Abstract
Background
High-intensity, risk-based therapeutic strategies for childhood cancer have resulted in long-term survival rates that now approach 80%. However, the growing population of survivors is at a substantial risk for treatment-related complications that can significantly impact quantity and quality of survival. It is increasingly recognized that many of these complications result from complex interactions between therapeutic exposures and genetic susceptibility.
Objective
This review is designed to increase general clinician awareness of the ongoing efforts by investigators to understand the interactions between therapeutic exposures and genetic susceptibility to therapy-related complications.
Results
Most studies have relied on a biologically plausible candidate gene approach to evaluate genetic susceptibility. This has resulted in the identification of unique genetic polymorphisms that could alter metabolic pathways of therapeutic agents associated with specific adverse events. We highlight some of these findings and discuss their implications for future prevention strategies, as well as their role in elucidating the pathophysiology of these complex diseases.
Conclusion
Research exploring the role of genetic susceptibility in the development of therapy-related adverse outcomes is still in its infancy. There is a need for continued efforts to study these outcomes in the context of complex gene-gene and gene-environment interactions unique to cancer survivors. A better understanding of the pathogenesis of these outcomes will help develop effective targeted prevention strategies.
KEY WORDS: childhood cancer, genetic predisposition, late effects, survivorship
INTRODUCTION
Risk-based therapy for childhood cancer has resulted in marked improvements in survival, with current 5-year survival rates approaching 80%.1 However this improvement in outcome is not enjoyed equally by all. The cumulative incidence of severe or life-threatening chronic health conditions exceeds 40% for childhood cancer survivors surviving 30 years after primary diagnosis.2 Research on survivorship issues has clearly demonstrated well-established associations between specific therapeutic exposures and adverse outcomes such as subsequent malignant neoplasms, cardiopulmonary dysfunction, avascular necrosis, endocrinopathies, and neurocognitive disorders.2–4
While a third of these cancer survivors are less than 20 years, 46% are between the ages of 20 and 39 years, and 20% are over 40 years of age.5 Thus, the long-term care of these survivors is increasingly being provided by family practitioners and general internists. The Children’s Oncology Group Long-Term Follow-Up (COG-LTFU) Guidelines provide healthcare professionals with a framework to standardize the screening of childhood cancer survivors at risk for therapy-related adverse outcomes.6 These are risk-based, exposure-related clinical practice guidelines that rely on the epidemiological evidence of the association between therapeutic exposures and specific adverse outcomes, and are grounded in the collective experience of experts in the field of cancer survivorship.
It is increasingly recognized, however, that for a given therapeutic exposure, marked heterogeneity exists in the prevalence and severity of many of the long-term adverse outcomes experienced by cancer survivors. There is emerging data to suggest that genetic susceptibility could play a role in modifying individual response to therapeutic exposures.7–9 Using a biologically plausible candidate gene approach, investigators have begun to identify polymorphisms that could alter metabolic pathways of therapeutic agents associated with specific adverse events. Many of these genomic variables, when fully established, could potentially play an important role in understanding the pathogenesis of the subsequent therapy-related adverse outcomes, and facilitate implementation of targeted prevention strategies. We present here an overview of the current knowledge regarding established associations between therapeutic exposures and these genomic variables in three commonly occurring complications in childhood cancer survivors—congestive heart failure, avascular necrosis and obesity. We believe that a clear understanding of those at highest risk due to established risk factors (age, gender and therapeutic exposures) as well as emerging risk factors (genetic susceptibility) will provide the necessary tools to internists and pediatricians to titrate the intensity of follow-up and provide appropriate interventions.
