Abstract
High fat meals induce oxidative stress, which is associated with the pathogenesis of disease. Obese individuals have elevated resting biomarkers of oxidative stress compared to non-obese. We compared blood oxidative stress biomarkers in obese (n = 14; 30 ± 2 years; BMI 35 ± 1 kg•m−2) and non-obese (n = 16; 24 ± 2 years; BMI 23 ± 1 kg•m−2) women, in response to a high fat meal. Blood samples were collected pre-meal (fasted), and at 1, 2, 4 and 6 hours post meal, and assayed for trolox equivalent antioxidant capacity (TEAC), xanthine oxidase activity (XO), hydrogen peroxide (H2O2), malondialdehyde (MDA), triglycerides (TAG), and glucose. An obesity status effect was noted for all variables (p < 0.001; MDA p = 0.05), with obese women having higher values than non-obese, except for TEAC, for which values were lower. Time main effects were noted for all variables (p ≤ 0.01) except for TEAC and glucose, with XO, H2O2, MDA and TAG increasing following feeding with a peak response at the four or six hour post feeding time point. While values tended to decline by six hours post feeding in the non-obese women (agreeing with previous studies), they were maintained (MDA) or continued to increase (XO, H2O2 and TAG) in the obese women. While no interaction effects were noted (p > 0.05), contrasts revealed greater values in obese compared to non-obese women for XO, H2O2, MDA, TAG and glucose, and lower values for TEAC at times from 1–6 hours post feeding (p ≤ 0.03). We conclude that young, obese women experience a similar pattern of increase in blood oxidative stress biomarkers in response to a high fat meal, as compared to non-obese women. However, the overall oxidative stress is greater in obese women, and values appear to remain elevated for longer periods of time post feeding. These data provide insight into another potential mechanism related to obesity-mediated morbidity.
Key words: free radicals, nutrition, body mass, triglycerides, oxidative stress, obesity
Introduction
Reactive oxygen and nitrogen species (RONS) are produced in the body via the activity of radical generating enzymes and inflammatory cells,16 as well as through cellular metabolism involving the processing of macronutrients.42 RONS produced in vivo exert both positive and negative effects on physiological function.46 The positive effects refer to the known role of RONS in promoting cellular signaling, aiding in phagocytic immune defense, as well as promoting apoptosis.46 The negative effects refer to the RONS-induced oxidative damage to various biomolecules, which occurs under conditions of accelerated RONS production, often coupled with impaired antioxidant defense.16 Such a condition is referred to as oxidative stress16 and has been shown to result in oxidative damage to nucleic acids, lipids and proteins, which over time has the potential to contribute to the development of disease.11 In fact, oxidative stress has been suggested to play a primary or secondary role in the pathophysiological mechanisms of multiple (>100) acute and chronic human illnesses/diseases.11
Overproduction of RONS can indeed result from exposure to a variety of stimuli, including environmental pollutants such as ozone15 and cigarette smoke,32 physical stress such as acute aerobic51 and anaerobic6 exercise as well as consumption and processing of dietary energy.34,42 In relation to the latter, several reports indicate an increase in oxidative stress biomarkers following both high fat27,30,45 and high carbohydrate10,28,39,41 feedings. This “postprandial oxidative stress” has been shown to be exacerbated in diseased (diabetes, cardiovascular disease) individuals, as compared to healthy controls.10,26,41
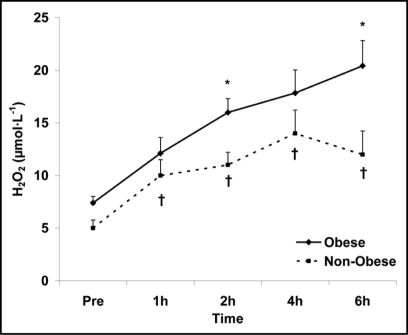
Obesity, a condition with strong associations to both type II diabetes and cardiovascular disease,14 may contribute to further increases in the oxidative stress response to feeding. This is because obese individuals are known to present with higher resting/fasting levels of oxidative stress biomarkers compared to those who are non-obese (reviewed in ref. 47), as well as experience a greater magnitude of exercise-induced oxidative stress compared to their non-obese counterparts.48,50 Moreover, such individuals are also known to have impaired glucose38 and lipid23 metabolism, likely mediated by problems with GLUT 4 translocation,31 insulin-insulin receptor binding17 and post-receptor signaling,36 in addition to decreased lipoprotein lipase activity,9 respectively. The finding of metabolic dysfunction appears important with regards to postprandial oxidative stress, as RONS production is dependent on the extent of both postprandial glycemia29 and lipemia.2,3,45 Hence, any condition that results in delayed clearance of glucose and/or TAG during the postprandial period would be expected to result in an exacerbated similar pattern for both obese and non-obese women was observed [indicating no interaction effect (p > 0.05)], overall values were greater for obese women compared to non-obese women. Date for H2O2 can be viewed in Figure 3.
