Abstract
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder in which the underlying pathophysiology is poorly understood; however, increased intestinal permeability in diarrhea-predominant IBS patients has been reported. Here we demonstrate diarrhea-predominant IBS patients (D-IBS) that display increased intestinal permeability. We have also found that increased intestinal membrane permeability is associated with visceral and thermal hypersensitivity in this subset of D-IBS patients. We evaluated 54 D-IBS patients and 22 controls for intestinal membrane permeability using the lactulose / mannitol method. All subjects ingested 5 g laclulose and 2 g mannitol in 100 ml of water after which their urine was collected. We also evaluated the mean mechanical visual analogue (MVAS) pain rating to nociceptive thermal and visceral stimulation in all subjects. All study participants also completed the FBDSI scale. Approximately 39% of diarrhea-predominant IBS patients have increased intestinal membrane permeability as measured by the lactulose / mannitol ratio. These IBS patients also demonstrated higher M-VAS pain intensity reading scale. Interestingly, the IBS patients with hypersensitivity and increased intestinal permeability had a higher FBDSI score (100.8±5.4) compared to IBS patients with normal membrane permeability and sensitivity (51.6±12.7) and controls (6.1 ± 5.6) (p<0.001). A subset of D-IBS patients have increased intestinal membrane permeability that is associated with an increased FBDSI score and increased hypersensitivity to visceral and thermal nociceptive pain stimuli. Thus, increased intestinal membrane permeability in D-IBS patients may lead to more severe IBS symptoms and hypersensitivity to somatic and visceral stimuli.
Keywords: Irritable bowel syndrome (IBS), visceral and thermal hypersensitivity, intestinal membrane permeability, functional bowel disease severity index (FBDSI)
INTRODUCTION
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder seen by gastroenterologists and is estimated to affect up to 20% of the U.S. population. Even though the pathophysiology of IBS is unclear, visceral hypersensitivity is an accepted biological marker of the disorder [22,38]. Despite the fact that IBS is one of the most common gastrointestinal disorders in the United States, the pathophysiological mechanisms of pain and hypersensitivity in IBS are not well understood. It is now well established that some patients with IBS demonstrate enhanced perception in response to brief phasic distension of the gut lumen, or visceral hypersensitivity [22,27,39,41]. Visceral hypersensitivity is a biological marker in some IBS patients and may account for symptoms of urgency, bloating, and abdominal pain experienced by patients with IBS. The cause of visceral hypersensitivity is unknown but several mechanisms have been postulated and include triggering events such as inflammation, psychological or environmental stress, or post-injury sensitization [12,24]. The effects these triggering events have on primary visceral afferents are now starting to be better understood.
Although hypersensitivity has been thought to be limited to the gut, many patients with IBS frequently complain of pain in body regions somatotopically distinct from the gut, suggesting that central hyperalgesic mechanisms may be involved [12,22,40]. Interestingly, several studies have shown that IBS patients demonstrate hyperalgesia to nociceptive stimuli applied to somatic tissues [4,10,39,41]. These results suggest that visceral and somatic nociceptive processing overlap, particularly in the lumbosacral distribution, as a result of viscerosomatic convergence. Thus, tonic input from the gut may sensitize spinal cord neurons that have viscerosomatic convergence and exhibit somatotopic overlap with the gut.
Several studies have now shown that patients with diarrhea-predominant IBS have increased intestinal membrane permeability [9,35,36]. This increased intestinal permeability may be due a number of factors including low-grade inflammation that has been reported in mucosal biopsies of some diarrhea-predominant (D-IBS) and post-infectious (PI-IBS) patients, but not constipation-predominant (C-IBS) patients [9]. It has been well established that patients with inflammatory conditions such as inflammatory bowel disease, celiac sprue, and acute alcoholic gastroenteritis have increased gut permeability [3,17,18]. The acute symptoms usually coincide with the acute inflammation that leads to chronic abdominal pain, diarrhea, and bloating. Transient inflammation of the gut may also cause sensitization of enteric neurons which persists long after resolution of the inflammation, similar to that demonstrated in recent animal models of functional gastrointestinal disorders [2,23,44].
