsal rhizotomy; (4) had a history of tendon transfer to a target muscle; or (5) had a known orthopedic, neuromuscular, or cardiovascular condition (or secondary condition for the children with motor impairment).
TABLE 1.
Subject demographic information.
| ID | Age (months) | Gender | BMI (kg/m2) | Walking experience (months) | Diagnosis | Assistive device |
|---|---|---|---|---|---|---|
| 1 | 64 | F | 11.2 | 52 | TD | None |
| 2 | 41 | F | 16.6 | 21 | TD | None |
| 3 | 47.5 | F | 17.0 | 38 | TD | None |
| 4 | 16 | M | 13.3 | 5 | TD | None |
| 5 | 22 | M | 17.9 | 12 | TD | None |
| 6 | 67.5 | M | 15.8 | 58 | TD | None |
| 7 | 59 | M | 17.6 | 29 | CP | PRW |
| 8 | 53 | M | 22.9 | 35 | CP | PRW |
| 9 | 38 | M | 16.4 | 10 | CP | PRW |
| 10 | 28 | F | 16.5 | 12.5 | TD | None |
| 11 | 13 | F | 18.8 | 1 | TD | None |
| 12 | 51 | F | 17.5 | 42.5 | TD | None |
| 13 | 44 | F | 15.7 | 31 | TD | None |
| 14 | 75 | F | 16.0 | 42 | CP | None |
| 15 | 47.5 | M | 16.3 | 11.5 | CP | FC |
BMI, body mass index; CP, cerebral palsy; TD, typical development; PRW, posterior rolling walker; FC, forearm crutches.
Data Acquisition
Spatiotemporal information, including heel strike events, were recorded as the children walked down a 15-ft long by 3-ft wide padded walkway (GAITRite®, CIR Systems, Havertown, PA, USA) at a self-selected pace. Concurrent surface EMG (sEMG) activity was acquired from the following muscles bilaterally: middle trapezius, erector spinae, rectus abdominus, external abdominal oblique, gluteus maximus, gluteus medius, rectus femoris, and semitendinosus. The Myomonitor III recording system (Delsys Inc., Boston, MA, USA) was employed with pre-amplified silver–silver chloride parallel bar surface electrodes with a 10.0 mm inter electrode distance. The sEMG data was collected at a sampling rate of 1200 Hz, pre-amplified with a gain of 10, and filtered with a high pass filter of 20 Hz to remove motion artifact, and low pass filter of 450 Hz. Placement for the abdominal electrodes was performed using the methods described by Ng et al.,20 while placement for all other muscles (back, gluteal, and thigh) was performed in accordance with the SENIAM recommendations.8 Synchronization between the walkway and the EMG data collection system was achieved through a trigger signal from the walkway to initiate EMG data collection. A resting static EMG trial was collected in supine prior to walking trials in order to establish baseline muscle activity. The same baseline recording was used as a reference for all three methods of onset/offset detection.
Onset/Offset Detection
The sEMG signal generated by an active muscle contraction contains information about the type of motor units active within the recording area of the electrode. As such, there is a great deal of information in regards to amplitude and frequency that is contained within the unprocessed signal.29 Onset detection, which has principally focused on changes in the amplitude of the sEMG signal, fails to take into account the accompanying frequency changes that must occur as a result of motor unit recruitment.
In the original paper by Kaiser11 describing the calculation of the energy of a signal, he pointed out that with a simple harmonic system described by:
| (1) |
where A is the amplitude of the oscillation, ω is the frequency of the oscillation, and φ is the arbitrary initial phase, the overall energy of the system, E, is proportional to the amplitude squared multiplied by the frequency squared. While the sEMG signal is not a simple harmonic, the concept of calculating the energy of the sEMG signal taking into account both amplitude and frequency alterations could potentially improve our ability to detect onset and offset of muscle activity.