Figure 3.
Plasma hydrogen peroxide in obese and non-obese women before and following intake of a high fat meal. Note: Obesity status (p = 0.009) and time (p < 0.0001) main effects; †Significant difference from pre meal value (p < 0.05). *Significant difference between obese and non-obese (p = 0.03).
MDA is a three carbon chain aldehyde produced during the decomposition of a lipid hydroperoxide and is commonly used as an indicator of lipid peroxidation.11 As with XO and H2O2, both a time (p < 0.0001) and obesity status (p = 0.05) main effect were noted for MDA, as values increased in response to the test meal, with a greater overall increase being observed in obese women. Date for MDA can be viewed in Figure 4.
Figure 4.
Plasma malondialdehyde in obese and non-obese women before and following intake of a high fat meal. Note: Obesity status (p =0.05) and time (p < 0.0001) main effects; †Significant difference from pre meal value (p < 0.05). *Significant difference between obese and non-obese (p = 0.03).
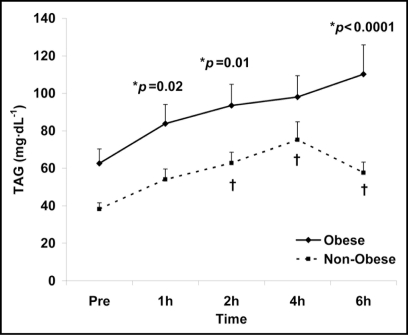
In addition to the above biomarkers used to assess the degree of feeding-induced oxidative stress, the measurement of TAG and glucose was also utilized to assess the overall metabolic load of the test meal. Similar to XO, H2O2 and MDA, both a time (p = 0.0006) and obesity status (p < 0.0001) main effect were noted for TAG. Values increased in response to the test meal, peaking at 4–6 hours post and higher overall in the obese women. A time main effect was not observed for glucose (p = 0.07); however, an obesity status main effect was noted (p < 0.0001), indicating higher glucose levels for obese women throughout all time points. Results for TAG and glucose can be viewed in Figures 5 and 6, respectively. Due to the similar pattern of response for obese and non-obese women, no interaction effects were noted for any variable (p > 0.05).
Figure 5.
Serum TAG in obese and non-obese women before and following intake of a high fat meal. Note: Obesity status (p < 0.0001) and time (p = 0.0006) main effects; †Significant difference from pre meal value (p < 0.05). *Significant difference between obese and non-obese.
Figure 6.
Serum glucose in obese and non-obese women before and following intake of a high fat meal. Note: Obesity status (p < 0.0001) main effect; *Significant difference between obese and non-obese.