To further evaluate visceral and somatic hypersensitivity in patients with IBS, we measured intestinal membrane permeability; thermal and visceral pain sensitivity; and the FBDSI score in diarrhea-predominant IBS patients compared to healthy subjects. The aims of this study were: (1) to evaluate intestinal membrane permeability in diarrhea-predominant IBS patients compared to controls; (2) to compare the FBDSI score in IBS patients with increased membrane permeability compared to IBS patients with normal membrane permeability and controls; and (3) to compare thermal and visceral hypersensitivity in diarrhea-predominant IBS patients vs. controls.
METHODS
Participants
A total of 54 patients (mean age 29.5 ± 7.33 years) with diarrhea-predominant IBS and 22 control subjects (mean age 28.3 ± 5.32 years) participated in the study. The study was approved by the University of Florida and the Ohio State University Institutional Review Boards. All subjects signed informed consent prior to the start of the study.
None of the control subjects had any evidence of acute or chronic somatic/abdominal pain or IBS based on a questionnaire and complete physical exam by an experienced gastroenterologist. Also, controls were free of any systemic disease or conditions that could affect sensory responses. All IBS patients had symptoms for at least 5 years and had upper and lower endoscopy with normal biopsies. The diagnosis of IBS was made by the same gastroenterologist who examined patients based on the ROME III criteria and exclusion of organic disease [20]. Fibromyalgia (FM) was excluded in all IBS patients using the 1990 American College of Rheumatology criteria for FM [13]. None of the participants were on pain medications, serotonin uptake inhibitors, serotonin antagonists, or tricyclic antidepressants for at least 3 weeks prior to the study. Also, all participants were required to refrain from ingesting NSAIDs and/or alcohol for 1 month before the study.
Functional Bowel Disorder Severity Index (FBDSI)
All subjects completed the Functional Bowel Disorder Severity Index (FBDSI) which comprises 3 variables: current pain (by visual analog scale), diagnosis of chronic abdominal pain, and number of physician visits in the past 6 months [33]. The Functional Bowel Disorder Severity Index is sensitive enough to distinguish among the different groups from healthy controls through IBS nonpatients, to patients with IBS only and, finally, IBS patients with concomitant fibromyalgia. Severity was rated as none (0 points-controls), mild (1-36 points-IBS nonpatients), moderate (37-110 points-IBS patients), and severe (>110 points-IBS and fibromyalgia). All participants in the study were characterized with this index.
Intestinal Membrane Permeability Testing (Lactulose/Mannitol)
The intestinal membrane permeability test that was used (Genova Diagnostics®, Asheville, NC) directly measures the ability of mannitol and lactulose (nonmetabolized sugar molecules) to permeate the intestinal mucosa. Mannitol is easily absorbed and serves as a marker of transcellular uptake, while lactulose is only slightly absorbed and serves as a marker for mucosal integrity. After an overnight fast, each subject voided to empty their bladder and then ingested 5 g of lactulose together with 2 g of mannitol dissolved in 100 ml of water. Urine was then collected in hourly samples for 24 hours and analyzed for lactulose and mannitol. An increased ratio of the excretion of lactulose / mannitol is suggestive of an increase in membrane permeability. Based on normative data from controls in our laboratory, a lactulose / mannitol ratio ≥0.07 is indicative of increased intestinal permeability.
Thermal/Visceral Pain Testing
All sessions were conducted between 9 AM and 6 PM to control for circadian rhythm effects. Subjects were instructed to refrain from use of any analgesic medication or caffeine for 48 hours before their sessions. Female subjects participated during the follicular phase of their cycles (i.e. 4-9 days post onset of menses). This cycle phase was chosen because it is characterized by the least sensitivity to pain and by minimal menstrual cycle related symptoms [11]. The menstrual cycle has been reported to alter pain perception and IBS symptoms in females [16,39]. The follicular phase was chosen as some female subjects will be using oral contraceptives (OC), and the follicular phase is the cycle phase during which women using OC and normally cycling women are the most similar in their responses to experimental pain [11].
Response Measure for Pain Stimuli
A mechanical visual analogue scale (M-VAS) was used to measure perceived pain intensity in all subjects during the thermal stimulation and rectal distension testing [30]. Each patient was asked to read a standardized set of instructions for the visual analog scales. The M-VAS is anchored by the descriptors “no pain sensation” at zero and “the most intense pain sensation imaginable” at 10. The M-VAS was used rather than a simple numerical scale because it has ratio scale characteristics and therefore provides accurate estimates of ratios of pain sensation intensity and % changes in pain intensity. Standard written instructions and practice in rating the thermal and rectal distension stimuli was given to all subjects prior to testing. All subjects rated their current clinical pain intensity on the M-VAS scale during the day of each testing session. After these instructions, each subject was queried to insure they understand the instructions for rating their pain intensity, and additional explanation was provided as needed. The experimental pain testing sessions were conducted by two research assistants, one of whom primarily interacted with the subject in administering the pain testing procedure, and the other operated the computer and other equipment. Subjects were randomly assigned within each group (Controls, D-IBS) to receive either thermal nociceptive stimulation to the left calf or rectal distension first in a random order that was counterbalanced across groups. Study subjects were given a 1 hour rest period in between the thermal and visceral experimental pain testing.