The algorithm, as originally proposed for the calculation of the energy of the signal, was employed here. We assumed that the sEMG signal, given its variations in amplitude and frequency, could also be considered as oscillatory body defined as:
| (2) |
where A is the amplitude, φ is the arbitrary initial phase in radians, n is the sample, and Ω is the digital frequency in radians/sample defined as:
| (3) |
where f is the analog frequency and fs is the sampling frequency. Using Eq. (2), taking three equally spaced adjacent samples of a given signal, the following three equations were derived:
| (4) |
| (5) |
| (6) |
Using the trigonometric identity of:
| (7) |
the cross term of x(n − 1)x(n + 1) can be written as:
| (8) |
and using another trigonometric identity:
| (9) |
The cross term of can be rewritten further as
| (10) |
As the first term on the right hand side is equal to the square of x(n):
| (11) |
or
| (12) |
Thus, using the three discrete sample points, it is possible at a given sample n to calculate the energy of the signal at that time instant, which is equivalent to the instantaneous amplitude squared, multiplied by the instantaneous frequency. This relationship, according to Kaiser, is true as long as the frequency is one-fourth of the sampling frequency, or in this case, for frequencies below 300 Hz (fs for the sEMG acquisition was 1200 Hz). As a majority of the information within the sEMG signal lies between 20 and 500 Hz,17 and with the earlier stages of muscle activity containing frequencies between 1 and 60 Hz,17 this relationship is exact for the sEMG signal and can thus be used.
Five representative gait cycles (ipsilateral heel strike to ipsilateral heel strike) were extracted from the sEMG recordings and processed using MATLAB (The MathWorks Inc., Natick, MA, USA). The TKE operator was applied to the sEMG for each gait cycle, filtered with a second-order low-pass Butterworth filter with phase correction and a cut-off frequency of 10 Hz, and averaged. To determine the onset/offset threshold, the TKE operator was applied to the static EMG baseline signal (5-s recording) that was recorded while all subjects were reclined on a mat in the supine position. The resulting output was then rectified, and the mean and standard deviation were calculated. The mean, plus one standard deviation, was used as the threshold level.
The assessment of the TKE operator for onset/offset detection was performed by comparing the duration of muscle activity obtained using the standard deviation (SD) threshold method. Comparison of the TKE to this method was done as it represented the most commonly used technique for clinical analysis and reporting of muscle activity, even though it is known to contain errors.6,9 For the SD method, the data were rectified and then smoothed using a second-order low-pass Butterworth filter with phase correction (fc = 10 Hz). A 20 ms long, moving average, square window was then applied to the output. If the mean voltage within the window was greater than the mean of the baseline sEMG signal plus three standard deviations, the muscle was identified as being active.
Recognizing that there is no accepted gold standard for EMG activity detection, we also compared both automated methods to visual analysis, which is also commonly used in clinical practice. Visual assessment of muscle activity was performed by two examiners, both having previous experience with visual analysis of sEMG. One examiner is commonly used when comparing visual analysis to automated techniques,1,9,15 if used at all. However two examiners were used in this case to demonstrate the common inconsistencies with visual analysis, and to give the reader better confidence that the TKE is useful in difficult cases. The signals for a given muscle were rectified and averaged across all five trials and displayed on a computer screen with the baseline mean and two standard deviations displayed concurrently. Using the graphical user interface commands with MATLAB, the examiners were able to indicate onset and offset times of the EMG signal with respect to the percent of the gait cycle to the nearest 0.1%. Each examiner determined muscle activity on the same set of traces on two separate days, 3 days apart to evaluate repeatability.
Analysis
The study was designed to examine the validity of the TKE method to assess hip and trunk muscle activity. However, the difficultly in establishing the validity of the TKE method in relation to the SD method and visual observation lies in the fact that the degree to which these methods also accurately reflect muscle activity in the hip and trunk musculature are also unknown. Therefore, to adequately compare all the methods, the relative and absolute reliability was calculated by using intraclass correlation coefficients (ICCs) and Bland-Altman plots using the visual observation as the basis for comparison.
Inter- and intra-rater reliability of the visual assessment method was established for the entire data set, as well as for the individual muscles, to establish the relative error of the visual raters and thus aid in comparison of the other techniques. All analyses were performed using SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA). An ICC2,k was selected since the raters were considered as representative of a larger population of similar raters, and assessment was based on the mean of several measures.23 ICC values above 0.8 were interpreted as good reliability, 0.6 to 0.8 as moderate, and below 0.6 as poor.23 The same model (ICC2,k) was then used to compared the SD and TKE methods to the raters assessment of activity. Agreement of muscle activity across the assessment methods was determined through the use of Bland-Altman limits of agreement.7
RESULTS
Initial analysis of muscle activity was performed with the data separated between the group of children with TD and the group of children with CP. As no trend was observed between the groups for the muscles, the data was combined for full analysis. The intra-rater reliability across all muscles for the two raters (R1, R2) between the first and second assessment days was 0.93 for R1 and 0.95 for R2, indicating reasonable consistency and a low error rate for each rater in determining muscle activity. Inter-rater reliability across all muscles on the first day assessment was good (ICC = 0.89), with individual muscle assessments presented in Table 2. Good reliability was exhibited for all muscles except the rectus abdominus (ICC = 0.69) and semitendinosus (0.57).