CONGESTIVE HEART FAILURE
Anthracyclines such as doxorubicin and daunomycin are widely used in the treatment of childhood cancer; nearly 60% of children with cancer receive anthracyclines as part of their treatment.10 Clinically, one of the most widely recognized side-effects of anthracycline therapy is dose-dependent cardiotoxicity. The cardiotoxicity may manifest as clinically symptomatic congestive heart failure (A-CHF) or asymptomatic cardiac dysfunction identified by abnormalities of cardiac function/structure on imaging studies.11 The incidence of A-CHF is less than 10% in patients exposed to under 500 mg/m2 of cumulative anthracycline exposure, and approaches 36% for doses exceeding 600 mg/m2.10,12 A-CHF often develops years after cessation of therapy, and its frequency increases with longer follow-up.3,10 Outcome following diagnosis of A-CHF is poor, with 5-year survival of less than 50%.13,14
Anthracycline cardiotoxicity is thought to be related to direct myocardial injury due to formation of free radicals.15 Well-recognized clinical risk factors for A-CHF include female sex, younger age at exposure, and radiation therapy involving the heart.11,16,17 However, these clinical risk factors do not fully explain the wide inter-individual variability in susceptibility to A-CHF. Significant cardiotoxicity has been reported at cumulative doses of less than 250 mg/m2,10 while doses that exceed 1000 mg/m2 have been tolerated without long-term sequelae by some.18 This heterogeneity could be explained, in part, by genetic susceptibility that alter the metabolism of anthracyclines, the myocardial response to the drug, as well as others thought to play a role in susceptibility to de novo disease.19–21
The strongest support for the role of these genes as regulators of response to anthracyclines has come from transgenic and knock-out mouse strains with altered sensitivity to the drugs. For example, transgenic overexpression of the multiple drug resistance gene (MDR1) is protective against the cardiotoxic effect of anthracyclines,22 while deficiency and overexpression of anthracycline metabolizing enzyme, carbonyl reductase (CBR) in mice had opposing effects (protecting and enhancing) on susceptibility to A-CHF.19,23,24 A limitation of information obtained from such genetically engineered mouse strains is the strong penetrance of introduced mutations, which likely does not reflect more subtle genetic variability in humans.20
Two recent studies in humans have evaluated the role that these genetic alterations play in the development of clinical A-CHF. These studies suggest that polymorphisms in genes involved in anthracycline metabolism, transport, and superoxide generation may have an impact on an individual’s risk of A-CHF. In the first study,21 82 genes involved in the metabolism of reactive oxygen species (ROS), DNA repair, drug transport and metabolism, endothelial physiology, the rennin-angiotensin system, muscle contraction and structure, and inflammation were evaluated. The risk of chronic cardiotoxicity was significantly increased (OR: 2.5) in individuals with a polymorphism for the NADPH oxidase subunit, NCF4, which is responsible for down-regulation of the enzyme involved in a multi-enzyme complex regulating ROS generation. Inherited NADPH oxidase deficiency may result in impaired ROS defense capacity and therefore lead to increased intracardiac ROS levels after anthracycline exposure.21
The second study19 examined polymorphisms in genes such as: catalase (CAT), superoxide dismutase 1 (SOD1), NADPH:quinone oxidoreductase (NQO1), and carbonyl reductase 3 (CBR3). A polymorphism in CBR3 (V244M) was associated with a markedly increased risk of A-CHF among homozygous and heterozygous individuals (OR: 8.2 and 5.4, respectively). It is believed that the functional CBR3 polymorphism may modulate intracardiac formation of cardiotoxic anthracycline alcohol metabolites, leading to myocardial injury and eventual cardiac dysfunction. These two studies illustrate how certain “at-risk” alleles in key candidate genes could identify individuals at the highest risk of developing anthracycline-related cardiotoxicity. If these findings are replicated and confirmed by others in independent study samples, they could set the stage for identifying a subgroup of patients up front who would perhaps need alternative treatment for management of their cancer; while for those who have already received the anthracyclines, identification of high-risk alleles would warrant closer surveillance for cardiotoxicity and use of medications that modulate cardiac function.
AVASCULAR NECROSIS
Avascular necrosis (AVN) presents with pain in weight bearing joints during or following completion of treatment. The diagnosis is made with plain radiographs, but magnetic resonance imaging (MRI) is more sensitive and can provide early detection. Estimates for the incidence of AVN vary and have ranged from 1%25–27 to 9%28, when based on clinical presentation, to nearly 15%29, when based on MRI screening. A recent large, multi-center, retrospective cohort study found that childhood cancer survivors were 6.2 times more likely than a sibling comparison to report the diagnosis of AVN.27 The risk was greatest among childhood leukemia survivors who had undergone allogeneic hematopoietic stem-cell transplantation (acute lymphoid leukemia [ALL], acute myeloid leukemia [AML]: Relative Risk=26.9, and 66.5, respectively). Among individuals with non-hematologic malignancies, the greatest risk was for those with history of bone sarcoma (RR 7.3).