Thermal Pain Stimuli
Thermal stimuli were delivered to the left calf using a computer-controlled Medoc Thermal Sensory Analyzer (TSA-2001, Ramat Yishai, Israel). This is peltier-element-based stimulator with a 3cm × 3cm surface area. Temperature levels were monitored by a contact-contained thermistor, and returned to a preset baseline of 32°C by active cooling at a rate of 10 deg C/Sec. The subject received a 47 °C stimulus to the left calf for 10 seconds. The subject then rated pain intensity on the M-VAS [30]. The thermal stimulus was repeated after a 2 minute interstimulus interval and the mean pain intensity rating of the 2 trials was recorded. The position of the thermode was altered slightly between the 2 trials in order to avoid either sensitization or habituation of cutaneous receptors on the calf.
Visceral Pain Testing
The balloon that was used for rectal distension consisted of a 500 ml polyethylene bag secured on a rectal catheter (Zinetics Medical, Inc., Salt Lake City, UT) using unwaxed dental floss and parafilm to ensure a tight seal. The subjects were placed in the left lateral decubitus position. The research technician described the procedure to the subject in detail. The physician and research technician performing the pain testing were not aware of what group (IBS, control) the study subject belonged to.
The rectal balloon was lubricated and placed into the rectum so that the attached end of the bag was 1 cm from the anal sphincter. A barostat (IsoBar 3: G & J Electronics Corp., Willowdale, Ontario) was used to inflate (phasic distension, 870 mL/min) the rectal balloon to a pressure plateau of 35 mmHg for 30 seconds followed by a 2 minute inter-stimulus interval (resting pressure of 5 mmHg). Following the rectal distension stimulus, the subject was asked to rate the perceived pain intensity using the M-VAS scale [30]. The trial was repeated and the mean pain intensity for the 2 trials was recorded for each subject.
Statistical Analysis
The statistical analysis has been performed using SAS software, Version 9.1.3 of SAS system Copyright © 2003 SAS institute Inc. and Prism version 6. Frequency distribution was used to classify the groups of patients for intestinal membrane permeability. Values are expressed as means ± standard deviation (SD). Pearson correlations between membrane permeability measured by the lactulose/mannitol ratio and VAS visceral intensity, VAS thermal intensity, and FBDSI were analyzed.
RESULTS
1. Subjects
A total of 76 participants were studied that included 54 patients (41 Females, 13 Males; mean age 29.5 ± 7.33 years) with diarrhea-predominant IBS (D-IBS); and 22 control subjects (16 Females, 6 Males; mean age 28.3±5.32 years). There was no difference in age or sex between the groups (controls, D-IBS). All of the patients had diarrhea-predominant (D-IBS) and met the Rome III criteria for irritable bowel syndrome.
2. Intestinal Membrane Permeability Testing
All IBS patients and controls underwent intestinal membrane permeability testing. Frequency distribution analysis was performed to compare the IBS patients to controls. A total of 21/54 (39%) of the IBS patients had an elevated lactulose / mannitol ratio ≥0.07, whereas, 33 / 54 (61%) of the IBS patients and all of the controls had a lactulose / mannitol ratio of <0.07 (See Figure 1).
Figure 1.
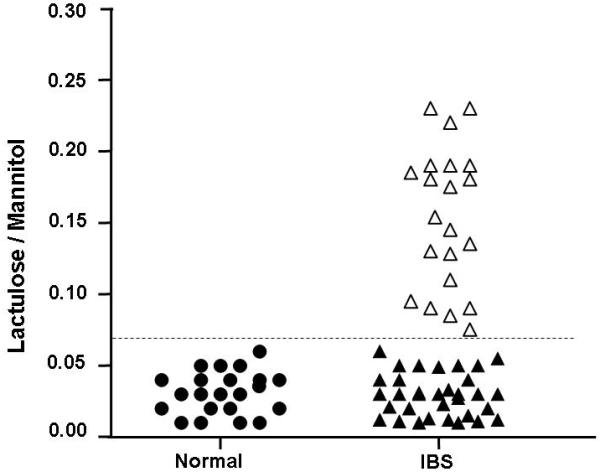
Frequency distribution of lactulose / mannitol ratio in controls and IBS patients. Black circles represent the controls. IBS patients with a lactulose / mannitol ratio of <0.07 are illustrated with black filled triangles. Empty triangles represent IBS patients with a lactulose / mannitol ratio of ≥0.07.