TABLE 2.
Intraclass correlation coefficients for individual muscles.
| Muscle | R1/R2 Day 1 | TKE/R1 | TKE/R2 | SD/R1 | SD/R2 |
|---|---|---|---|---|---|
| Trapezius | 0.92 | 0.89 | 0.89 | 0.30 | 0.57 |
| Erector spinae | 0.77 | 0.83 | 0.86 | 0.33 | 0.36 |
| Rectus abdominus | 0.69 | 0.51 | 0.53 | 0.17 | 0.20 |
| External abdominal oblique | 0.91 | 0.45 | 0.50 | 0.15 | 0.11 |
| Gluteus maximus | 0.83 | 0.51 | 0.72 | 0.56 | 0.76 |
| Gluteus medius | 0.84 | 0.76 | 0.82 | 0.41 | 0.60 |
| Rectus femoris | 0.89 | 0.76 | 0.89 | 0.11 | 0.12 |
| Semitendinosus | 0.57 | 0.70 | 0.65 | 0.11 | 0.40 |
R1, Rater 1; R2, Rater 2; TKE, Teager-Kaiser energy operator; SD, standard deviation threshold method.
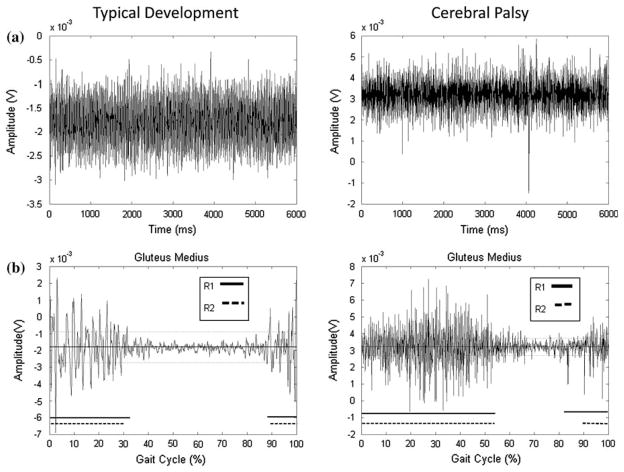
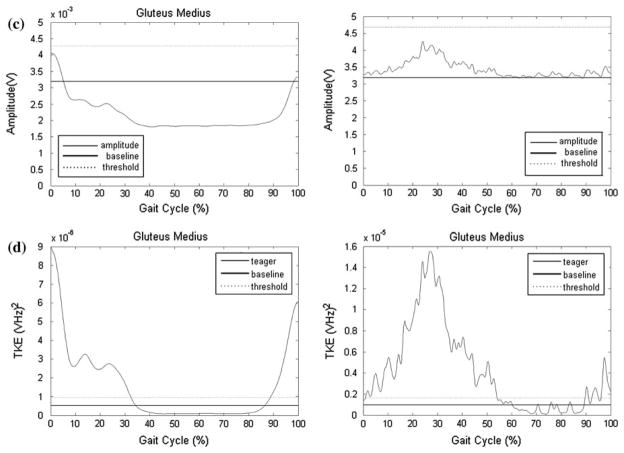
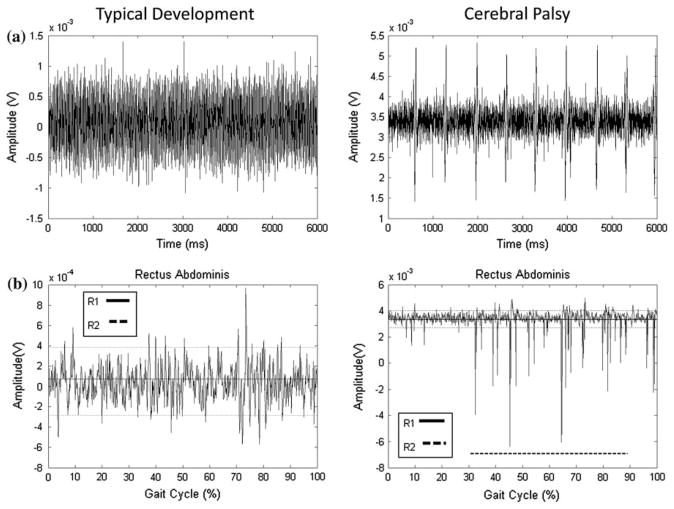
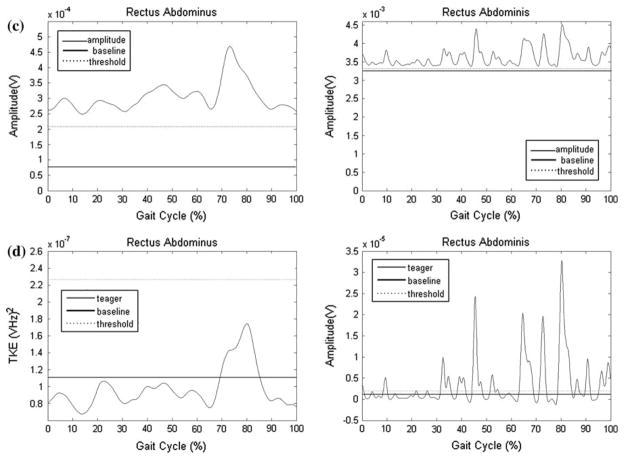
Examples of the TKE and SD curves for select muscles, and compared to the results determined by the raters, are presented Figs. 1 and 2. The data in Fig. 1 represent an example of an EMG recording with a high signal to noise ratio with clear bursts of activity (gluteus medius) for one child with TD and one with CP. There was high agreement between R1, R2, and the TKE method, while the SD method underestimated activation time. The data in Fig. 2 represent an example of a muscle with a low signal to noise ratio (rectus abdominus) characterized by low muscle activity and a variable firing pattern, for one child with TD and one with CP. R1, R2, and the TKE agreed with activation time for the child with TD (0%), while the SD method resulted in 100% activation. For the child with CP, R1 determined the muscle to be active for 0% of the gait cycle, R2 59%, the TKE 39%, and the SD method 100%. This example highlights limitations in the visual assessment method when activity bursts are not clear.
FIGURE 1.