It is well-recognized that glucocorticoids (GCs) substantially increase an individual’s susceptibility to AVN.28,30 Other clinical risk factors include older age at exposure, female gender, white race, and radiation.25,28,31,32 The risk of AVN due to GCs is especially high for survivors of childhood leukemia since GCs are a critical component their prolonged maintenance therapy. The mechanisms by which GCs cause AVN can be complex and may include suppression of osteoblasts, apoptosis of osteocytes, intra-medullary lipocyte proliferation (affecting sinusoidal circulation), and fat embolization to subchondral arteries.33–35 This has prompted a unifying theory of ‘cumulative cell stress’ to explain the complex pathophysiology of therapy-related AVN and is based on three components: anatomic location (weight bearing), systemic illness, and GC exposure.33 In this hypothesis, it is recognized that GCs play a ‘necessary but not sufficient’ role, and that clear host-exposure differences exist that account for heterogeneity of clinical presentation.
Relling and colleagues were one of the first to study the role of genetic polymorphisms in the risk of therapy-related AVN.29 They found two inherited polymorphisms, vitamin D receptor Fok1 polymorphism (VDR Fok1) and thymidylate synthase (TYMS) to independently predict risk of therapy-associated AVN (OR: 4.5 and 7.4, respectively). The TYMS 2/2 genotype is associated with low TYMS expression, rendering cells more susceptible to toxic and anti-cancer effects of drugs such as methotrexate. The serendipitous finding by Relling and colleagues helped shed light on additional therapy-related risk factors for osteonecrosis - suggesting that antifolate therapy and subsequent homocysteine dysregulation may contribute to the risk of osteonecrosis. These findings may provide one of the explanations for the increased incidence of AVN in bone sarcoma survivors, since high dose anti-metabolite therapy is a critical component of the treatment of osteogenic sarcoma.
To further explore the genetic predictors of AVN, Relling et al. studied 12 candidate polymorphisms in a separate cohort of patients with a relatively high incidence of AVN.36 Genetic polymorphisms of interest included the two previously identified (VDR Fok1, TYMS), plasminogen activator inhibitor (PAI-1), and others. They found a three-fold increased risk of AVN in individuals with a PAI-1 polymorphism. It was postulated that high levels of PAI-1, induced by corticosteroid treatment or through germline polymorphisms in PAI-1, lead to suppression of fibrinolysis, potentially resulting in increased intraosseous venous pressure and eventual bone death or osteonecrosis.37,38 These studies play a critical role in improving our understanding of the pathogenesis of therapy-related AVN, and therefore help us in developing interventional strategies. As in the case with anthracycline-related cardiotoxicity, once confirmed, these finding would help refine the identification of those at highest risk, where targeted prevention strategies could be instituted.