3. Functional Bowel Disorder Severity Index (FBDSI)
Clinical symptoms as measured by the FBDSI were positively correlated with increased membrane permeability. The mean FBDSI score was 100.8 ± 5.4 in IBS patients with a lactulose / mannitol ration ≥0.07. The mean FBDSI score was 51.6 ± 12.7 in IBS with a lactulose / mannitol ratio <0.07 and 6.1 ± 5.6 in controls (See Figure 2). Thus, more severe IBS symptoms were correlated with an increase in intestinal membrane permeability.
Figure 2.
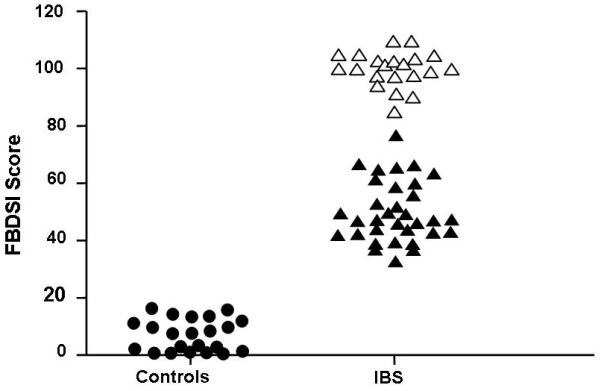
Frequency distribution of FBDSI score in controls and IBS patients. Black circles represent the controls. IBS patients with a lactulose / mannitol ratio in the normal range are illustrated with black filled triangles. Empty triangles represent IBS patients with a lactulose / mannitol ratio that is elevated.
4. Visceral Pain Testing
Interestingly, visceral hypersensitivity was positively correlated with an increase in intestinal membrane permeability. The IBS patients with a lactulose / mannitol ratio of ≥0.07 had significantly higher VAS intensity ratings to nociceptive rectal distension than those IBS patients and controls with a lactulose / mannitol ratio of <0.07 (See Figure 3). Thus, increased visceral hypersensitivity was correlated with an increase in intestinal membrane permeability.
Figure 3.
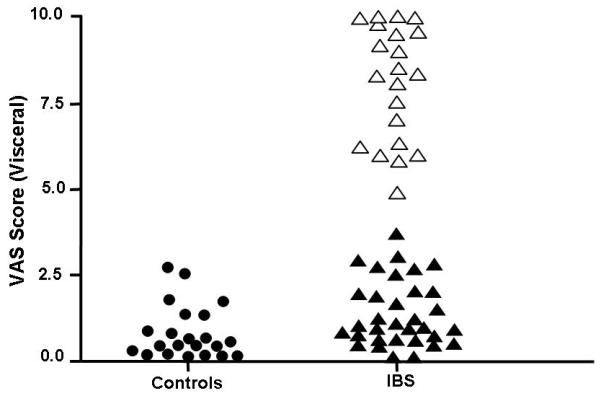
Frequency distribution of VAS score for visceral sensitivity in controls and IBS patients. Black circles represent the controls. IBS patients with a lactulose / mannitol ratio in the normal range are illustrated with black filled triangles. Empty triangles represent IBS patients with a lactulose / mannitol ratio that is elevated.
5. Thermal Pain Testing
Similar to visceral hypersensitivity, thermal hypersensitivity was positively correlated with an increase in intestinal membrane permeability. IBS patients with a lactulose / mannitol ratio of ≥0.07 had significantly higher VAS intensity ratings to nociceptive thermal stimulation of the calf than those IBS patients and controls with a lactulose / mannitol ratio of <0.07(See Figure 4). Thus, increased thermal hypersensitivity was correlated with an increase in intestinal membrane permeability.
Figure 4.
