Abstract
Objective
To evaluate whether penetration of a combination regimen into the central nervous system (CNS), as estimated by the CNS Penetration-Effectiveness (CPE) rank, is associated with lower cerebrospinal fluid (CSF) viral load.
Design
Data were analyzed from 467 participants who were human immunodeficiency virus (HIV) seropositive and who reported antiretroviral (ARV) drug use. Individual ARV drugs were assigned a penetration rank of 0 (low), 0.5 (intermediate), or 1 (high) based on their chemical properties, concentrations in CSF, and/or effectiveness in the CNS in clinical studies. The CPE rank was calculated by summing the individual penetration ranks for each ARV in the regimen.
Results
The median CPE rank was 1.5 (interquartile range, 1–2). Lower CPE ranks correlated with higher CSF viral loads. Ranks less than 2 were associated with an 88% increase in the odds of detectable CSF viral load. In multivariate regression, lower CPE ranks were associated with detectable CSF viral loads even after adjusting for total number of ARV drugs, ARV drug adherence, plasma viral load, duration and type of the current regimen, and CD4 count.
Conclusions
Poorer penetration of ARV drugs into the CNS appears to allow continued HIV replication in the CNS as indicated by higher CSF HIV viral loads. Because inhibition of HIV replication in the CNS is probably critical in treating patients who have HIV-associated neurocognitive disorders, ARV treatment strategies that account for CNS penetration should be considered in consensus treatment guidelines and validated in clinical studies.
Over the last 10 years, the use of combination antiretroviral therapy has led to declines in the incidence of complications of human immunodeficiency virus (HIV) infection.1–3 During this same period, the prevalence of neurologic disease has remained stable or increased,4,5 suggesting that treatment of central nervous system (CNS) disease may be suboptimal. One explanation for this may be poor antiretroviral (ARV) penetration into the CNS. Pharmacodynamic models1 and in vivo evidence2 suggest that the tissue-specific concentrations of ARV drugs are important determinants of their effectiveness in that tissue. Whether this is true for specific tissues in humans has been difficult to demonstrate because of technical limitations in sampling and determining tissue drug concentrations and viral loads (VLs). Many investigators have speculated that ARV concentrations in the CNS may be particularly important in treating nervous system disease, but no widely accepted or clinically useful approach to estimating CNS effectiveness exists. This has impeded clinical progress in managing the neurologic complications of HIV.
To address this need, we devised a practical method for quantifying the penetration of the many different ARV drug regimens currently prescribed. This method uses publicly available information about ARV drug characteristics, measured cerebrospinal fluid (CSF) concentrations, and effectiveness in the CNS to rank drugs relative to one another and provides a way to rank combination drug regimens by algorithmically combining the individual drug rankings. As a step toward validating this method for actual clinical use, we evaluated reductions in CSF VL as a function of the resulting CNS penetration-effectiveness (CPE) ranks. We hypothesized that patients treated with regimens having lower CPE ranks would be more likely to show evidence of residual viral replication in CSF (detectable CSF VL) than those treated with regimens having higher CPE ranks.
METHODS
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study is a multicenter, prospective, observational study designed to recruit a cohort that is similar to the US population of individuals infected with HIV and to determine the effects of ARV therapy on the nervous system. The research was conducted at 6 North American locations. All research was approved by institutional review boards at each site. The study’s procedures included comprehensive neuropsychological and neuromedical assessments, phlebotomy, and lumbar puncture. Data from baseline evaluations that occurred between October 2003 and January 2006 were included in this cross-sectional analysis.
At the time of analysis, 833 HIV-positive individuals had enrolled in the CHARTER study. Venipuncture and lumbar puncture were successfully performed on 659 subjects (79%). Of these, 467 (71%) met eligibility criteria for this analysis by reporting current ARV drug use and having HIV VL measured in both plasma and CSF.
Plasma and CSF HIV VLs were quantified by reverse transcriptase–polymerase chain reaction (Amplicor, Roche Diagnostic Systems, Indianapolis, Indiana) using the ultrasensitive assay (nominal lower limit of quantitation, 50 c/mL). Blood CD4+ cell counts were measured by flow cytometry.