OBESITY
The high rate of obesity (BMI≥30 kg/m2) in adult survivors of childhood cancer is especially concerning since increased BMI has a modifying effect on other well-described therapy-related complications such as cardiopulmonary disease, diabetes, and musculoskeletal disorders. Depending on the definition and methods used for measurement, the reported prevalence of obesity in survivors ranges from 11–40%.39–43 Survivors of childhood ALL and central nervous system (CNS) tumors are at the highest risk, largely as a consequence of cranial radiation therapy (CRT). 39,44–46 A report from a large cohort of childhood cancer survivors found that compared to siblings, adult survivors of childhood ALL who received CRT at a dose 20 Gy or greater had an up to three-fold increased risk of obesity.39 The risk was not increased in individuals who received chemotherapy only or in those who received CRT doses less than 20 Gy. In brain tumor survivors, the risk of obesity is greatest in females who received any CRT and were less than 10 years of age at exposure.44
A possible mechanism of radiation-related obesity is leptin-insensitivity and/or growth hormone deficiency (GHD) that can result from CRT-mediated disruption of the hypothalamic-pituitary axis (HPA). Leptin is a hormone secreted almost exclusively by adipocytes, and plays an important role in long-term regulation of body weight and metabolism.47 Leptin is thought to act as a satiety signal in a feedback loop with hypothalamic centers that control feeding behavior and hunger, energy expenditure, and body temperature.48 Mice with inactivating mutations in the gene encoding leptin (LEP) or its receptor (LEPR) are phenotypically characterized as obese, with nearly three times the body weight and five times the fat mass of genotypically normal mice.49
It is increasingly recognized, however, that obesity in humans is most likely a polygenic trait, with complex gene-environment and gene-gene interactions that are now being studied.50–52 Ross and colleagues recently examined the potential association between the Gln223Arg polymorphism in the LEPR gene and risk of obesity in a large cohort of ALL survivors.53 Survivors with BMI ≥25 kg/m2 were significantly more likely to be Arg homozygous compared with those with BMI <25 kg/m2, an observation that was limited to females. The risk was especially great for Arg homozygous females who received ≥20 Gy CRT (OR: 6.1). These preliminary findings support the role for the LEPR polymorphism in modifying risk of obesity. However, the association is limited to a subset of female survivors of childhood ALL exposed to cranial radiation, reinforcing the complex nature of a likely polygenic trait.
We know now that radiation to the brain is associated with an increased risk of morbid obesity. It is also clear that the risk is higher in girls, and in those who receive higher doses of radiation, especially at a young age. Evidence is now emerging that favors the existence of “at-risk” alleles in certain genes that place these individuals at a much higher risk. These studies need to be confirmed and a more comprehensive examination of genes that could be implicated in this pathway need to be examined. This research is of particular importance because it may have implications in understanding and addressing the rising prevalence of obesity in the general population.
FUTURE DIRECTIONS
Recent advances in the sequencing of the human genome54,55 have created many opportunities for investigating the relationship between polymorphic genetic variants present in the human population and their impact on the pathogenesis of various medical conditions.56–58 It is now becoming increasingly recognized that risks for many diseases result from an interaction between inherited gene variants and environmental factors, including chemical, physical, and behavioral factors, which raises the possibility of targeted disease prevention and health promotion efforts for individuals at high risk because of their genetic makeup.59 The diseases discussed in the current review illustrate that research exploring the role of genetic susceptibility in the development of therapy-related adverse outcomes is still in its infancy, and that there is indeed an urgent need for continued efforts to study therapy-related adverse outcomes in the context of the complex gene-gene and gene-environment interactions unique to the growing population of cancer survivors.
A comprehensive approach to understanding the pathogenesis of therapy-related adverse outcomes is not without its challenges. It requires integration of changing technologies, adoption of new concepts of complex statistical modeling, and multi-disciplinary collaborations.60–62 Advances in molecular biology and biotechnology have provided inexpensive and rapid laboratory methods required to conduct large population-based studies.59,63 The traditional approach has been to use the candidate gene approach, in which a gene or pathway is targeted as potentially important based on a priori hypotheses about their etiological role in development of disease.64 However, the sequencing of the human genome has greatly expanded the ability of researchers to broaden the focus of genomic studies and perform genome-wide association studies (GWAS), allowing the study of genetic variation across the entire genome and risk of disease.65 The choice of one approach over the other depends on a number of variables including, but not limited to the following: sample size, study design, availability of resources, and outcomes of interest.
The advantage of the candidate gene approach is that it allows us to ask hypothesis-driven questions using relatively modest sample sizes; it has the potential to provide a more thorough understanding of the genetic variation within a gene or pathway and its functional consequence; and it may be easily incorporated into statistical models that already include numerous clinical and treatment-related exposures. If the detected association is strong enough, investigators can quickly move a SNP into a clinical model for risk-based intervention. The disadvantages of the candidate gene approach is that due to budgetary and statistical considerations, often small numbers of genes are evaluated at a time, possibly missing genes in pathways that are not a part of the a priori hypothesis, but may be in linkage disequilibrium; genetic associations have been difficult to replicate for complex diseases unless the magnitude of effect is significantly great—a rare occurrence when looking at high frequency polymorphisms with low penetrance; and lastly, it would be difficult to adopt the candidate gene approach when the fundamental physiological defects of the disease are unknown.