Discussion
Data from the present investigation indicate that obese women experience an exaggerated oxidative stress and TAG response to a high fat meal when compared to non-obese women of similar age, dietary intake and activity profile. This is the first study to our knowledge to report postprandial oxidative stress and TAG data in reference to a sample of obese women. Our findings provide additional insight into another possible mechanism related to obesity-mediated morbidity. It should be noted that these data were obtained using a young population of women. It is possible that older obese women, or obese men for that matter, may experience a more robust oxidative stress response to feeding. This is because aging is associated with increased oxidative stress,13 and men have been reported to have higher oxidative stress levels as compared to women.5 Related to the age associated increase in oxidative stress, it should be understood that this appears present only when large disparities exists between groups in terms of age [e.g., young (8 month) vs. old (24 month) animals or young (∼20 years) vs. old (∼65 years) humans].4 With respect to humans, trials using participants of similar age (e.g., range of 20–40 years or 40–60 years) demonstrate little or no difference in oxidative stress biomarkers and have shown no independent effect of age on oxidative stress biomarkers.20 Therefore, our moderate group age difference, despite being statistically significant, would not be oxidative stress response. Considering the above, it is certainly possible that obese individuals may experience a greater rise in postprandial oxidative stress than those of normal weight.
To our knowledge, no investigation has been conducted focused on postprandial oxidative stress in a homogenous sample of obese individuals. Therefore, in the present investigation we compared blood oxidative stress biomarkers, as well as glucose and TAG, in obese and non-obese women in response to a high fat test meal standardized to body mass. We hypothesized that obese individuals would experience a heightened oxidative stress response to feeding as compared to non-obese women.
Results
As expected, several differences were apparent between the two groups with regards to descriptive characteristics (p < 0.05; Table 1). With regards to dietary intake, no differences were noted for total kcal, macro or micronutrient intake between the two groups (p > 0.05), with the exception of a higher percentage of protein being consumed by non-obese women (p = 0.026; Table 2). The total amount of self reported light (1.1 ± 0.6 vs. 0.5 ± 0.5 hrs; p = 0.71), moderate (0.5 ± 0.4 vs. 0.8 ± 0.5 hrs; p = 0.42), and strenuous (0.4 ± 0.5 vs. 0.9 ± 0.5 hrs; p = 0.32) physical activity performed by obese and non-obese women did not differ during the week prior to the test meal.
Table 1.
Descriptive characteristics of obese and non-obese women
| Variable | Obese (n = 14) | Non-obese (n = 16) | p value* |
| Age (yrs)* | 30 ± 2 | 24 ± 2 | 0.017 |
| Height (cm) | 167 ± 2 | 164 ± 2 | 0.210 |
| Weight (kg)* | 97 ± 3 | 60 ± 3 | <0.0001 |
| BMI (kg·m−2)* | 35 ± 1 | 23 ± 1 | <0.0001 |
| Body fat (%)* | 30 ± 1 | 22 ± 1 | <0.0001 |
| Waist (cm)* | 108 ± 3 | 68 ± 2 | <0.0001 |
| Hip (cm)* | 124 ± 2 | 97 ± 2 | <0.0001 |
| Waist:Hip* | 0.87 ± 0.01 | 0.70 ± 0.01 | <0.0001 |
| Resting HR (bpm) | 76 ± 3 | 71 ± 3 | 0.251 |
| Resting SBP (mmHg) | 118 ± 3 | 112 ± 3 | 0.171 |
| Resting DBP (mmHg)* | 81 ± 2 | 71 ± 2 | 0.001 |
| Total Cholesterol (mg·dL−1) | 189 ± 15 | 166 ± 8 | 0.171 |
| HDL-C (mg·dL−1) | 51 ± 7 | 57 ± 4 | 0.320 |
| LDL-C (mg·dL−1)* | 127 ± 11 | 99 ± 8 | 0.041 |
Data are mean ± SEM.
Table 2.