Representative data for the gluteus medius for one child with typical development (TD) and one with cerebral palsy (CP); (a) Raw EMG traces of the static baseline trial; (b) Raw signals during walking used by raters for visual assessment. Thick solid and dashed lines parallel to x-axis represent muscle activation as determined by R1 and R2, respectively. Percent activation was determined to be 44 and 41% by R1 and R2, respectively, for the child with TD and 72 and 63% for the child with CP; (c) SD amplitude curve. The dashed lines indicate the threshold level determined from comparison to the baseline EMG. All activity above the threshold was considered “on,” which resulted in 0% activation for both children; (d) TKE curves. The dashed lines indicate the threshold level determined from comparison to the baseline EMG. All activity above the threshold was considered “on,” which resulted in 43.5% activation for the child with TD and 61.2% for the child with CP.
FIGURE 2.
Representative data for the rectus abdominus for one child with typical development (TD) and one with cerebral palsy (CP); (a) Raw EMG traces of the static baseline trial; (b) Raw signals during walking used by raters for visual assessment. Thick solid and dashed lines parallel to x-axis represent muscle activation as determined by R1 and R2, respectively. Thick dashed line parallel to x-axis represents muscle activation as determined by R2, 0% for the child with TD and 59% of the gait cycle for the child with CP. R1 did not determine that the muscle was activated during any period of the gait cycle for either child; (c) SD amplitude curve. The dashed lines indicate the threshold level determined from comparison to the baseline EMG. All activity above the threshold was considered “on,” which resulted in 100% activation for both children; (d) TKE curves. The dashed lines indicate the threshold level determined from comparison to the baseline EMG. All activity above the threshold was considered “on,” which resulted in 0% activation for the child with TD and 39% for the child with CP.