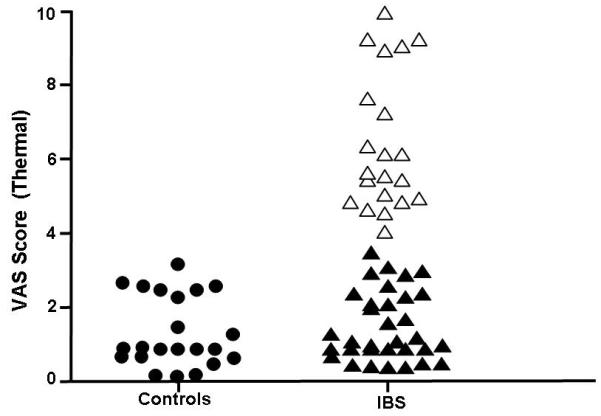
Frequency distribution of VAS score for thermal sensitivity in controls and IBS patients. Black circles represent the controls. IBS patients with a lactulose / mannitol ratio in the normal range are illustrated with black filled triangles. Empty triangles represent IBS patients with a lactulose / mannitol ratio that is elevated.
6. Membrane Permeability Correlations
Pearson correlations between membrane permeability and the main outcome measures of VAS visceral intensity, VAS thermal intensity, and FBDSI were analyzed. In the IBS patients, there was a significant positive correlation between the lactulose / mannitol ratio and VAS visceral intensity, VAS thermal intensity, and FBDSI (Table 1 left panel). For the control group, there was no correlation between the lactulose / mannitol ratio and VAS visceral intensity, VAS thermal intensity, and FBDSI (Table 1 right panel).
Table 1.
Person correlation coefficients for IBS patients and controls.
| Person Correlation Coefficients IBS patients n=54 |
Person Correlation Coefficients Normal Controls n=22 |
||
|---|---|---|---|
| Lactulose /Mannitol | Lactulose /Mannitol | ||
| VAS Visceral | 0.74909 p < 0.0001 |
VAS Visceral | − 0.04094 p = 0.4400 |
| VAS Thermal | 0.79641 p < 0.0001 |
VAS Thermal | − 0.05389 p =0.8117 |
| FBDSI | 0.76321 p < 0.0001 |
FBDSI | −0.03125 p = 0.8902 |
DISCUSSION
The results of our current study indicate that approximately 39% of diarrhea-predominant IBS patients have increased intestinal membrane permeability as measured by the lactulose / mannitol ratio. These results support previous studies that have shown that patients with diarrhea-predominant IBS have increased intestinal membrane permeability [9,35,36]. A unique finding of our work is that diarrhea-predominant IBS patients with increased membrane permeability also have a higher FBDSI score along with increased visceral and thermal hypersensitivity to experimental nociceptive pain stimuli. To the best of our knowledge, the relationship between increased intestinal membrane permeability and hypersensitivity in IBS patients has not been studied previously.
The major functions of the gastrointestinal tract are to act as an absorptive organ and as a barrier to bacteria, macromolecules, and toxic compounds [3,21]. Disruption of this barrier can lead to local gastrointestinal dysfunction as well as systemic abnormalities such as bacterial translocation and sepsis. Abnormalities of the immune or mechanical barriers lead to enhanced uptake of inflammatory luminal macromolecules and pathogenic bacteria. Increased membrane permeability of the intestinal mucosal barrier appears to correlate with a number of clinical disorders including: inflammatory bowel disease, food allergies, allergic disorders, rheumatoid arthritis, celiac disease, and several chronic dermatological conditions [28]. The most recent literature has focused on increased membrane permeability as a potential etiologic factor in IBS patients [5].
Several studies have shown that 20-25% of patients develop IBS symptoms following enteric infection of the gut [15,25,26,34,35,36]. The acute symptoms will usually resolve within a week, however, abdominal pain, diarrhea, and bloating persist. Transient small bowel and colonic inflammation may cause sensitization of the gut which persists long after resolution of the inflammation, similar to that demonstrated in animal models of functional gastrointestinal disorders [2,22,23,44]. Although previous studies suggest certain triggering events may lead to chronic visceral hypersensitivity, little is known about the specific peripheral and/or colonic afferents that are sensitized and lead to chronic visceral hypersensitivity.