Penetration of ARV drugs was characterized using a hierarchical approach based on the best available evidence. Available data on chemical characteristics, CSF pharmacology, and effectiveness in the CNS were reviewed for ARV drugs approved by the US Food and Drug Administration using package inserts, drug references, published manuscripts, and conference abstracts. Using this approach, ARV drugs were classified into 3 categories. We considered an ARV drug to be in the lowest penetration category if (1) its chemical properties supported relatively poor penetration into the CNS (eg, the large molecular weight of enfuvirtide3), (2) either its concentrations in CSF were unmeasurable in CSF in human or animal studies (eg, nelfinavir4) or CSF concentrations were measurable (eg, didanosine5) but consistently did not exceed the population median inhibitory concentration (IC50) for wild-type HIV as defined by Monogram Biosciences (South San Francisco, California), or (3) clinical studies demonstrated its ineffectiveness in reducing CSF VL or improving cognition (eg, saquinavir6). We considered an ARV drug to be in the high penetration category if (1) its molecular and pharmacologic properties supported relatively high penetration into the CNS (eg, nevirapine7), (2) its concentrations in CSF were measurable in CSF in human or animal studies and concentrations consistently exceeded the population IC50 for wild-type HIV (eg, ritonavir-boosted lopinavir8), or (3) clinical studies demonstrated its effectiveness in reducing CSF VL or improving cognition (eg, zidovudine9). A third, intermediate category was assigned if (1) clinical studies did not consistently detect the drug in CSF (eg, atazanavir10), (2) its concentrations were measurable but did not consistently exceed the population IC50 (eg, stavudine11), or (3) their chemical properties did not clearly support penetration (eg, efavirenz6). These rules were applied in a hierarchical manner: clinical effectiveness studies were considered the strongest evidence, pharmacokinetic studies were considered the next strongest evidence, and chemical data were considered in the absence of the other 2 types of evidence. For the purposes of categorization, protease inhibitors that were boosted with ritonavir were considered as a single drug distinct from the unboosted parent protease inhibitor. Individual ARV drugs were assigned a rank based on penetration category (0 = lowest penetration, 0.5 = intermediate penetration, 1 = highest penetration). The CPE rank was then determined by summing the individual penetration ranks for each ARV drug in a regimen.
Regimens of ARV drugs were categorized into groups based on the number and mechanism of action of the component ARV drugs: (1) nonnucleoside reverse transcriptase inhibitor (NNRTI)–based regimens included at least 1 NNRTI with at least 2 other ARVs and no protease inhibitors; (2) protease inhibitor–based regimens included at least 1 protease inhibitor with at least 2 other ARVs and no NNRTIs; (3) other regimens containing 3 or more drugs; (4) 2-drug regimens; and (5) 1-drug regimens. Table 1 lists the number of subjects taking each type of regimen. All study subjects completed the ACTG ARV Adherence Questionnaire. Subjects who reported skipping at least 1 ARV dose over the previous 4 days were considered to have reduced adherence.
Table 1.