Researchers have begun to increasingly advocate the use of a GWAS for the study of complex chronic diseases such as cardiovascular disease, asthma, and diabetes.65,66 GWAS is an approach whereby rapidly scanning markers across complete sets of DNA, or genomes, of many people are used to find genetic variations associated with a particular disease. The advantage of GWAS is that it allows for the study of multiple genes, potentially identifying known and previously unknown genetic variants contributing to phenotype and outcome; investigators are able to use information obtained from preliminary GWAS analyses for more detailed investigation of genes and pathways of interest. While at one point, the GWAS approach was costly, genotyping costs are likely to decrease, allowing for the simultaneous investigation of numerous studies. The disadvantages of GWAS are that it often leads to a high false discovery rate and may miss rare but important alleles that are less well represented in large SNP databases; once a set of SNPs is identified, investigators are faced with the dilemma of how best to prioritize the ones which deserve further study and eventually take into the clinical intervention model; lastly, due to the large number of associations being tested, GWAS is best reserved for studies with large numbers of patients with outcomes that occur at greater frequency than those occurring in the cancer survivors population.
CONCLUSIONS
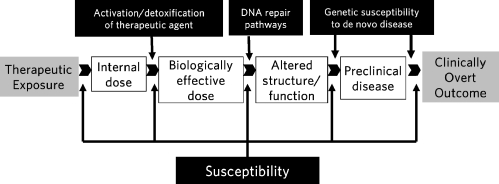
Advances in the treatment of childhood cancer have led to a growing number of survivors at risk for long-term adverse outcomes. During the last two decades there has been a sustained effort to try and identify the clinical and treatment-related risk factors for these outcomes. However, there continue to be large gaps in knowledge with regards to the pathogenesis of therapy-related adverse events. These gaps can be filled only by approaching these problems in a systematic, comprehensive manner that not only helps further the understanding of disease biology but also identifies those at highest risk of these adverse outcomes (Fig. 1). This approach requires multidisciplinary teams and access to large patient populations. One such initiative is currently ongoing within the Children’s Oncology Group (Funding: Lance Armstrong Foundation and Leukemia-Lymphoma Society)—where a mechanism has been successfully established to identify key adverse events and matched controls, obtain detailed therapeutic exposure data and obtain biospecimens, with the goal to understand the molecular pathogenesis of these outcomes.
Figure 1.
Biological markers and proposed areas of genetic susceptibility along the continuum from therapeutic exposure to clinically overt disease.
Acknowledgements
Funding/support: Lymphoma/Leukemia Society Scholar Award for Clinical Research 2191–02 (S. Bhatia).
Conflict of Interest None disclosed.
Footnotes
Funding/support: Lymphoma/Leukemia Society Scholar Award for Clinical Research 2191–02 (S. Bhatia).
References
- 1.Ries L, Eisner M, Kosary C, et al. Cancer Statistics Review, 1975–2002, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2002/, Accessed April 2009.
