Abstract
Immunization of jirds with Bm-alt-2 elicited partial protection against challenge infection with the filarial parasite Brugia malayi. In this study, we initially compared the protective immune responses elicited following immunization with recombinant Bm-ALT-2 protein regimen and Bm-alt-2 DNA regimen. These studies showed that protein vaccination conferred approximately 75% protection compared to DNA vaccination that conferred only 57% protection. Analysis of the protective immune responses showed that the protein immunization promoted a Th2-biased response with an increase in IL-4, IL-5 and IgG1 responses, whereas, the DNA vaccine promoted a Th1-biased response with profound IFN-γ and IgG2a responses. Since protein vaccination gave better results than DNA vaccination, we then wanted to evaluate whether a prime-boost vaccination that combined DNA prime and protein boost will significantly increase the protective responses induced by the protein vaccine. Our results suggest that prime-boost vaccination had no added advantage and was comparatively less effective (64% protection) than the Bm-ALT-2 protein alone vaccination. Prime boost vaccination generated mixed Th1/Th2 responses with a slightly diminished Th2 responses compared to protein vaccination. Thus, our results suggest that Bm-ALT-2 protein vaccination regimen may be slightly better than prime-boost vaccine regimen and the mechanism of protection appears to be largely mediated by a Th2-biased response.
Keywords: Brugia malayi, Filariasis, Bm-alt-2, DNA prime-protein boost vaccination
1. Introduction
Lymphatic filariasis, caused by Wuchereria bancrofti and Brugia malayi, affects more than 120 million people worldwide causing major public health problems especially in the tropics. Clinically, disease is classified into distinct stages including microfilaraemic (MF) where individual is asymptomatic but microfilaraemic; chronic pathology (CP), where individual is with clinical evidence of lymphatic obstruction but amicrofilaraemic. In addition, individuals who are asymptomatic amicrofilaraemic and believed to be resistant to disease are designated as endemic normals (EN).
Chemotherapy and vector based control measures are widely employed in many endemic regions (Curtis et al., 2002; Fraser et al., 2005; Maxwell et al., 1999; Ramzy et al., 2006). However, these control methods have limited utility, because of several factors including the development of possible drug and pesticide resistance, high cost of chemotherapy programs and inaccessibility of many years of required drug treatment to infected population of poor regions (Molyneux et al., 2003). Hence, development of control measures such as vaccination would be best additional strategy to eliminate filariasis. Previous studies showed that vaccination with recombinant paramyosin could offer partial protection to larval challenge (Li et al., 1993). However, vaccine studies using BmVAL-1 (Murray et al., 2001), heat shock protein 70, myosin, and α1-type IV collagen (Peralta et al., 1999) did not yield encouraging results. Among all the recombinant vaccine candidates analyzed, ALT (Abundant Larval Transcript) family of proteins appeared to elicit significantly high protective responses against B. malayi (Gnanasekar et al., 2004; Gregory et al., 2000). The expression of these proteins starts at late L2 stage and peaks at L3 stage. Thus, Bm-ALT-2 protein is abundantly synthesized in the infective stages of the parasite and believed to play a major role in the transmission and infectivity of the filarial parasite (Gregory et al., 2000; Gomez-Escobar et al., 2005). Interestingly, sera from endemic normal individuals (EN), but not asymptomatic microfilaremic (MF) or chronic lymphatic obstruction/elephantiasis (chronic pathology, CP) patients, have significantly high levels of circulating antibodies against Bm-ALT-2 (Gnanasekar et al., 2004; Ramachandran et al., 2004). Since EN subjects are considered as putatively immune individuals, several studies were conducted to evaluate the potential of Bm-ALT-2 as a potential vaccine candidate (Gnanasekar et al., 2004; Ramachandran et al., 2004). These studies showed that immunization of mice or jirds with recombinant Bm-ALT-2 can confer over 70% protection. These findings coupled with the fact that there are no human homologues for Bm-ALT-2 suggests that Bm-ALT-2 may be an ideal candidate for vaccine development against human lymphatic filariasis.
DNA vaccination is an attractive approach to study protective immunity against filarial parasites. Even though DNA vaccines were initially thought to be ideal for vaccination only against intracellular pathogens (Gardner et al., 1996; Hoffman et al., 1997; Walker et al., 1998), recent findings show that DNA vaccine could also be efficiently utilized for vaccination against extracellular parasites (Harrison and Bianco, 2000; Harrison et al., 1999; Kalinna, 1997). In fact, recent studies from our group (Ramachandran et al., 2004) showed that DNA vaccine coding for Bm-alt-2 could generate partial protection against the filarial parasite B. malayi in a mouse model.
Currently, various strategies are being investigated to optimize vaccines against infectious diseases using recombinant DNA vaccination technology. Heterologous prime-boost vaccination is one of the approaches in which after priming with DNA, a different agent like recombinant virus or protein is used in as a ‘boost’ dose to obtain enhanced protective efficacy (Ferraz et al., 2004; Santra et al., 2004; Vordermeier et al., 2003; Wang et al., 2004). Several studies show that the strategy of using DNA to prime the immune system and recombinant protein to boost immune responses could lead to better cellular and antibody response in vaccinated animals compared to homologous protein or DNA vaccination regimens (Jones et al., 2001; Letvin et al., 1997; Ruitenberg et al., 2000). In the present study, we compared the degree of protective immunity conferred by Bm-alt-2 DNA prime-Bm-ALT-2 protein-boost vaccination (prime boost) regimen with DNA prime-DNA boost (DNA vaccine) or protein prime–protein boost (protein vaccine) regimens.