Participant Characteristics
| Entire Cohort (n=467) |
CSF VL Undetectable (n=389) |
CSF VL Detectable (n=78) |
P Value | |
|---|---|---|---|---|
| White, No. (%) | 205 (44) | 182 (46.8) | 23 (29.5) | <.01 |
| Male, No. (%) | 379 (81) | 318 (82) | 61 (78) | NS |
| Mean age (SD), y | 44 (8) | 44 (8) | 42 (7) | .03 |
| Education, mean (SD), y | 12 (2) | 13 (2) | 12 (2) | NS |
| AIDS, No. (%) | 359 (77) | 295 (76) | 64 (82) | NS |
| CD4 count, median (IQR) | 406 (230–587) | 428 (268–612) | 224 (106–434) | <.001 |
| CD4 nadir, median (IQR) | 116 (27–209) | 118 (27–210) | 99 (25–200) | NS |
| Viral load, median (IQR) c/mL | ||||
| Plasma (log10) | 1.7 (1.7–2.6) | 1.7 (1.7–2.1) | 3.9 (2.6–4.7) | <.001 |
| CSF (log10) | 1.7 (1.7–1.7) | All ≤ 1.7 | 2.5 (1.71–3.0) | |
| ARV drugs being taken, median (IQR), No. | 3 (3–4) | 3 (3–4) | 3 (3–3) | NS |
| Receiving current regimen ≥3 mo, No. (%)a | 327 (74) | 282 (78) | 45 (58) | <.001 |
| Type of ARV regimen, No. (%) | .01 | |||
| PI-based | 135 (29) | 108 (28) | 27 (35) | |
| NNRTI-based | 171 (37) | 153 (39) | 18 (23) | |
| 3-Drug regimen | 150 (32) | 120 (31) | 30 (38) | |
| 2-Drug regimen | 10 (2) | 7 (2) | 3 (4) | |
| 1-Drug regimen | 1 (<1) | 1 (<1) | 0 |
Abbreviations: ARV, antiretroviral; CSF, cerebrospinal fluid; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NS, not significant; PI, protease inhibitor; VL, viral load.
Data available for n=439.
Plasma and CSF HIV VLs were log10-transformed prior to analysis to improve their distribution. The relationship between CPE ranks and VL was evaluated by Spearman ρ. Viral loads were dichotomized as suppressed (≤50 c/mL) or unsuppressed (>50 c/mL) and CPE ranks as above vs at or below 1.5 (the median) for some analyses. Univariate analyses of these categorical variables were performed using the χ2 test. Multivariate analyses of CSF viral suppression were performed by logistic regression. An initial, full model contained all covariates considered in this analysis (plasma VL, CD4 count, total number of ARV drugs, type and duration of current ARV regimen, and adherence). A final, reduced model was determined by performing a series of stepwise, multivariate logistic regressions predicting detectable CSF VL, identifying the best-fit model that explained the greatest variance with the sparsest number of covariates. All analyses were performed using the JMP statistical package (version 5.1.1, SAS Institute Inc, Cary, NC).
RESULTS
Demographic and clinical characteristics are summarized in Table 1. Of the 467 subjects included in this analysis, 201 (43%) had detectable HIV VL in plasma and 78 (17%) had detectable HIV VL in CSF. The median number of ARV drugs that study participants reported using at the time of their visit was 3 (interquartile range, 3–4). The median CPE rank was 1.5 (interquartile range, 1–2). The 467 subjects in this analysis took 166 different combination regimens. The most commonly used regimen was tenofovir-emtricitabine-atazanavir-ritonavir, which was used by 28 individuals.
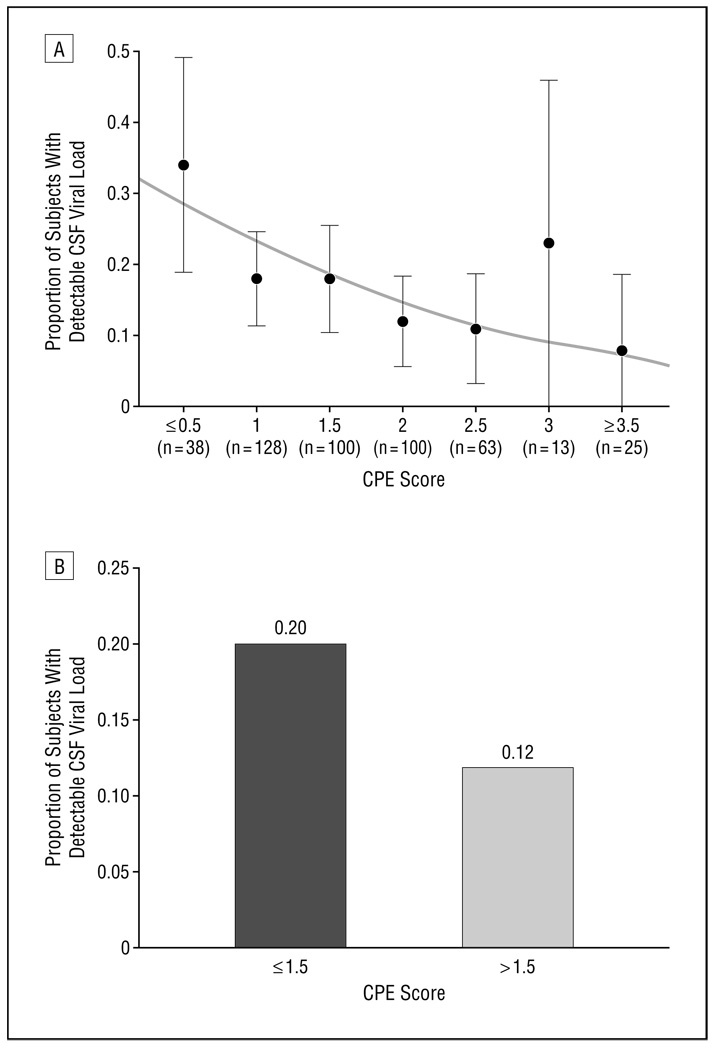