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. [DOI] [PubMed]
- 3.Green DM. Late effects of treatment for cancer during childhood and adolescence. Curr Probl Cancer. 2003;27:127–42. [DOI] [PubMed]
- 4.Bhatia S. Late effects among survivors of leukemia during childhood and adolescence. Blood Cells Mol Dis. 2003;31:84–92. [DOI] [PubMed]
- 5.Childhood cancer survivorship: improving care and quality of life. Washington, D.C., National Academy Press, 2003 [PubMed]
- 6.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–90. [DOI] [PubMed]
- 7.Johnson LA, Ross JA. Host factors and consequence of chemotherapy in pediatric cancer patients. Pediatr Blood Cancer. 2008;51:320–6. [DOI] [PubMed]
- 8.Hartford CM, Dolan ME. Identifying genetic variants that contribute to chemotherapy-induced cytotoxicity. Pharmacogenomics. 2007;8:1159–68. [DOI] [PMC free article] [PubMed]
- 9.Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer. 2001;1:99–108. [DOI] [PubMed]
- 10.van Dalen EC, van der Pal HJ, Kok WE, et al. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–8. [DOI] [PubMed]
- 11.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. [DOI] [PubMed]
- 12.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Hematol Oncol Clin North Am. 2008;22:305–18. vii. [DOI] [PubMed]
- 13.Armenian SH, Sun CL, Francisco L, et al. Late Congestive Heart Failure After Hematopoietic Cell Transplantation. J Clin Oncol 26:In Press, 2008 [DOI] [PMC free article] [PubMed]
- 14.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. [DOI] [PubMed]
- 15.Horenstein MS, Vander Heide RS, L'Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab. 2000;71:436–44. [DOI] [PubMed]
- 16.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–43. [DOI] [PubMed]
- 17.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44:600–6. [DOI] [PubMed]
- 18.Henderson IC, Allegra JC, Woodcock T, et al. Randomized clinical trial comparing mitoxantrone with doxorubicin in previously treated patients with metastatic breast cancer. J Clin Oncol. 1989;7:560–71. [DOI] [PubMed]
- 19.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–95. [DOI] [PubMed]
- 20.Deng S, Wojnowski L. Genotyping the risk of anthracycline-induced cardiotoxicity. Cardiovasc Toxicol. 2007;7:129–34. [DOI] [PubMed]
- 21.Wojnowski L, Kulle B, Schirmer M, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–62. [DOI] [PubMed]
- 22.Dell'Acqua G, Polishchuck R, Fallon JT, et al. Cardiac resistance to adriamycin in transgenic mice expressing a rat alpha-cardiac myosin heavy chain/human multiple drug resistance 1 fusion gene. Hum Gene Ther. 1999;10:1269–79. [DOI] [PubMed]
- 23.Gonzalez-Covarrubias V, Kalabus JL, Blanco JG. Inhibition of polymorphic human carbonyl reductase 1 (CBR1) by the cardioprotectant flavonoid 7-monohydroxyethyl rutoside (monoHER). Pharm Res. 2008;25:1730–4. [DOI] [PMC free article] [PubMed]
- 24.Lakhman SS, Ghosh D, Blanco JG. Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3). Drug Metab Dispos. 2005;33:254–7. [DOI] [PubMed]
- 25.Burger B, Beier R, Zimmermann M, et al. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)–experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44:220–5. [DOI] [PubMed]
- 26.Bomelburg T, von Lengerke HJ, Ritter J. Aseptic osteonecroses in the treatment of childhood acute leukaemias. Eur J Pediatr. 1989;149:20–3. [DOI] [PubMed]
- 27.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:3038–45. [DOI] [PMC free article] [PubMed]
- 28.Mattano LA Jr., Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children's Cancer Group. J Clin Oncol. 2000;18:3262–72. [DOI] [PubMed]
- 29.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–6. [DOI] [PubMed]
- 30.Mattano LA Jr., Sather HN, Nachman JB. Modified dexamethasone (DXM) reduces the incidence of treatment-related osteonecrosis (ON) in children and adolescents with higher risk acute lymphoblastic leukemia: A report of CCG-1961. Blood. 2003;102:221a. (abstr 777).