2. Materials and methods
2.1. Animals
A total of thirty 4–6 week-old, male jirds (Meriones unguiculatus) were used in this study. Animals were obtained from Mahatma Gandhi Institute of Medical Sciences, India. Jirds were handled in accordance with the institutional guidelines, and was approved by an Institutional Animal Care Committee.
2.2. Parasites
Aedes aegypti mosquitoes were infected by feeding them with B. malayi microfilariae in jird's blood. Twelve days after infection, B. malayi L3 larvae were obtained by crushing the insects (Suzuki and Seregeg, 1979) and carefully removing the L3s. Larvae were counted under a microscope and used for challenge infection.
2.3. Bm-ALT-2 protein
T7 expression vector pRSET B (Invitrogen, Carlsbad, CA) was used to express recombinant Bm-ALT-2 as histidine tagged protein (Gnanasekar et al., 2004). Plasmid was transformed to Escherichia coli BL21 (DE3) pLysS for expression. Bacterial cultures were induced with 1 mM β-d-1-isopropyl thiogalactoside and subsequently incubated for 4 h. Histidine-tagged Bm-ALT-2 protein was purified from pelleted bacteria using chelating sepharose fast flow chromatography (Amersham Biosciences, Uppsala, Sweden) as per manufacturer's recommendations. Prior to immunization, endotoxin content in the protein preparations were determined by limulus amebocyte lysate assay (E-toxate, Sigma, St. Louis, MO) and were found to be less than 1 EU/mg.
2.4. Bm-alt-2 DNA vaccine
Bm-alt-2 expressing plasmid, designated as pVBmALT-2, was constructed by inserting Bm-alt-2 gene into pVR1020 vector (Ramachandran et al., 2004). pVR1020 empty plasmid was used as a control in all immunizations. Plasmids were maintained and propagated in E. coli DH5α cells. Subsequently, plasmids were purified using endotoxin free plasmid extraction kit (Qiagen, Hilden, Germany) as per manufacturer's instructions. DNA was analyzed by agarose gel electrophoresis and quantified by spectrophotometry (OD260/OD280, ratio > 1.8). DNA was finally diluted in endotoxin free phosphate buffered saline (PBS). Bm-alt-2 DNA did not have any detectable levels of endotoxin as determined by the amebocyte lysate assay (Sigma).
2.5. Immunization
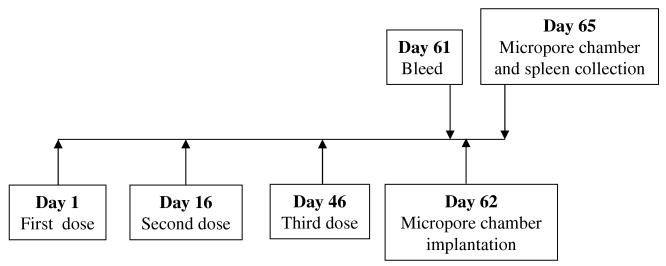
A total of thirty 4–6 week-old, male jirds in five groups were used for immunization and each jird received three doses on days 1, 16 and 46. Fig. 1 shows a schematic representation of the vaccination protocol used in these experiments. Jirds were immunized using three different regimens. Doses of Bm-alt-2 DNA and Bm-ALT-2 protein used in these studies was selected based on previous publications (Gregory et al., 2000; Li et al., 1999). Regimen 1 used a DNA vaccination protocol. This regimen used 100 μg of pVBmALT-2 intramuscularly (i.m.) for DNA prime and same amount of DNA for boosting. Regimen 2 used a protein vaccination protocol. In this regimen, jirds were primed with 25 μg of alum adsorbed Bm-ALT-2 protein given intraperitoneally (i.p.) and boosted with the same amount of protein (i.p.). Regimen 3 used a prime-boost vaccine protocol. In this regimen, jirds were immunized with two doses of pVBmALT-2 (100 μg of DNA each given i.m.) on day 1 and 16, respectively, and a booster dose consisting of 25 μg of alum adsorbed Bm-ALT-2 given i.p. on day 46. Two groups of jirds representing control groups received either 100 μg of blank pVR1020 vector DNA (i.m.) or alum/PBS (i.p.). Vaccine-induced protection was evaluated on day 62 after first immunization using micropore chamber method (Fig. 1).
Fig. 1.
Schematic representation of the immunization protocol used in this study.