Inter-rater reliability (Table 2) ranged from 0.45 and 0.89 between the TKE and R1, and from 0.50 to 0.89 between TKE and R2, indicating poor to good agreement. The SD method, in comparison, indicated poor to moderate agreement across the data set with values ranging between 0.11 and 0.56 in relation to R1, and from 0.11 to 0.76 in relation to R2. Overall, the TKE method performed better in comparison to the raters than the SD method, with the poorest correlations for TKE method for the rectus abdominus, external abdominal oblique, and semitendinous.
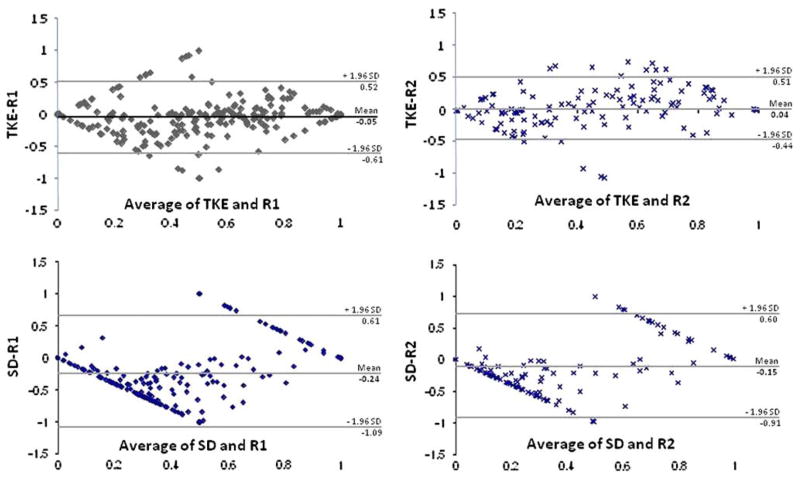
Bland-Altman plots of the TKE and SD methods in comparison to the raters for all the muscles are shown in Fig. 3. Bland-Altman plots indicated that most points (95.0% for R1 and R2 respectively) lie within the 95% limits of agreement for the TKE method, and for the SD method (97.5 and 98.1% for R1 and R2 respectively). However, the 95% limits of agreement were wide in relation to the mean difference of the two methods (TKE to R1: 113%; TKE to R2: 95%; SD to R1: 170%; SD to R2: 151%), but were lower for the TKE method. The biases for the TKE method (TKE to R1: − 5%, TKE to R2: 4%) were also lower than for the SD method (SD to R1: − 24%, SD to R2: − 15%). The negative bias for the SD method to both raters indicated a consistent underestimation of muscle activity.
FIGURE 3.
Bland-Altman plots for the TKE and SD methods, which examine the agreement between the methods and the two raters (R1 and R2) with respect to overall muscle activity The y-axis represents the subtraction of method from the rater, whereas the x-axis represents the average of the two. The 95% limits of agreement are represented as grey lines, and mean bias is represented as a solid black line.
DISCUSSION
Accurate detection of muscle activity from the hip and trunk musculature during walking in the pediatric population, both with and without disabilities, is of great importance to understand how these muscles work to maintain upright posture during the maturation of gait in the pathological condition. With this knowledge, researchers and clinicians may be able to develop interventions that address deficits in core muscle activation. However, the extraction of muscle activity patterns from these muscles is complicated by the fact that in children and, in particular, those with a pathological condition, the ability to recruit the muscle could be compromised,25 and the quality of the signal could be compromised by internal or external noise sources.1
Because of these complications, a majority of the methods proposed in the literature that rely upon amplitude measurements, such as the use of a standard deviation (SD) threshold with respect to a resting baseline,5,9 pattern recognition and template matching, 18,31 probabilistic criterion matching,2,19,24,28 and visual assessment, may under or over report the activity level in the hip and trunk muscles. Likewise, the generalized likelihood ratio and the approximated generalized likelihood ratio methods,2,24,28 which work well in cases of poor signal to noise ratio, or low levels of muscle activity, depend upon a probabilistic change in the amplitude level of the signal to detect bursts of activity, and may fail if the muscle is tonically active with respect to amplitude. Therefore, the examination of amplitude changes, as well as alterations within the frequency components of the sEMG signal, becomes necessary to accurately determine of the level of muscle activity. The TKE operator,10,11,16,26 considers both amplitude and frequency changes in the EMG signal by calculating the overall change in the energy of the signal. By taking frequency into consideration for the detection of muscle activity for the hip and trunk muscles, the ability to correctly detect intervals of muscle activity may be enhanced, particularly in light of recent studies on the sensitivity of frequency information in the sEMG signal12–14,30 to detect differences across groups as well as the effects of a clinical intervention.