- 31.Arico M, Boccalatte MF, Silvestri D, et al. Osteonecrosis: An emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica. 2003;88:747–53. [PubMed]
- 32.Strauss AJ, Su JT, Dalton VM, et al. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19:3066–72. [DOI] [PubMed]
- 33.Sala A, Mattano LA Jr., Barr RD. Osteonecrosis in children and adolescents with cancer - an adverse effect of systemic therapy. Eur J Cancer. 2007;43:683–9. [DOI] [PubMed]
- 34.Shusterman S, Meadows AT. Long term survivors of childhood leukemia. Curr Opin Hematol. 2000;7:217–22. [DOI] [PubMed]
- 35.Assouline-Dayan Y, Chang C, Greenspan A, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed]
- 36.French D, Hamilton LH, Mattano LA Jr., et al. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111:4496–9. [DOI] [PMC free article] [PubMed]
- 37.Van Veldhuizen PJ, Neff J, Murphey MD, et al. Decreased fibrinolytic potential in patients with idiopathic avascular necrosis and transient osteoporosis of the hip. Am J Hematol. 1993;44:243–8. [DOI] [PubMed]
- 38.Glueck CJ, Fontaine RN, Gruppo R, et al. The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis. Clin Orthop Relat Res. 1999;133–46 [DOI] [PubMed]
- 39.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–65. [DOI] [PubMed]
- 40.Rogers PC, Meacham LR, Oeffinger KC, et al. Obesity in pediatric oncology. Pediatr Blood Cancer. 2005;45:881–91. [DOI] [PubMed]
- 41.Odame I, Reilly JJ, Gibson BE, et al. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71:147–9. [DOI] [PMC free article] [PubMed]
- 42.Craig F, Leiper AD, Stanhope R, et al. Sexually dimorphic and radiation dose dependent effect of cranial irradiation on body mass index. Arch Dis Child. 1999;81:500–4. [DOI] [PMC free article] [PubMed]
- 43.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: role of cranial irradiation. Med Pediatr Oncol. 2000;35:91–5. [DOI] [PubMed]
- 44.Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88:4731–9. [DOI] [PubMed]
- 45.Nathan PC, Jovcevska V, Ness KK, et al. The prevalence of overweight and obesity in pediatric survivors of cancer. J Pediatr. 2006;149:518–25. [DOI] [PubMed]
- 46.Lustig RH, Post SR, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88:611–6. [DOI] [PubMed]
- 47.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. [DOI] [PubMed]
- 48.Stephens TW, Basinski M, Bristow PK, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–2. [DOI] [PubMed]
- 49.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. [DOI] [PubMed]
- 50.Clement K, Ferre P. Genetics and the pathophysiology of obesity. Pediatr Res. 2003;53:721–5. [DOI] [PubMed]
- 51.Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–51. [DOI] [PubMed]
- 52.Rankinen T, Perusse L, Weisnagel SJ, et al. The human obesity gene map: the 2001 update. Obes Res. 2002;10:196–243. [DOI] [PubMed]
- 53.Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:3558–62. [DOI] [PubMed]
- 54.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291:1304–51. [DOI] [PubMed]
- 55.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. [DOI] [PubMed]
- 56.Loktionov A. Common gene polymorphisms and nutrition: emerging links with pathogenesis of multifactorial chronic diseases (review). J Nutr Biochem. 2003;14:426–51. [DOI] [PubMed]
- 57.Subramanian G, Adams MD, Venter JC, et al. Implications of the human genome for understanding human biology and medicine. Jama. 2001;286:2296–307. [DOI] [PubMed]
- 58.Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–56. [DOI] [PubMed]
- 59.Khoury MJ. Genetic epidemiology and the future of disease prevention and public health. Epidemiol Rev. 1997;19:175–80. [DOI] [PubMed]
- 60.Austin MA, Peyser PA, Khoury MJ. The interface of genetics and public health: research and educational challenges. Annu Rev Public Health. 2000;21:81–99. [DOI] [PubMed]
- 61.Ioannidis JP, Bernstein J, Boffetta P, et al. A network of investigator networks in human genome epidemiology. Am J Epidemiol. 2005;162:302–4. [DOI] [PubMed]
- 62.Khoury MJ, Little J, Gwinn M, et al. On the synthesis and interpretation of consistent but weak gene-disease associations in the era of genome-wide association studies. Int J Epidemiol. 2007;36:439–45. [DOI] [PubMed]
- 63.Khoury MJ, Millikan R, Little J, et al. The emergence of epidemiology in the genomics age. Int J Epidemiol. 2004;33:936–44. [DOI] [PubMed]
- 64.Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet. 2002;3:391–7. [DOI] [PubMed]
- 65.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. [DOI] [PubMed]
- 66.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–3. [DOI] [PubMed]