Lower CPE ranks correlated with higher HIV VLs in CSF, whether expressed as a continuous (Spearman ρ = −0.12, P = .008) or a categorical measure (P = .03) (Figure). Subjects who had CPE ranks at or below the median (1.5) had 88% increased odds of having detectable CSF VLs compared with those above the median (20% vs 12%, detectable P = .02). In contrast, CPE ranks were not associated with HIV VLs in plasma, whether VL was expressed as a continuous (Spearman ρ = −0.02, P = .64) or a categorical measure (P = .89). Plasma VLs were also not different between subjects with CPE ranks greater than 1.5 compared with those with lower CPE ranks (42% detectable vs 44%, P = .64). Subjects who reported reduced adherence (at least 1 skipped ARV dose during the previous 4 days) were more likely to have a higher CSF VL (P<.001) but did not have different CPE ranks compared with those who did not report reduced adherence (P = .20). Subjects who had treatment durations of less than 3 months were also more likely to have detectable CSF VLs (42% vs 22%, P<.001).
Figure.
Subjects who had lower central nervous system Penetration-Effectiveness (CPE) ranks were more likely to have detectable cerebrospinal fluid (CSF) viral load when CPE rank was analyzed as a continuous variable (A) or as a categorical variable (B). A, Proportion of subjects with detectable CSF viral load decreases with CPE rank. The proportions (black circles) and 95% confidence interval (vertical bars) were calculated from observations at each CPE rank level (1 subject with a CPE rank of 0 and 2 with a CPE rank of 4 were grouped with the adjacent groups). The solid curve represents predicted proportions of CSF viral suppression from the univariate logistic regression. B, Subjects with a CPE rank greater than 1.5 were 1.88 times more likely to have a detectable CSF viral load.
Because higher plasma VLs, lower CD4 counts, type and duration of ARV regimen, and ARV adherence were associated with higher CSF VLs, a multivariate analysis predicting CSF VL was used to evaluate the variance explained by these factors. The total numbers of ARV drugs were also included because combining ARV drugs can be associated with better virologic response and because, as expected, a larger number of total ARV drugs correlated with higher CPE ranks (Spearman ρ = 0.47, P < .001). In the initial model that included all covariates, lower CPE ranks were associated with detectable CSF VL (model R2 = 0.42, P < .001). In the best-fit model, as determined by performing a series of multiple logistic regressions, detectable CSF VL was associated with lower CPE ranks (P < .001) and lower plasma VL (P < .001, model R2 = 0.37, P < .001). In this model, each unit decrease in CPE rank was associated with a 2.43-fold increase in the odds of having detectable CSF VL (adjusted odds ratio = 2.43, 95% confidence interval, 1.56–3.93) (Table 2). Alternatively, CPE ranks at or below 1.5 were associated with more than a 3-fold increase in the odds of having detectable CSF VL (odds ratio = 3.32, 95% confidence interval, 1.62–6.71).
Table 2.