2.6. Parasite challenge studies using micropore chambers
Immunized animals were challenged using micropore chamber technique as described elsewhere (Abraham et al., 1989; Lange et al., 1993). Micropore chambers consisted of plexiglas ring covered with 5.0 μm polycarbonate membranes (Millipore Corporations, Bedford, MA). Cyanoacrylate adhesive was used to bind the membranes to the rings. Chambers were sterilized at 80 °C for 10 h. For parasite challenge, 20 infective L3s suspended in RPMI1640 medium supplemented with 15% heat inactivated fetal calf serum (FCS), were inoculated into the chambers and the chambers were sealed with MF cement (Millipore). Chambers were then implanted into the peritoneum of each jird under anesthesia on day 62 post immunization. Aseptic conditions were followed for the surgical procedures. The skin was sutured and animals were allowed to recover. After 72 h of implantation (i.e. 65 days after immunization), animals were sacrificed and chambers were recovered from peritoneum. Contents of each chamber were examined microscopically for cell adherence and death of parasites. The parasite was considered dead if it was not motile and had several adherent cells on the surface. Percentage of protection was calculated using the following formula: number of dead larvae ÷ number of larvae placed into the micropore chamber × 100.1
2.7. Detection of antibody responses
Sera were collected from immunized jirds on day 61 post immunization (Fig. 1). Titer of anti-Bm-ALT-2 antibodies in the sera were analyzed by ELISA as described previously (Gnanasekar et al., 2004; Ramachandran et al., 2004) with minor modifications. Briefly, wells of 96 well microtiter plates were coated with 100 ng of recombinant Bm-ALT-2 protein suspended in 100 μl of coating buffer (NaHCO3/Na2CO3, 0.067 M pH 9.6) and incubated overnight at 4 °C. After washing with PBST (PBS containing 0.5% triton X-100), 100 μl of 3% BSA was added per well to block the non-specific sites and incubated for 2 h at 37 °C. After washing with PBST, serially diluted jird sera were added and incubated for 2 h at 37 °C. To determine the isotype of antibodies, goat anti-mouse IgG1 or IgG2a were added and incubated for 1 h at 37 °C. Wells were washed with PBST and alkaline phosphatase labeled goat anti-mouse IgG (Sigma) was added. After incubation for an hour at 37 °C, color was developed using p-nitrophenyl phosphate substrate (1 mg/ml) in substrate buffer (100 mM Tris–Cl, pH 9.5, 100 mM NaCl, 5 mM MgCl2) and 100 μl of 3 N NaOH was added to the wells to stop the reaction. Absorbance was measured at 405 nm in a microplate reader.
2.8. Splenocyte proliferation assay
Single cell suspension of spleen cells were prepared from both immunized and control jirds on day 65 post immunization. Approximately 2 × 106 cells per well suspended in one ml of RPMI1640 supplemented with 10% heat inactivated FCS, 0.5% gentamicin, 1% glutamine and 0.4% HEPES were cultured in six-well plates. Cells cultured in triplicate wells were stimulated with Bm-ALT-2 protein (10 μg/ml) or cultured with medium alone. Plates were then incubated at 37 °C in a CO2 incubator. After 3 days, 3H-Thymidine (0.5 μCi per well, Amersham Biosciences) was added to each well and further incubated. Cells were harvested 16 h later and 3 H-thymidine uptake was measured in a liquid scintillation counter and expressed as stimulation index (SI) = (counts per min of stimulated cultures/counts per min of unstimulated cultures).
2.9. RT-PCR for cytokine analysis
Jird cytokine mRNA in the cell pellets were analyzed by RT-PCR. Briefly, spleen cells from immunized and control jirds were cultured as above at a concentration of 2 × 106 cells/ml in six-well plates in the presence of Bm-ALT-2 antigen (10 μg/ml). After 3 days of culture, cells were centrifuged (1000 rpm for 5 min) and supernatant was discarded. Cells cultured in media alone served as controls. Total RNA was extracted from cell pellets using Trizol reagent (Gibco BRL, Life technologies, Carlsbad, CA) in accordance with the manufacturer's instructions. Following preparation and subsequent washes with ethanol, RNA pellets were dissolved in diethyl-pyrocarbonate (Sigma) treated water. After treatment with DNase I, total RNA was quantified using spectrophotometer at 260 nm, and the quality of RNA was determined by the ratio of optical density at 260 nm to that at 280 nm. Reverse transcription of total RNA was performed using a ProtoScript first strand cDNA synthesis kit (New England Biolabs, Beverly, MA) as per manufacturer's recommendations. The cDNA was then subjected to PCR using primers for jird IL-4, IL-5 and IFN-γ (Table 1). β-actin was used as internal control. PCR was performed using a Minicycler (MJ Research, MA, USA). First step consisted of denaturation at 95 °C for 10 min and the following cycles consisted of 1 min denaturation step at 95 °C, 1 min annealing step at 58 °C and extension at 72 °C with final extension at 72 °C for 10 min. PCR amplification for 28 cycles was carried out to ensure that the products lie within the linear portion of the curve. PCR products were visualized by electrophoresis on 2% agarose gels containing ethidium bromide. Gels were scanned to determine band intensity of PCR products. Densitometry values for each cytokine mRNA were expressed as percentages of mean intensities of that for β-actin in each group (Ausiello et al., 1995, 1996).
Table 1.
Primers details of jirds cytokines
| Primers | Primer sequences | Product size (bp) | Accession No./Reference |
|---|---|---|---|
| IL-4 | |||
| Forward | 5′CACATCCCTGACGGTAGAGTT3′ | 424 | L37779 |
| Reverse | 5′TAGGCGTCCCAGGAAGTCATT3′ | ||
| IL-5 | |||
| Forward | 5′ATTCTAACTCTCGCCTGGGTCTGG3′ | 315 | L37780 |
| Reverse | 5′GAACTGCCGTGCTCTCCGTCTC3′ | ||
| IFN-γ | |||
| Forward | 5′CTTTGGGCCCTCTGACTTCGTA3′ | 519 | L37782 |
| Reverse | 5′TTTCCGCTTCCTTAGGGTGACTC3′ | ||
| β-Actin | |||
| Forward | 5′GCACCACACCTTCTACAATGAG3′ | 163 | Takashima et al. (2001) |
| Reverse | 5′ATAGCACAGCCTGGATAGCAAC3′ |
2.10. Statistical analysis
Mann–Whitney U test was applied to analyze data from vaccination studies. Probability values (p) of <0.05 were considered statistically significant.