The findings of this study further support our previous work that a subset of IBS patients have evidence of somatic hypersensitivity as a result of central or peripheral mechanisms [40,41]. In contrast to our current findings, a few studies have indicated lack of somatic hypersensitivity in IBS patients compared to controls [1,8,42,45]. One possible explanation for this could be differences in the type of pain stimulus, as previous studies have used electrical stimuli, mechanical pressure, and cold immersion. Another study reported that female IBS patients showed significantly higher pressure pain thresholds than female controls in response to a randomly administered series of fixed stimuli, but no group differences emerged for threshold assessed using ascending stimuli [7]. Consistent with the present findings, other investigators have reported somatic hypersensitivity in IBS patients using cold pain [4,43], and we have shown similar results with heat immersion [39,41]. Wilder-Smith and colleagues reported that approximately half of their IBS population showed somatic hypersensitivity, defined as being below the 95% confidence interval of the control population [43]. These investigators also reported a strong association between somatic hypersensitivity and visceral hypersensitivity as we have in our current study.
The presence of increased membrane permeability in some diarrhea-predominant IBS patients may set up a chronic nociceptive drive from the gut to the spinal cord which could then lead to central sensitization. Thus, our results demonstrate that a subset of IBS patients with increased thermal sensitivity also have increased intestinal membrane permeability. These novel findings are important as they may shed light on the underlying pathophysiology of somatic pain in IBS patients.
There is mixed evidence regarding the association between visceral hypersensitivity and clinical symptoms in IBS [6,19,29,31,37]. Our present study reports an association between IBS symptom severity as measured by the FBDSI and the presence of visceral and thermal hypersensitivity. It is interesting that the IBS patients with visceral and somatic hypersensitivity had increased intestinal membrane permeability. It is possible that disruption of the intestinal barrier can lead to both local gastrointestinal dysfunction and symptoms. Increased intestinal permeability allows the passage of bacteria and antigens through the mucosal layer of the gut. This may then lead to activation of mucosal immune responses and subsequent chronic diarrhea and abdominal pain seen in IBS patients [34]. Our current data indicated that a subset of IBS patients have increased intestinal membrane permeability associated with visceral and thermal hypersensitivity. In these patients, increased membrane permeability may allow the passage of bacteria and inflammatory agents through the mucosal layer of the gut leading to sensitization of the myenteric plexus and the common spinal segments of the central nervous system. This spinal sensitization in IBS patients could be created and/or maintained by tonic impulse input from the colon to the spinal cord. Several studies have shown that IBS patients have hypersensitivity in response to experimental nociceptive somatic stimuli in addition to visceral hypersensitivity further supporting the presence of central sensitization [4,14,39]. The spatial distribution of somatic hyperalgesia in IBS patients exists in a gradient of hyperalgesia with the most pronounced hyperalgesia occurring at lumbosacral levels where colonic and lower extremity nociceptive afferents are likely to converge on common spinal segments (i.e. viscerosomatic convergence) [39,41].
Work by our laboratory and others suggests that patients with functional pain disorders such as IBS may be characterized by abnormalities in both peripheral and central pain processing mechanisms [4,12,40,41]. Many patients with IBS also exhibit a wide variety of somatic symptoms including back pain, migraine headaches, heartburn, dyspareunia, and muscle pain. Collectively, these somatic symptoms suggest that IBS patients may also suffer from central hyperalgesic dysfunction [10,39,40,41]. Similar to IBS, patients with other chronic pain disorders such as temporomandibular disorders have been shown to have generalized hyperalgesia [32]. The role of central sensitization in IBS patients is now further supported by our human data that suggests IBS patients have somatic hypersensitivity in response to experimental nociceptive thermal stimuli in addition to visceral hypersensitivity.
Conclusions
Our study indicates that a subset of IBS patients with increased intestinal membrane permeability have visceral and thermal hypersensitivity. These findings are unique and add further support that some IBS patients have visceral and somatic hypersensitivity similar to what we have recently shown in an animal model [44]. The FBDSI score in IBS patients was also highly correlated with both an increase in intestinal membrane permeability and visceral and thermal hypersensitivity. Further studies are warranted to determine if the FBDSI score could be used as a predictor of visceral and thermal hypersensitivity in IBS patients along with increased intestinal permeability. IBS patients with a high FBDSI and increased membrane permeability may have central sensitization that would warrant alternative treatment modalities instead of the standard IBS therapies that mainly target the gut.
Acknowledgements
This study was supported by a NIH RO1-NS053090 award (PI: GN Verne) and a VA Merit Review Award (PI: GN Verne) from the Medical Research Service at the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial or other relationship to report that might lead to a conflict of interest.