Decreased CPE Ranks and Increased Likelihood of Having Undetectable Cerebrospinal Fluid VL
| P Value | OR (95% CI) | |
|---|---|---|
| CPE rank | < .001 | 2.43 (1.56–3.93) |
| Plasma HIV VL (log10) | < .001 | 0.22 (0.16–0.30) |
Abbreviations: CI, confidence interval; CPE, central nervous system Penetration Effectiveness; HIV, human immunodeficiency virus; OR, odds ratio; VL, viral load.
COMMENT
In this cross-sectional analysis from the CHARTER cohort, poorer ARV penetration, as estimated by lower CPE ranks, was associated with detectable CSF VL, whether expressed continuously (log10 c/mL) or categorically (≤50 c/mL or <50 c/mL). This relationship was independent of the systemic potency of the regimen (as estimated by plasma VLs), ARV adherence, current CD4 count, total number of ARV drugs, and type and duration of current regimen. Because CSF VL suppression is associated with improvement among individuals who have HIV-associated neurocognitive impairment,12 the CPE scoring system may be clinically relevant, particularly in impaired individuals. Thus, this study’s findings provide further evidence that penetration of ARV drugs into the CNS is important for CSF HIV viral suppression and demonstrates successful use of a new metric designed to consider multiple factors related to ARV effectiveness in the CNS.
Univariate analyses demonstrated that CPE ranks explained 12% of the variance in CSF VLs. This modest correlation may reflect the cross-sectional design of this study, which cannot fully account for time-dependent variables, such as time to virologic suppression. Other potential contributors are the highly positively skewed distribution of CSF VLs in CHARTER (83% undetectable), which is similar to other cohorts with a high prevalence of ARV use, and the importance of other predictors, such as plasma VL, in determining CSF VL. Because of the skewed distribution, the predictive ability of the CPE rank may be more accurately demonstrated by the categorical analysis, which indicated that a CPE rank lower than the median (1.5) was associated with nearly double (odds ratio = 1.88) the odds of having detectable CSF VL.
Because ARV therapy does not immediately suppress VL to undetectable levels, detectable CSF VLs might simply be due to insufficient time receiving therapy. In previous clinical trials,13 3 months of combination ARV therapy has been shown to be sufficient to suppress plasma VL levels to undetectable in most individuals. To address this potential confound, treatment duration (categorized as at least 3 months vs less than 3 months) was included in a multivariate model predicting detectable CSF VL. This model demonstrated that lower CPE ranks remained a significant predictor independent of the effects of treatment duration.
Numerous studies have assessed the relationships between penetration of ARV drugs into the CNS and either neurocognitive response or CSF VL (Table 3). These studies used varying definitions of CNS penetration and diverse outcome measures, making direct comparisons difficult. For example, to assess penetration, some studies focused on individual drugs (eg, zidovudine or indinavir), others counted the number of penetrating ARV drugs, and others directly measured ARV concentrations in CSF. Clinical outcome measures in these studies were even more diverse and included CSF VL, clinical staging of impairment (eg, Memorial Sloan-Kettering AIDS Dementia Complex), neuropsychological testing (either brief or comprehensive batteries), magnetic resonance spectroscopy, or evoked potentials. Even when studies were prospectively accrued, they were uncontrolled or had CNS response as only a secondary objective. Not surprisingly, these varying methods yielded inconsistent results, which have failed to resolve the controversy about the importance of CNS penetration. Despite these caveats, larger studies (ie, those with ostensibly greater power) and those that were prospective (vs retrospective) or controlled (included an internal comparison) were more likely to identify that penetration was associated with improved CNS outcomes.
Table 3.