3. Results
3.1. Antibody response in jirds to Bm-alt-2 vaccination
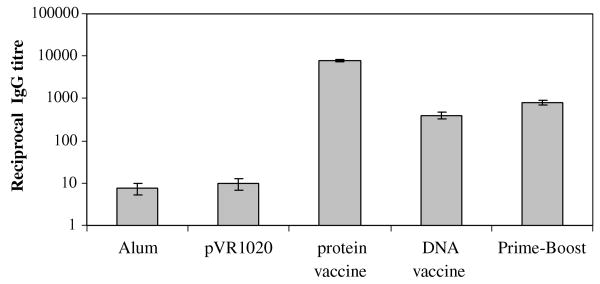
Sera were collected on day 61 (i.e. 15 days after the final booster dose) to determine antibody titers. Control group of animals had no detectable Bm-ALT-2 specific antibody in their sera. In contrast, animals that were immunized with Bm-ALT-2 protein, DNA or prime-boost vaccines showed Bm-ALT-2 specific antibodies in their sera (Fig. 2). Bm-alt-2 prime-boost vaccine regimen induced significantly higher titer of antibodies compared to DNA vaccine alone (p < 0.05). Bm-ALT-2 protein vaccine induced the highest level of Bm-ALT-2 specific IgG titer.
Fig. 2.
Bm-ALT-2 specific IgG titer in jirds immunized by different protocols. Jirds were bled 15 days after the last immunization and Bm-ALT-2-specific IgG titer was detected by ELISA. Optical density was measured at 405 nm. Antibody titer is defined as reciprocal dilution of the sera yielding a one-half maximal absorbance at 405 nm. Data represent mean titers (in logarithmic scale) and standard errors for each group of animals.
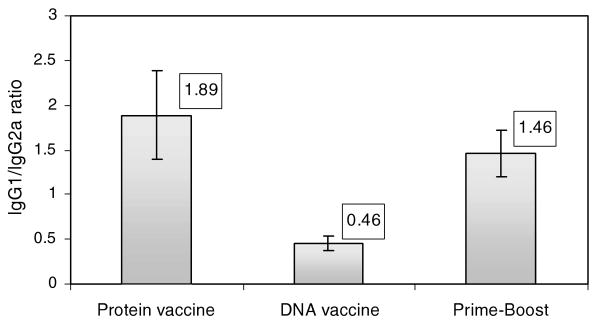
We also investigated the isotype of anti-Bm-ALT-2 antibodies generated following various immunization regimens to determine whether we can correlate protection with any one group of immunoglobulin isotypes. Furthermore, the antibody isotype also offers a good surrogate marker of Th1 and Th2 immune responses (Rizzo et al., 1992). IgG2a levels correlate with Th1 and IgG1 levels correlate with Th2. Thus, we could also determine the relationship between Th1 and Th2 responses following various immunization regimens by calculating the IgG1/IgG2a ratio (Fig. 3). An increase in the IgG1/IgG2a ratio suggests a Th2-biased response, whereas, a reversed IgG1/IgG2a ratio suggests a Th1-biased response. Animals vaccinated with Bm-ALT-2 protein alone had high IgG1/IgG2a ratio suggesting a Th2-biased response, whereas, animals immunized with Bm-alt-2 DNA alone had a low ratio of IgG1/IgG2a suggesting a Th1-biased response. Interestingly however, prime-boost vaccinated animals had high IgG1/IgG2a ratios comparable to Bm-ALT-2 protein vaccinated animals. These findings suggested that immunization with Bm-ALT-2 protein even as a booster dose increases the IgG1 titers in DNA primed animals pushing the responses to a Th2-biased pathway.
Fig. 3.
Ratio of IgG1 titer to IgG2a titer in different groups. The ratio of IgG1/IgG2a was obtained by dividing the titer of IgG1 by that of IgG2a. Data presented are means (in boxes) and SD values.
3.2. Antigen specific cellular immune response
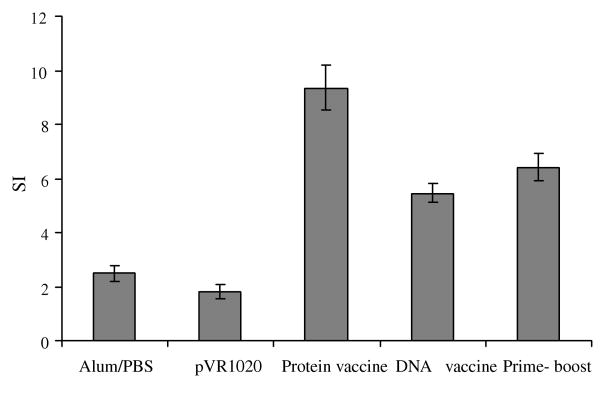
To determine the cellular immune responses, spleen cells were cultured in the presence of Bm-ALT-2 protein and their proliferative responses and cytokine profiles were evaluated. As shown in Fig. 4, spleen cells from animals immunized with Bm-ALT-2 protein, Bm-alt-2 DNA or prime-boost vaccination showed significant proliferative responses to the antigen (p < 0.05), whereas, proliferative response of cells from control groups were near background levels. Cells from prime-boost vaccinated group showed significant proliferative responses to Bm-ALT-2 antigen compared to DNA vaccinated group (p < 0.05). Interestingly, cells from Bm-ALT-2 protein vaccinated group showed significantly higher cell proliferate responses to Bm-ALT-2 than the prime-boost vaccinated group (p < 0.05).
Fig. 4.
Lymphocyte proliferative responses to Bm-ALT-2. Spleen cells were collected from immunized animals and stimulated with Bm-ALT-2. Data shown are means ± standard deviations of stimulation index.