This study demonstrated that the TKE method of muscle activity detection more closely resembled the results obtained from visual analysis than the SD amplitude threshold method, and thus may be a more desirable, automated method of calculating muscle activity in the hip and trunk muscles of children (Figs. 1 and 2, Table 2). Automated processing of muscle activity substantially reduces the time needed from clinicians and researchers to analyze sEMG data, and can provide a reliable, objective analysis. A perceived additional advantage of the TKE method is the relatively minimal degree of computational complexity, which may make it more clinically feasible to implement and more accepted for use. The ease of use may be why the SD and visual methods are still reported for muscle activity assessment, in spite of the more robust methods that have been proposed.2,18,19,24,28,31
Recording and interpreting EMG signals from small children is a challenge. Placement must be accurate to avoid crosstalk from adjacent muscles. In addition, abnormal motor unit firing patterns in individuals with neurological impairment can increase the variability and decrease the clarity of the signal. Our results are based on the assumption that visual analysis is an accurate method of determining the onset and offset of muscle activity. It is apparent from the representative data for the rectus abdominus (Fig. 2) that this is not always the case (R1 vs. R2 disagreement). While visual analysis is a clinically accepted standard, it is not a flawless method. Visual analysis, like most automated methods, primarily considers only the amplitude component of the EMG signal which is prone to error.6 In instances where the TKE method is not consistent with the visual analysis of one or both raters, it remains unclear which method is more accurate and further investigation is needed.
CONCLUSION
Clinicians and researchers in numerous fields of work investigate muscle activity. A reliable, objective, and accurate method of EMG analysis is necessary to make sound clinical decisions and appropriately maintain the rigor of scientific research. The TKE method of onset and offset detection appears a valid tool to meet these goals, particularly in instances of low signal to noise ratio. Likewise, investigations into the affects of signal filtering and sample rate on the accuracy of TKE method, and comparison to a larger group of raters, needs to be explored. The application of this method to other clinical populations and other movement patterns will further establish its feasibility and allow for more detailed recommendations on its use in those areas.
Acknowledgments
Support for this study was provided by NIH/NINDS Grant # R03 NS044875.
References
- 1.Allison GT. Trunk muscle onset detection technique for EMG signals with ECG artefact. J Electromyogr Kinesiol. 2003;13(3):209–216. doi: 10.1016/S1050-64110300019-1. [DOI] [PubMed] [Google Scholar]
- 2.Buurke JH, Hermens HJ, Roetenberg D, Harlaar J, Rosenbaum D, Kleissen RF. Influence of hamstring lengthening on muscle activation timing. Gait Posture. 2004;20(1):48–53. doi: 10.1016/S0966-63620300092-4. [DOI] [PubMed] [Google Scholar]
- 3.Damiano DL, Martellotta TL, Sullivan DJ, Granata KP, Abel MF. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Arch Phys Med Rehabil. 2000;81(7):895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- 4.Davis MF, Worden K, Clawson D, Meaney J, Duncan B. Confirmatory factor analysis in osteopathic medicine: fascial and spinal motion restrictions as correlates of muscle spasticity in children with cerebral palsy. J Am Osteopath Assoc. 2007;107:226–232. [PubMed] [Google Scholar]
- 5.Di Fabio RP. Reliability of computerized surface electromyography for determining the onset of muscle activity. Phys Ther. 1987;67(1):43–48. doi: 10.1093/ptj/67.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Farina D. Interpretation of the surface electromyogram in dynamic contractions. Exerc Sport Sci Rev. 2006;34(3):121–127. doi: 10.1249/00003677-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hanneman SK. Design, analysis, and interpretation of method-comparison studies. AACN Adv Crit Care. 2008;19(2):223–234. doi: 10.1097/01.AACN.0000318125.41512.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/S1050-641100 00027-4. [DOI] [PubMed] [Google Scholar]