Studies Comparing Effects of CNS Penetrating/Nonpenetrating ARV Drugs on CSF Viral Load
| Source | Design | No. | Penetration Measurea | Effect on CSF VL |
|---|---|---|---|---|
| von Giesen, 200514 | C-S | 71 | ZDV, d4T | Similar |
| Letendre, 200412 | P | 31 | No. of penetrators | Lower |
| Eggers, 200315 | P | 40 | Multiple | Similar |
| Solas, 200316 | C-S | 41 | IDV | Similar |
| Marra, 200317 | P | 25 | ZDV, IDV | Similar |
| Lafeuillade, 200218 | C-S | 41 | IDV vs LPV-r or NFV | Similar |
| Robertson, 200213 | C-S | 98 | No. of penetrators | Similar |
| Antinori (a), 200219 | C-S | 75 | IDV | Lower |
| Antinori (b), 200219 | P | 29 | ≥3 Penetrators | Lower |
| De Luca (a), 200220 | C-S | 134 | No. of penetrators | Similar |
| De Luca (b), 200220 | P | 50 | No. of penetrators | Lower |
| Letendre, 200121 | C-S | 1239 | NNRTI- vs PI-based | Lower |
| Gisolf, 200022 | Pb | 27 | SQV-r + d4T vs SQV-r | Lower |
| Murphy, 200023 | Pb | 27 | APV-ZDV-3TC vs APV | Lower |
Abbreviations: 3TC, lamivudine; APV, amprenavir; CNS, central nervous system; C-S, cross-sectional; CSF, cerebrospinal fluid; d4T, stavudine; IDV, indinavir; LPV-r, lopinavir-ritonavir; NFV, nelfinavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; P, prospective, longitudinal; PI, protease inhibitor; SQV-r, saquinavir-ritonavir; VL, viral load; ZDV, zidovudine.
The method of estimating penetration substantially differed between studies.
Controlled prospective studies.
In our recently published article,12 we examined the CNS effectiveness of ARV regimens that contained CNS penetrating drugs. We found that subjects who took greater numbers of CNS penetrating drugs showed significantly greater reduction in CSF VL. Subjects who attained viral suppression in CSF demonstrated greater neurocognitive improvement than those who did not. The current analysis confirms the prior finding that CNS targeted therapy is important for reducing CSF viral load while using a more detailed approach to describing penetration. This analysis improves on the previous analysis because the study sample is larger (467 in the current analysis vs 31 in the prior analysis) and more generally representative of the HIV-infected clinic population and because the ARV regimens analyzed are more representative of contemporary prescribing practices.
The CPE rank is intended to be a practical approach to estimate the CNS effectiveness of ARV drug regimens taken by HIV patients in the United States. An inherent limitation of the CPE rank approach is the paucity of available information on ARV penetration into the CNS. Because ARV drug concentrations and virologic suppression cannot be directly measured in brain tissue, surrogate markers are used instead, such as chemical characteristics and CSF drug levels. However, the availability of data on ARV drug levels in CSF varies, significantly limiting the ability to make direct comparisons between different drugs. To deal with these limitations in available data, we incorporated an additional class of information into the CPE rank: the degree to which a drug, in previous clinical studies, independently reduced CSF VL or improved neurocognitive impairment, ie, its effectiveness in the CNS. Although the validity of suppression of HIV levels in CSF as a surrogate for treatment of the brain is unknown, considerable evidence supports it.
This initial analysis focused on the relationship between CPE ranks and CSF VL, but future analyses from the CHARTER cohort will examine the relationship between CPE ranks and neurocognitive performance. A more powerful method to assess this relationship would be a clinical trial that randomizes subjects to receive regimens with either low or high penetration. Observational analyses can be confounded by a variety of difficult-to-control factors, such as the severity, duration, and reversibility of neurocognitive impairment and the potential influence of the impairment itself on the type of regimens prescribed (eg, the impaired individuals might be more likely to receive more convenient, but less well-penetrating regimens). Another limitation of this study should be noted. Many of the study subjects were recruited from HIV clinics at North American tertiary care centers, which may limit the generalizability of the findings to other populations.