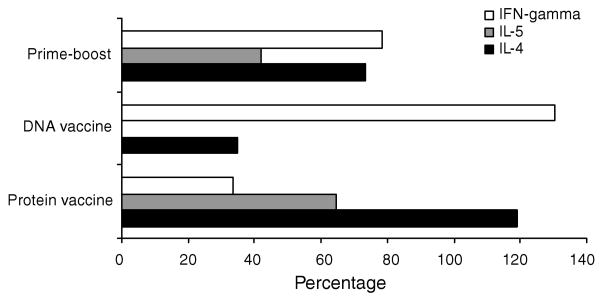
Since the cells were proliferating significantly to the recombinant antigen, cytokine profiles of the proliferating cells were investigated. RT-PCR was used to determine the message levels of Th1 (IFN-γ) and Th2 cytokines (IL-4 and IL-5). Values are expressed as percentage increase over the mean intensities of β-actin products for each group. Fig. 5 summarizes IFN-γ, IL-4 and IL-5 mRNA expression in spleen cells stimulated with Bm-ALT-2 antigen. Spleen cells of control groups did not show detectable cytokines expression at message level when stimulated with Bm-ALT-2 protein (data not shown). Spleen cells collected from animals immunized with Bm-ALT-2 protein vaccine alone had prominent IL-4 and IL-5 mRNA expression, whereas, spleen cells from jirds immunized with only Bm-alt-2 DNA vaccine expressed high levels of IFN-γ compared to IL-4. Thus, there was a clear dichotomy in the cytokine responses between animals vaccinated with protein or DNA. However, when the DNA primed animals were boosted with Bm-ALT-2 protein, there was an increased expression of IL-4, IL-5 and IFN-γ mRNA. Thus, a single booster injection of Bm-ALT-2 protein in the prime-boost vaccination regimen was sufficient to induce a robust Th2 response in a Th1 environment generated by DNA priming dose.
Fig. 5.
Cytokine mRNA levels. Spleen cells were stimulated with 10 μg/ml of Bm-ALT-2 protein for 72 h. Cytokine mRNA expression was analyzed by RT-PCR. The actual densitometric values for each cytokine mRNA were normalized by using β-actin densitometry as the 100% reference, and all other values are expressed as percentages of β-actin.
3.3. Parasite challenge using micropore chambers
Micropore chambers were implanted into the peritoneal cavity of immunized jirds on day 62 post immunization (2 weeks after the final booster dose). After 72 h of implantation, the micropore chambers were harvested and percent of dead worms were determined. Use of micropore chambers significantly alleviated the problem of recovering parasites from the peritoneal cavity and allowed us to count the exact number of larval death. Microscopic examination of worms recovered from micropore chambers revealed large numbers of macrophages and polymorph nuclear cells adhered to dead larvae. Numbers of dead and live larvae within the micropore chambers were counted and percent protection was calculated. Our results showed that immunization with Bm-alt-2 DNA alone could induce 57% protection in vaccinated animals (Table 2). A comparison of the protective efficacy of the Bm-ALT-2 protein vaccine and the prime-boost vaccination regimen showed that Bm-ALT-2 protein vaccination alone was enough to elicit maximum protection levels. Jirds immunized with prime-boost vaccination had less than 40% live larvae, significantly less than that of animals immunized with DNA vaccine alone (57%, p < 0.05). Thus, more than 60% of protection could be achieved by prime-boost vaccination. Interestingly, immunization with Bm-ALT-2 protein alone was equally effective in eliciting significant larval killing responses and induced 75% protection (p < 0.05).
Table 2.
Percentage of protection against B. malayi infective larvae
| Groups | Protection (% mean ± SD) |
|---|---|
| Alum/PBS | 6.2 ± 2.77 |
| pVR1020 | 0 ± 0 |
| Protein vaccine | 75.4 ± 7.89b |
| DNA vaccine | 57 ± 4.47a |
| Prime-boost | 64.4 ± 4.39a |
Statistically significant in comparison with each other at p < 0.05 (Mann–Whitney test).
Statistically significant in comparison to all other groups at p < 0.05 (Mann–Whitney test).
4. Discussion
In the present study, we have compared the protective efficacy of a prime-boost (Bm-alt-2 DNA prime followed by Bm-ALT-2 protein boost) vaccination with DNA vaccination regimen alone or recombinant protein vaccination alone in a jird model of B. malayi. Our results show that Bm-ALT-2 protein vaccination is much superior to DNA vaccine alone or prime-boost vaccination. Analysis of the antibody and cytokine responses suggested that Th2-biased immune responses predominated in Bm-ALT-2 protein vaccination.
Filariais is recognized as one of the major poverty promoting disease in tropical countries (Hotez and Ferris, 2006). An effective vaccine would serve as a valuable addition to the existing control measures and advance efforts for elimination of this disease and reduction of poverty. Several efforts have been made to develop protective vaccines against filariasis (Li et al., 1993; Gregory et al., 2000) and our efforts are focussed on potential vaccine candidate Bm-ALT-2. Using the phage-display based immuno screening and analysis, we have recently demonstrated the potential of Bm-ALT-2 as a candidate vaccine antigen (Gnanasekar et al., 2004). Our subsequent studies showed that Bm-ALT-2 given as a protein vaccine alone gave better results than DNA vaccine alone in mouse model (Ramachandran et al., 2004). Therefore, in this study we evaluated whether a DNA prime–protein boost vaccination has any added advantage over protein vaccination alone.