- 9.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996;101(6):511–519. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 10.Hortobagyi T, Solnik S, Gruber A, Rider P, Steinweg K, Helseth J, DeVita P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture. 2009 doi: 10.1016/j.gaitpost.2008.12.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Kaiser JF. On a simple algorithm to calculate the energy of a signal. ICASSP-90. 1990:381–384. [Google Scholar]
- 12.Lauer RT, Johnston TE, Smith BT, Lee SCK. Muscle activity during cycling in adolescents with and without cerebral palsy. Clin Biomech. 2008;23(4):442–449. doi: 10.1016/j.clinbiomech.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer RT, Smith BT, Shewokis PA, McCarthy JJ, Tucker CA. Time-frequency changes in electromyographic signals after hamstring lengthening surgery in children with cerebral palsy. J Biomech. 2007;40(12):2738–2743. doi: 10.1016/j.jbiomech.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Lauer RT, Stackhouse CA, Shewokis PA, Smith BT, Tucker CA, McCarthy J. A time-frequency based electromyographic analysis technique for use in cerebral palsy. Gait Posture. 2007;23(3):420–427. doi: 10.1016/j.gaitpost.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Lee AS, Cholewicki J, Reeves NP. The effect of background muscle activity on computerized detection of sEMG onset and offset. J Biomech. 2007;40(15):3521–3526. doi: 10.1016/j.jbiomech.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng. 2007;35(9):1532–1538. doi: 10.1007/s10439-007-9320-z. [DOI] [PubMed] [Google Scholar]
- 17.de Luca CJ. Electromyography. In: Webster JG, editor. Encyclopedia of Medical Devices and Instrumentation. New York: Wiley Sons; 2006. pp. 98–109. [Google Scholar]
- 18.Merlo A, Farina D, Merletti R. A fast and reliable technique for muscle activity detection from surface EMG signals. IEEE Trans Biomed Eng. 2003;50(3):316–323. doi: 10.1109/TBME.2003.808829. [DOI] [PubMed] [Google Scholar]
- 19.Micera S, Sabatini AM, Dario P. An algorithm for detecting the onset of muscle contraction by EMG signal processing. Med Eng Phys. 1998;20(3):211–215. doi: 10.1016/S1350-45339800017-4. [DOI] [PubMed] [Google Scholar]
- 20.Ng JK, Kippers V, Richardson CA. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyogr Clin Neurophysiol. 1998;38(1):51–58. [PubMed] [Google Scholar]
- 21.Nicholson JH, Morton RE, Attfield S, Rennie D. Assessment of upper-limb function and movement in children with cerebral palsy wearing lycra garments. Dev Med Child Neurol. 2001;43(6):384–391. doi: 10.1017/S001216220100072X. [DOI] [PubMed] [Google Scholar]
- 22.Policy JF, Torburn L, Rinsky LA, Rose J. Electromyographic test to differentiate mild diplegic cerebral palsy and idiopathic toe-walking. J Pediatr Orthop. 2001;21(6):784–789. doi: 10.1097/00004694-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Stamford: Appleton & Lange; 1993. [Google Scholar]
- 24.Roetenberg D, Buurke J, Veltink P, Cordero A, Hermens H. Surface electromyography analysis for variable gait. Gait Posture. 2003;18(2):109–117. doi: 10.1016/S0966-63620300005-5. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 26.Solnik S, DeVita P, Rider P, Long B, Hortobagyi T. Teager-Kaiser operator improves the accuracy of EMG onset detection independent of signal to noise ratio. Acta Bieng Biomech. 2008;10(2):65–68. [PMC free article] [PubMed] [Google Scholar]
- 27.Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31(5):594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staude G, Wolf W. Objective motor response onset detection in surface myoelectric signals. Med Eng Phys. 1999;21(6–7):449–467. doi: 10.1016/S1350-45339900067-3. [DOI] [PubMed] [Google Scholar]
- 29.Wakeling JM. Motor units are recruited in a task dependent fashion during locomotion. J Exp Biol. 2004;207:3883–3890. doi: 10.1242/jeb.01223. [DOI] [PubMed] [Google Scholar]
- 30.Wakeling J, Delaney R, Dudkiewicz I. A method for quantifying dynamic muscle dysfunction in children and young adults with cerebral palsy. Gait Posture. 2007;25(4):580–589. doi: 10.1016/j.gaitpost.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Wong YM, Ng GY. The double peak-to-peak analysis for determining EMG onset of muscle contraction. Electromyogr Clin Neurophysiol. 2005;45(5):267–271. [PubMed] [Google Scholar]