In this observational study, ARV regimens were selected by the subjects and their care providers. Thus, the low CPE ranks (median 1.5) probably reflect current clinical practice. These low values indicate that the penetration of many clinical regimens can be considerably improved, which could, in turn, have favorable neurologic effects. For example, a 1-unit increase in the CPE rank was associated with a 2.5-fold increase in the odds of an undetectable CSF VL. If the penetration estimate of an ARV regimen could be improved by 2 units (eg, from 1.0 to 3.0, which is clinically feasible), the odds of reaching an undetectable CSF VL would improve by 6-fold. Thus, our data suggest that the CNS effectiveness of ARV regimens could be substantially improved for a significant proportion of those receiving ARV therapy. Previous studies have suggested that improving the treatment of CNS HIV infection can benefit neurological outcomes and reduce overall disability due to neurocognitive impairment in HIV infection12
Acknowledgments
Funding/Support: This study was supported by award N01 MH22005 from the National Institutes of Health. CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) Investigators: The CHARTER group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, and Washington University, St Louis; is headquartered at the University of California, San Diego; and includes the following individuals: Director: Igor Grant, MD; Codirectors: J. Allen McCutchan, MD, Ronald J. Ellis, MD, PhD, and Thomas D. Marcotte, PhD; Center Manager: Shondra Neumayer, RN, FNP; Neuromedical Component: Ronald J. Ellis, MD, PhD (principal investigator [PI]), J. Allen McCutchan, MD, and Terry Alexander, RN; Laboratory, Pharmacology, and Immunology Component: Scott Letendre, MD (PI), Edmund Capparelli, PharmD, and Janis Durelle, BS; Neurobehavioral Component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, and Donald Franklin, BS; Virology Component: Joseph K. Wong, MD (PI), and Caroline Ignacio, BA; Imaging Component: Terry Jernigan, PhD (PI), Michael J. Taylor, PhD, and Rebecca Theilmann, PhD; Data Management Unit: Anthony C. Gamst, PhD (PI), Clint Cushman, BA, and Michelle Frybarger, BA; Statistics Unit: Ian Abramson, PhD (PI), and Deborah Lazzaretto, MS; Protocol Coordinating Component: Thomas D. Marcotte, PhD (PI), and Rodney von Jaeger, MPH; Johns Hopkins University Site: Justin C. McArthur, MBBS (PI); Mount Sinai School of Medicine Site: Susan Morgello, MD (Co-PI), and David Simpson, MD (Co-PI); University of California, San Diego, Site: J. Allen McCutchan, MD (PI); University of Washington, Seattle, Site: Ann Collier, MD (Co-PI), and Christina Marra, MD (Co-PI); University of Texas, Galveston, Site: Benjamin Gelman, MD, PhD (PI); and Washington University, St Louis, Site: David Clifford, MD (PI), and Muhammad Al-Lozi, MD.
Footnotes
Financial Disclosure: Dr Letendre has worked on clinical studies with funding provided by Gilead Sciences, Abbott, and Merck; has served as a consultant for Schering-Plough; and has received honoraria for educational talks from Abbott and GlaxoSmithKline. Dr Best has received funding for an investigator-initiated study from Abbott and has served as a consultant for ARK Diagnostics. Dr Clifford has served as a consultant for Biogen Idec, Genzyme, Millennium Pharmaceuticals, Schering-Plough, Roche, Elan, Novartis, and Genentech and as a speaker for Boehringer-Ingelheim. Dr Clifford has received research support from Savient Pharmaceuticals, NeurogesX, Bavarian Nordic, Tibotec, Pfizer, Schering-Plough, and Ortho Biotech. Dr Collier has had research contracts with Boehringer-Ingelheim, Tibotec-Virco, and Gilead Sciences and has participated on advisory boards for GlaxoSmithKline and Pfizer. Dr Collier has a family member who owns stock in Bristol Myers Squibb.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
REFERENCES
- 1.Strazielle N, Ghersi-Egea JF. Factors affecting delivery of antiviral drugs to the brain. Rev Med Virol. 2005;15(2):105–133. doi: 10.1002/rmv.454. [DOI] [PubMed] [Google Scholar]
- 2.Clements JE, Li M, Gama L, et al. The central nervous system is a viral reservoir in simian immunodeficiency virus–infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neurovirol. 2005;11(2):180–189. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 3.Nutley NJ. Fuzeon [package insert] Morrisville, NC: Roche Laboratories Inc; and Morrisville, NC: Trimeris Inc.; [Google Scholar]