Bm-alt-2 has several important characteristics of a vaccine candidate. These include high levels of expression in the infective larval stages presenting as an abundant target for the immune system and more importantly there is no known homolog in the mammalian species. In fact, our pervious studies using Bm-ALT-2 protein as vaccine candidate antigen gave promising results in animal model. One of these studies also used adult worm establishment as a read out for vaccine-induced protection in immunised jirds (Gnanasekar et al., 2004). All these studies showed that vaccination with Bm-ALT-2 protein could induce more than 70% protection. Similar results were observed in the present study using a more accurate micropore chamber approach confirming previous observations that Bm-ALT-2 is an excellent vaccine candidate.
Encouraged by the success of recombinant protein vaccines, efforts were subsequently initiated for the development of Bm-alt-2 DNA vaccine. DNA-based vaccine has been attempted with varying success using other vaccine candidates against lymphatic filariasis. For example, vaccination studies using paramyosin DNA did not confer significant protection (Li et al., 1999). However, one of our previous studies suggested that Bm-alt-2 DNA vaccine could induce partial protection in mice model against B. malayi infection (Ramachandran et al., 2004). We repeated similar studies in jirds, which is a permissive host. Our results also confirmed that Bm-alt-2 DNA vaccination could confer significant, but less protection compared to Bm-ALT-2 protein vaccination.
Recent developments in vaccine research suggest that heterologous prime-boost protocol is advantageous over homologous prime-boost protocols (Jones et al., 2001; Letvin et al., 1997; Ruitenberg et al., 2000; Vordermeier et al., 2003; Wang et al., 2004). In the present work, we analyzed whether a similar approach could be used to improve the protective effect of Bm-alt-2 DNA or protein vaccination. As expected, our results showed that prime-boost vaccination regimen conferred significantly enhanced protection against B. malayi infection (64%), compared with DNA vaccine alone (57%). Nevertheless, protective response induced by prime-boost vaccination was not as effective as the protein vaccine alone.
Analysis of the characteristics of protective immune responses showed that in prime-boost vaccinated animals there was a high IgG1/IgG2a ratio in their sera suggesting Th2 biased responses. However, this was not truly reflected when the antigen-specific cytokine profile was evaluated by RT-PCR. When cells from these animals were stimulated with recombinant Bm-ALT-2 protein, nearly equal amount of IL-4 and IFN-γ was produced suggesting that both Th1/Th2 type responses may be important in the prime-boost mediated protective responses. Similar discrepancy between isotype and cytokines has been reported previously by MacDonald et al. (2005).
Analysis of the IgG isotype and cytokine profile in vaccinated animals revealed that immunization with alum-adsorbed Bm-ALT-2 protein vaccine promoted Th2 type immune responses as evidenced by an increase in the serum IgG1 levels and a high IgG1/IgG2a ratio. This Th2 bias might also be partially due to the alum in the preparation. It is well established that alum adjuvant preferentially stimulates a Th2-mediated immune response (Brewer et al., 1999). Earlier studies showed that Th2-biased responses are associated with protective immune responses against various nematode parasites (Babu et al., 2000; Le Goff et al., 2000; Martin et al., 2000; Volkmann et al., 2003). A role for IL-5 (Th2 cytokine) has been suggested in these protective responses. In the present study also, we observed an increase in IL-5 mRNA expression in animals immunized with protein vaccine. No such increases in IL-5 responses were seen in animals immunized with DNA vaccine alone. However, in prime-boost vaccinated animals there was an increase in IL-5 suggesting that this response might be mediated by the protein boost. Previous studies also suggest that IL-5 may be essential for vaccine-mediated protection in filarial infection in mice (Le Goff et al., 2000). Increased expression of IL-5 in protein vaccinated animals suggests that IL-5 mediated responses may also have a role in jirds, a permissive host. DNA vaccination predominantly generated a Th1-biased response as evidenced by the high IgG2a levels, decreased IgG1/IgG2a ratio and high IFN-γ. This response was slightly diminished in the prime-boost vaccination. In fact, the prime-boost vaccinated animals showed a mixed Th1–Th2 response. DNA component in the prime-boost regimen is probably responsible for the Th1 responses. The protein component in prime-boost regimen was responsible for the increased levels of Bm-ALT-2 specific IgG1 serum antibodies. Nevertheless, these responses were not sufficient enough to down regulate the IFN-γ induced by the DNA vaccine in the prime-boost regimen.
A strong IgG1 response in both protein and prime-boost vaccinated animals suggest that IgG1 antibodies may have a significant role in the vaccine-induced protection. The focus of our present study was not to characterize the role of antibodies. However, further passive transfer experiments using various isotype of antibodies would be useful in determining the role of each isotype of antibodies, especially IgG1 in the vaccine-induced immunity.
In conclusion, our studies show that vaccination using alum adsorbed Bm-ALT-2 vaccination is superior in inducing protective responses against B. malayi in jirds compared to Bm-alt-2 DNA alone or DNA prime and protein-boost vaccination regimens. Analysis of the antibody and cytokine responses suggests that a Th2-biased response may be more important in the protection induced by the Bm-ALT-2 protein. Further studies to enhance the Th2 responses induced by the Bm-ALT-2 protein vaccination with other Th2 driving adjuvants might enhance the protective effect.
Acknowledgments
The authors are thankful to Department of Biotechnology, Government of India and University Grants Commission (UGC—under DRS Scheme), New Delhi, India for providing the financial support to this study. S. Thirugnanam was the recipient of research fellowship from the UGC, India. We thank Balaji Ganesh and Bhandary Y.P. for their assistance in animal studies. We also thank Sabarinathan Ramachandran and Pankaj Kumar Mishra for their help with the studies.