REFERENCES
- 1.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108(3):636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 2.Al Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 4.Bouin M, Meunier P, Riberdy-Poitras M, Poitras P. Pain hypersensitivity in patients with functional gastrointestinal disorders: a gastrointestinal-specific defect or a general systemic condition? Dig Dis Sci. 2001;46(11):2542–48. doi: 10.1023/a:1012356827026. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol. Motil. 2007;19:545–52. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 6.Castilloux J, Noble A, Faure C. Is visceral hypersensitivity correlated with symptom severity in children with functional gastrointestinal disorders? J Pediatr Gastroenterol Nutr. 2008;45(3):272–278. doi: 10.1097/MPG.0b013e31814b91e7. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84(23):297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 8.Cook IJ, van Eeden A, Collins SM. Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. Gastroenterology. 1987;93(4):727–733. doi: 10.1016/0016-5085(87)90434-3. [DOI] [PubMed] [Google Scholar]
- 9.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–94. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 10.Dunphy RC, Bridgewater L, Price DD, Robinson ME, Zeilman CJ, 3rd, Verne GN. Visceral and cutaneous hypersensitivity in Persian Gulf War veterans with chronic gastrointestinal symptoms. Pain. 2003;102:79–85. doi: 10.1016/s0304-3959(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 11.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 12.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. AmJGastrointest. Liver Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 13.Geel SE. The fibromyalgia syndrome: musculoskeletal pathophysiology. Semin Arthritis Rheum. 1994;23(5):347–353. doi: 10.1016/0049-0172(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 14.Geisser ME, Robinson ME, Pickwren WE. Differences in cognitive coping strategies among pain-sensitive and pain-tolerant individuals on the cold pressor test. Beh Ther. 1992;23:31–42. [Google Scholar]
- 15.Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347(8995):150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 16.Heitkemper MM, Jarrett M. Pattern of gastrointestinal and somatic symptoms across the menstrual cycle. Gastroenterology. 1992;102(2):505–513. doi: 10.1016/0016-5085(92)90097-i. [DOI] [PubMed] [Google Scholar]
- 17.Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. 1994;89(12):2205–2211. [PubMed] [Google Scholar]
- 18.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22(2):157–164. doi: 10.1111/j.1365-2036.2005.02524.x. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald TT, Montelenone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 22.Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008 Mar;57(3):384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122(7):2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 24.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 25.McKendrick MW, Read NW. Irritable bowel-syndrome—post salmonella infection. J Infect. 1994;29(1):1–3. doi: 10.1016/s0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- 26.Mearin F, Pérez-Oliveras M, Perelló A, Vinyet J, Ibañez A, Coderch J, Perona M. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Naliboff BD, Munakata J, Fullerton s, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41(4):505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porras M, Martín MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm. Bowel Dis. 2006;12:843–852. doi: 10.1097/01.mib.0000231571.88806.62. [DOI] [PubMed] [Google Scholar]
- 29.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simrén M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133(4):1113–23. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical raing scales. Pain. 1994 Feb;56(2):217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 31.Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with the irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28(4):484–490. doi: 10.1111/j.1365-2036.2008.03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temoromandibular disorders patients. Pain. 2003;102(3):221–6. doi: 10.1016/S0304-3959(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 33.Sperber AD, Carmel S, Atzmon Y, Weisberg I, Shalit Y, Neumann L, Fich A, Friger M, Buskila D. Use of the Functional Bowel Disorder Severity Index (FBDSI) in a study of patients with the irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2000;95(4):995–998. doi: 10.1111/j.1572-0241.2000.01977.x. [DOI] [PubMed] [Google Scholar]
- 34.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 35.Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42(Suppl XVII):41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 36.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Veek PP, Van Rood YR, Masclee AA. Symptom severity but not psychopathology predicts visceral hypersensitivity in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6(3):321–328. doi: 10.1016/j.cgh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Verne GN, Cerda JJ. Irritable bowel syndrome. Streamlining the diagnosis. Postgrad. Med. 1997;102:197–208. doi: 10.3810/pgm.1997.09.322. [DOI] [PubMed] [Google Scholar]
- 39.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103(12):99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 40.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4(4):322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- 41.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93(1):7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 42.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–92. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 43.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroeneterol. 2007;13(27):3699–704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats of rats following TNBS-Induced colitis. Pain. 2008;134(12):9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci. 1995;40(4):819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]