- 4.Aweeka F, Jayewardene A, Staprans S, et al. Failure to detect nelfinavir in the cerebrospinal fluid of HIV-1–infected patients with and without AIDS dementia complex. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(1):39–43. doi: 10.1097/00042560-199901010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Burger DM, Kraayeveld CL, Meenhorst PL, et al. Study on didanosine concentrations in cerebrospinal fluid: implications for the treatment and prevention of AIDS dementia complex. Pharm World Sci. 1995;17(6):218–221. doi: 10.1007/BF01870615. [DOI] [PubMed] [Google Scholar]
- 6.Sustiva [package insert] New York, NY: Bristol Myers Squibb.; [Google Scholar]
- 7.Ridgefield CT. Viramune [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; [Google Scholar]
- 8.Capparelli EV, Holland D, Okamoto C, et al. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS. 2005;19(9):949–952. doi: 10.1097/01.aids.0000171409.38490.48. [DOI] [PubMed] [Google Scholar]
- 9.Yarchoan R, Berg G, Brouwers P, et al. Response of human-immunodeficiency-virus-associated neurological disease to 3′-azido-3′-deoxythymidine. Lancet. 1987;1(8525):132–135. doi: 10.1016/s0140-6736(87)91968-4. [DOI] [PubMed] [Google Scholar]
- 10.Best B. Low atazanavir concentrations in cerebrospinal fluid. Paper presented at: 13th Annual Conference on Retroviruses and Opportunistic Infections; February 5–8, 2006; Denver, CO.. [Google Scholar]
- 11.Haworth SJ, Christofalo B, Anderson RD, Dunkle LM. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(3):235–238. doi: 10.1097/00042560-199803010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56(3):416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 13.Robertson KR, Fiscus S, Robertson WT, Meeker R, Hall CD. CSF HIV RNA and CNS penetrating antiretroviral regimens. Paper presented at: 9th Conference on Retroviruses and Opportunistic Infections; February 24–28, 2002; University of North Carolina, Chapel Hill.. [Google Scholar]
- 14.von Giesen HJ, Adams O, Koller H, Arendt G. Cerebrospinal fluid HIV viral load in different phases of HIV-associated brain disease. J Neurol. 2005;252(7):801–807. doi: 10.1007/s00415-005-0749-4. [DOI] [PubMed] [Google Scholar]
- 15.Eggers C, Hertogs K, Sturenburg HJ, van Lunzen J, Stellbrink HJ. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17(13):1897–1906. doi: 10.1097/00002030-200309050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Solas C, Lafeuillade A, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47(1):238–243. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology. 2003;60(8):1388–1390. doi: 10.1212/01.wnl.0000058768.73358.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafeuillade A, Solas C, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. Differences in the detection of three HIV-1 protease inhibitors in non-blood compartments: clinical correlations. HIV Clin Trials. 2002;3(1):27–35. doi: 10.1310/WMWL-6W9Y-PXV2-X148. [DOI] [PubMed] [Google Scholar]
- 19.Antinori A, Giancola ML, Grisetti S, et al. Factors influencing virological response to antiretroviral drugs in cerebrospinal fluid of advanced HIV-1-infected patients. AIDS. 2002;16(14):1867–1876. doi: 10.1097/00002030-200209270-00003. [DOI] [PubMed] [Google Scholar]
- 20.De Luca A, Ciancio BC, Larussa D, et al. Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology. 2002;59(3):342–347. doi: 10.1212/wnl.59.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Letendre S, Ellis R, Grant I, McCutchan A HNRC Group. The relationships between CSF HIV RNA levels and other markers in 1,239 samples. Paper presented at: Eighth Conference on Retroviruses and Opportunistic Infections; February 4–8, 2001; San Francisco, CA. [Google Scholar]
- 22.Gisolf EH, van Praag RM, Jurriaans S, et al. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25(5):426–443. doi: 10.1097/00042560-200012150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Murphy R, Currier J, Gerber J, et al. Antiviral activity and pharmacokinetics of amprenavir with or without zidovudine/3TC in the cerebral spinal fluid of HIV-infected adults. Paper presented at: Conference on Retroviruses and Opportunistic Infections; January 30 to February 4, 2000; San Francisco, CA.. [Google Scholar]