Dietary data of obese and non-obese women during 7 days preceding a test meal
| Variable | Obese (n = 14) | Non-obese (n = 16) | p value* |
| Kcal | 2101 ± 185 | 2084 ± 172 | 0.744 |
| Protein (g) | 72 ± 7 | 89 ± 6 | 0.123 |
| % Protein* | 14 ± 1 | 17 ± 1 | 0.026 |
| CHO (g) | 253 ± 22 | 249 ± 21 | 0.893 |
| % CHO | 49 ± 3 | 49 ± 4 | 0.891 |
| Fat (g) | 87 ± 10 | 77 ± 9 | 0.588 |
| % Fat | 37 ± 3 | 34 ± 2 | 0.222 |
| Vitamin C (mg) | 62 ± 11 | 79 ± 10 | 0.257 |
| Vitamin E (mg) | 6 ± 2 | 11 ± 2 | 0.107 |
| Vitamin A (RE) | 3700 ± 1254 | 6077 ± 1173 | 0.177 |
Data are mean ± SEM.
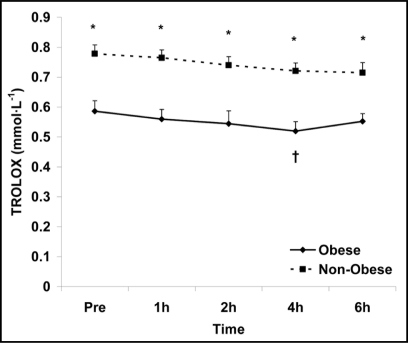
With regards to the biochemical variables, results are discussed herein relative to each biomarker. TEAC is a commonly used biomarker utilized to estimate the body's antioxidant capacity, in which trolox is used as a standard. In general, it provides an estimation of the lipid soluble antioxidants present within the circulation and/or tissue sampled.52 In the present study, no time main effects were noted for TEAC (p = 0.17), meaning that values did not change in response to the test meal; however, an obesity status main (p < 0.0001) was observed. This is evident by lower levels in the obese women, and can be viewed in Figure 1.
Figure 1.
Serum trolox equivalent antioxidant capacity in obese and non-obese women before and following intake of a high fat meal. Note: Obesity status (p < 0.0001) main effect; †Significant difference from pre meal value (p < 0.05). *Significant difference between obese and non-obese (p < 0.0001).
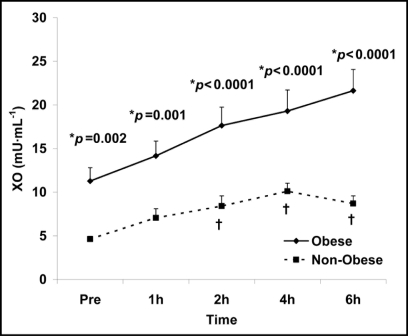
XO generates superoxide (O2-•), primarily in response to conditions of ischemia followed by reperfusion, by catalyzing the oxidation of hypoxanthine to xanthine and xanthine to uric acid.12 Both a time (p < 0.0001) and obesity status (p < 0.0001) main effect were noted for XO, meaning that the activity of XO increased in response to the test meal, with values peaking at 4 or 6 hours post ingestion for non-obese and obese women, respectively. Although a similar pattern of increase occurred for both obese and non-obese women [no interaction effect (p > 0.05)], contrast revealed a greater overall increase in the obese women (i.e., exacerbated XO-mediated superoxide production). Data for XO can be viewed in Figure 2.
Figure 2.
Plasma xanthine oxidase activity in obese and non-obese women before and following intake of a high fat meal. Note: Obesity status (p < 0.0001) and time (p < 0.0001) main effects; †Significant difference from pre meal value (p < 0.05). *Significant difference between obese and non-obese.
H2O2 is a non-radical species primarily produced during the dismutation of superoxide anion via superoxide dismutase; however, direct production of H2O2 can also occur by way of several oxidase enzymes (e.g., glycolate oxidase, xanthine oxidase).16 Similar to XO, both a time (p < 0.0001) and obesity status (p = 0.009) main effect were noted for H2O2, meaning that values increased in response to the test meal (peaking at 4–6 hours post ingestion). Although a expected to impact our outcome measures, as all women in both groups were relatively young (range: 20–42 years).