Index Descriptors and Abbreviations
- alt-2
abundant larval transcript-2 gene
- ALT-2
Abundant Larval Transcript-2 protein
- Bm
Brugia malayi
- CP
chronic pathology patients
- EN
endemic normal individuals
- IFN-γ
interferon gamma
- IL
Interleukin
- L2
Larval stage 2
- L3
Larval stage 3 (Infective larvae)
- MF
asymptomatic microfilaremic patients
- Th
T helper cells
References
- Abraham D, Grieve RB, Holy JM, Christensen BM. Immunity to larval Brugia malayi in BALB/c mice: protective immunity and inhibition of larval development. The American Journal of Tropical Medicine and Hygiene. 1989;40:598–604. doi: 10.4269/ajtmh.1989.40.598. [DOI] [PubMed] [Google Scholar]
- Ausiello CM, Urbani F, la Sala A, Funaro A, Malavasi F. CD38 ligation induces discrete cytokine mRNA expression in human cultured lymphocytes. European Journal of Immunology. 1995;25:1477–1480. doi: 10.1002/eji.1830250554. [DOI] [PubMed] [Google Scholar]
- Ausiello CM, la Sala A, Ramoni C, Urbani F, Funaro A, Malavasi F. Secretion of IFN-γ, IL-6, granulocyte-macrophage colony-stimulating factor and IL-10 cytokines upon CD38 ligation. Cellular Immunology. 1996;173:192–197. doi: 10.1006/cimm.1996.0267. [DOI] [PubMed] [Google Scholar]
- Babu S, Ganley LM, Klei TR, Shultz LD, Rajan TV. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infection and Immunity. 2000;68:3034–3035. doi: 10.1128/iai.68.5.3034-3035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. The Journal of Immunology. 1999;163:6448–6454. [PubMed] [Google Scholar]
- Curtis CF, Malecela-Lazaro M, Reuben R, Maxwell CA. Use of floating layers of polystyrene beads to control populations of the filaria vector Culex quinquefasciatus. Annals of tropical medicine and parasitology. 2002;96:S97–S104. doi: 10.1179/000349802125002446. [DOI] [PubMed] [Google Scholar]
- Ferraz JC, Stavropoulos E, Yang M, Coade S, Espitia C, Lowrie DB, Colston MJ, Tascon RE. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infection and Immunity. 2004;72:6945–6950. doi: 10.1128/IAI.72.12.6945-6950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M, Taleo G, Taleo F, Yaviong J, Amos M, Babu M, Kalkoa M. Evaluation of the program to eliminate lymphatic filariasis in Vanuatu following two years of mass drug administration implementation: results and methodologic approach. The American Journal of Tropical Medicine and Hygiene. 2005;73(4):753–758. [PubMed] [Google Scholar]
- Gardner MJ, Doolan DL, Hedstrom RC, Wang R, Sedegah M, Gramzinski RA, Aguiar JC, Wang H, Margalith M, Hobart P, Hoffman SL. DNA vaccines against malaria: immunogenicity and protection in a rodent model. Journal of Pharmaceutical Sciences. 1996;85:1294–1300. doi: 10.1021/js960147h. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, Ramaswamy K. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infection and Immunity. 2004;72:4707–4715. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory WF, Atmadja AK, Allen JE, Maizels RM. The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infection and Immunity. 2000;68:4174–4179. doi: 10.1128/iai.68.7.4174-4179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA, Bianco AE. DNA immunization with Onchocerca volvulus genes, Ov-tmy-1 and OvB20: serological and parasitological outcomes following intramuscular or Gene Gun delivery in a mouse model of onchocerciasis. Parasite Immunology. 2000;22:249–257. doi: 10.1046/j.1365-3024.2000.00304.x. [DOI] [PubMed] [Google Scholar]
- Harrison RA, Wu Y, Egerton G, Bianco AE. DNA immunisation with Onchocerca volvulus chitinase induces partial protection against challenge infection with L3 larvae in mice. Vaccine. 1999;18:647–655. doi: 10.1016/s0264-410x(99)00274-1. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Ferris MT. The antipoverty vaccines. Vaccine. 2006;24:5787–5799. doi: 10.1016/j.vaccine.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Doolan DL, Sedegah M, Aguiar JC, Wang R, Malik A, Gramzinski RA, Weiss WR, Hobart P, Norman JA, Margalith M, Hedstrom RC. Strategy for development of a pre-erythrocytic Plasmodium falciparum DNA vaccine for human use. Vaccine. 1997;15:842–845. doi: 10.1016/s0264-410x(96)00273-3. [DOI] [PubMed] [Google Scholar]
- Jones TR, Narum DL, Gozalo AS, Aguiar J, Fuhrmann SR, Liang H, Haynes JD, Moch JK, Lucas C, Luu T, Magill AJ, Hoffman SL, Sim BK. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. The Journal of Infectious Diseases. 2001;183:303–312. doi: 10.1086/317933. [DOI] [PubMed] [Google Scholar]
- Kalinna BH. DNA vaccines for parasitic infections. Immunology and Cell Biology. 1997;75:370–375. doi: 10.1038/icb.1997.58. [DOI] [PubMed] [Google Scholar]
- Lange AM, Yutanawiboonchai W, Lok JB, Trpis M, Abraham D. Induction of protective immunity against larval Onchocerca volvulus in a mouse model. The American Journal of Tropical Medicine and Hygiene. 1993;49:783–788. doi: 10.4269/ajtmh.1993.49.783. [DOI] [PubMed] [Google Scholar]