Antioxidant capacity, which was far lower in obese compared to non-obese women (Fig. 1), declined slightly during the postprandial period, likely due to the consumption of serum antioxidants in an attempt to counteract the increased RONS production resulting from nutrient metabolism. We measured xanthine oxidase activity as a marker of RONS production, as well as hydrogen peroxide. These biomarkers are highly related and provide an overall representation of biological oxidative stress. Xanthine oxidase is a ubiquitous generator of radical species, ultimately leading to superoxide radical. Although in aqueous solutions the superoxide radical is poorly reactive, it can be converted into hydrogen peroxide through the activity of superoxide dismutase.25 As with superoxide radical, much of the cytotoxic effects of hydrogen peroxide are due to its conversion into the hydroxyl radical via either the Fenton reaction53 or the Haber-Weiss reaction.21 A rise in hydroxyl radical increases the potential for damage to various cellular structures, including lipids.1 Malondialdehyde is a representation of RONS-induced lipid peroxidation, involving degradation of polyunsaturated fatty acids and phospholipids through a chain reaction sequence,1 typically initiated by hydroxyl radical.
Our findings of increased TAG and oxidative stress biomarkers in response to the high fat meal agree with previous work, noting a peak in these variables at four hours following feeding.40,43 While the pattern of response for these variables was relatively similar between obese and non-obese women, the overall oxidative stress was greater for obese women (Figs. 2–5). Moreover, while values peaked at four hours post feeding for non-obese women, values continued to rise (for XO, H2O2 and TAG) at the six hour post feeding time point. It is unknown whether or not values would have continued to increase beyond this period, due to our cessation of sample collection at the six hour postprandial time. This is a limitation of the study.
Obesity induces oxidative stress through a variety of mechanisms, including hyperglycemia, increased energy expenditure resulting from excessive weight, elevated tissue lipid levels, inadequate antioxidant defenses (either from inadequate dietary intake and/or a sedentary lifestyle), chronic inflammation, endothelial RONS production, and hyperleptinemia (reviewed in ref. 49). It should be understood that the factors above may not be indicative of every obese individual and other contributing pathways may also exist. However, related to the issue of postprandial oxidative stress, it would appear that insulin insensitivity, resulting from chronic low grade inflammation, commonly observed in obese individuals is a significant contributor to RONS production both at rest and following feeding.33 Adipocytes express pro-inflammatory cytokines, including tumor necrosis factor-α (TNFα) and interleukin-6 (IL-6),14 both of which have been shown to promote insulin resistance via disruption of insulin receptor signaling through the insulin receptor substrate-1 pathway,17 and alterations of the expression/function of several key proteins involved in insulin signaling,36 respectively. Insulin resistance can then promote hyperglycemic conditions, as well as alter lipid metabolism and blood lipid levels, thus further promoting RONS production. This, along with other contributing factors, has lead to the finding of higher oxidative stress biomarkers in obese compared to non-obese individuals.47 Additionally, obese individuals have been reported to experience an exaggerated oxidative stress response to physical work.48,50 Based on these findings, it follows that obese individuals may experience a more pronounced increase in oxidative stress following feeding.
Following the consumption of an energy dense carbohydrate or lipid rich meal, an acute period of glycemia or lipemia occurs, evident by postprandial increases in blood glucose or triglycerides, respectively.42 This large influx of substrate (either glucose and/or TAG) in systemic circulation and subsequent processing via the electron transport chain appears to result in the increase in mitochondrial superoxide production from vascular endothelial cells.8 In support of this notion, increased superoxide production has been noted following both a carbohydrate (glucose)28 and lipid (whipping cream)27 rich meal. This initial accelerated production of superoxide anions appear to lay the foundation for the secondary activation of several other RONS generating pathways such as increased polyol pathway flux, activation of protein kinase C (PKC) and formation of advanced glycation end products (AGEs), apparently mediated by a reduction in the activity of a key glycolytic enzyme [glyceraldehyde-3 phosphate dehydrogenase (GAPDH)], thereby resulting in cellular oxidative damage and dysfunction.8 Because the production of RONS is strongly dependent both on the magnitude and rate of postprandial glycemia/lipemia,2,3,29,45 coupled with the fact that obesity is known to negatively impact both glucose38 and lipid23 metabolism, our findings appear well supported.