- Le Goff L, Loke P, Ali HF, Taylor DW, Allen JE. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infection and Immunity. 2000;68:2513–2517. doi: 10.1128/iai.68.5.2513-2517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Montefiori DC, Yasutomi Y, Perry HC, Davies ME, Lekutis C, Alroy M, Freed DC, Lord CI, Handt LK, Liu MA, Shiver JW. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BW, Chandrashekar R, Weil GJ. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. Journal of Immunology. 1993;150:1881–1885. [PubMed] [Google Scholar]
- Li BW, Zhang S, Curtis KC, Weil GJ. Immune responses to Brugia malayi paramyosin in rodents after DNA vaccination. Vaccine. 1999;18:76–81. doi: 10.1016/s0264-410x(99)00182-6. [DOI] [PubMed] [Google Scholar]
- MacDonald AJ, Cao L, He Y, Zhao Q, Jiang S, Lustigman S. rOv-ASP-1, a recombinant secreted protein of the helminth Onchocerca volvulus, is a potent adjuvant for inducing antibodies to ovalbumin, HIV-1 polypeptide and SARS-CoV peptide antigens. Vaccine. 2005;23:3446–3452. doi: 10.1016/j.vaccine.2005.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Le Goff L, Ungeheuer MN, Vuong PN, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infection and Immunity. 2000;68:3651–3656. doi: 10.1128/iai.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CA, Mohammed K, Kisumku U, Curtis CF. Can vector control play a useful supplementary role against Bancroftian filariasis? Bulletin of the World Health Organization. 1999;77:138–144. [PMC free article] [PubMed] [Google Scholar]
- Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. Mass drug treatment for lymphatic filariasis and onchocerciasis. Trends in Parasitology. 2003;19(11):516–522. doi: 10.1016/j.pt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Murray J, Gregory WF, Gomez-Escobar N, Atmadja AK, Maizels RM. Expression and immune recognition of Brugia malayi VAL-1, a homologue of vespid venom allergens and Ancylostoma secreted proteins. Molecular and Biochemical Parasitology. 2001;118:89–96. doi: 10.1016/s0166-6851(01)00374-7. [DOI] [PubMed] [Google Scholar]
- Peralta ME, Schmitz KA, Rajan TV. Failure of highly immunogenic filarial proteins to provide host-protective immunity. Experimental Parasitology. 1999;91:334–340. doi: 10.1006/expr.1998.4382. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Kumar MP, Rami RM, Chinnaiah HB, Nutman T, Kaliraj P, McCarthy J. The larval specific lymphatic filarial ALT-2: Induction of protection using protein or DNA vaccination. Microbiology and Immunology. 2004;48:945–955. doi: 10.1111/j.1348-0421.2004.tb03624.x. [DOI] [PubMed] [Google Scholar]
- Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367(9515):992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- Rizzo LV, DeKruyff RH, Umetsu DT. Generation of B cell memory and affinity maturation: Induction with Th1 and Th2 T cell clones. Journal of Immunology. 1992;148(12):3733–3739. [PubMed] [Google Scholar]
- Ruitenberg KM, Walker C, Love DN, Wellington JE, Whalley JM. A prime-boost immunization strategy with DNA and recombinant baculovirus-expressed protein enhances protective immunogenicity of glycoprotein D of equine herpesvirus 1 in naive and infection-primed mice. Vaccine. 2000;18:1367–1373. doi: 10.1016/s0264-410x(99)00400-4. [DOI] [PubMed] [Google Scholar]
- Santra S, Barouch DH, Korioth-Schmitz B, Lord CI, Krivulka GR, Yu F, Beddall MH, Gorgone DA, Lifton MA, Miura A, Philippon V, Manson K, Markham PD, Parrish J, Kuroda MJ, Schmitz JE, Gelman RS, Shiver JW, Montefiori DC, Panicali D, Letvin NL. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11088–11093. doi: 10.1073/pnas.0401954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Seregeg IG. A mass dissection technique for determining infectivity rate of filariasis vectors. The Japanese Journal of Experimental Medicine. 1979;49:117–121. [PubMed] [Google Scholar]
- Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765–773. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann L, Bain O, Saeftel M, Specht S, Fischer K, Brombacher F, Matthaei KI, Hoerauf A. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Medical Microbiology and Immunology. 2003;192:23–31. doi: 10.1007/s00430-002-0155-9. [DOI] [PubMed] [Google Scholar]
- Vordermeier HM, Lowrie DB, Hewinson RG. Improved immunogenicity of DNA vaccination with mycobacterial HSP65 against bovine tuberculosis by protein boosting. Veterinary Microbiology. 2003;93:349–359. doi: 10.1016/s0378-1135(03)00046-4. [DOI] [PubMed] [Google Scholar]
- Walker PS, Scharton-Kersten T, Rowton ED, Hengge U, Bouloc A, Udey MC, Vogel JC. Genetic immunization with glycoprotein 63 cDNA results in a helper T cell type 1 immune response and protection in a murine model of leishmaniasis. Human Gene Therapy. 1998;9:1899–1907. doi: 10.1089/hum.1998.9.13-1899. [DOI] [PubMed] [Google Scholar]
- Wang QM, Sun SH, Hu ZL, Yin M, Xiao CJ, Zhang JC. Improved immunogenicity of a tuberculosis DNA vaccine encoding ESAT6 by DNA priming and protein boosting. Vaccine. 2004;22:3622–3627. doi: 10.1016/j.vaccine.2004.03.029. [DOI] [PubMed] [Google Scholar]