The effects of obesity on blood glucose, although complex, are likely mediated by impaired GLUT 4 translocation,31 insulin-insulin receptor binding17 and post-receptor signaling.36 Pertaining to lipids, these effects appear mediated by the presence of fasting hypertriglyceridemia and insulin insensitivity,22 as well as decreased lipoprotein lipase activity.9 Although these aspects were not studied in the present investigation, it is possible that these may have contributed to our findings of elevated oxidative stress in our obese women.
It should be noted that although fasting/pre-meal TAG values were not statistically different between the obese and non-obese women (and surprisingly low for both groups), values were higher in obese women (Fig. 5). This may be important to consider, as fasting TAG is strongly correlated to the TAG response to feeding.22 Therefore, attempts to decrease fasting TAG may improve the oxidative stress response to feeding in such individuals. Likewise, fasting/pre-meal glucose was higher in obese compared to non-obese women (Fig. 6), which could help to explain the results for our oxidative stress biomarkers. Although no subject was a diabetic, obese women presented with fasting blood glucose values which would classify them as pre-diabetic (100–125 mg•dL−1) based on recent guidelines.37 As with TAG, strategies to reduce fasting blood glucose may prove effective in minimizing postprandial oxidative stress in obese individuals with elevated blood glucose.44 Future studies are indeed needed in this area of research.
In conclusion, we report for the first time that obese women experience an exaggerated oxidative stress and TAG response to a high fat meal standardized to body mass, when compared to non-obese controls. These findings add to the already apparent elevation in fasting and physical work-induced oxidative stress biomarkers in those who are obese, possibly providing additional mechanistic information related to obesity-mediated ill-health and disease. Treatment options, possibly involving chronic exercise, and/or the administration of antioxidant, lipid and glucose regulating agents, may prove effective in this regard.
Experimental Methods
Participants.
Obese (n = 14; BMI = 30–40 kg•m−2) and non-obese (n = 16; BMI < 25 kg•m−2) women met all enrollment criteria and volunteered to participate in this study. It should be noted that during the early phase of participant recruitment, we could not identify men who were interested in participating. Therefore, rather than risk having a very small percentage of men in our sample, we decided early on to recruit women exclusively, in attempt to keep our sample homogeneous. The women completed a health history and physical activity questionnaire and underwent a physical examination, including anthropometric testing. Body fat was assessed via 7-site skinfold determination.18 The women were sedentary to moderately active, normolipidemic (fasting TAG < 150 mg•dL−1), and did not have diagnosed cardiovascular, metabolic or pulmonary disease as defined by the American College of Sports Medicine.54 All women were non-smokers and reported no use of medications (e.g., anti-inflammatory or cardiovascular drugs), including oral contraceptives or nutritional supplements (e.g., antioxidants), all of which may have impacted our outcome measures. Following the screening procedures, women were scheduled for the test meal and given detailed instructions and data forms related to the recording of both dietary and physical activity data during the seven days before the test meal. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. All experimental procedures were approved by the University Human Participants Review Board and women provided verbal and written consent. Participant characteristics are presented in Table 1.
Test meal.
The women reported to the laboratory in the morning (0600–1000 hours) following a minimum ten-hour overnight fast. In addition, women reported for testing during the early follicular phase of their menstrual cycle in order to avoid any potential antioxidant effect of estrogen, as circulating estrogen is low during this time. A pre-meal blood sample was collected following a ten minute quiet rest period. The women then consumed the test meal (within 15 minutes). The meal consisted of a milkshake made with a combination of whole milk, ice cream and whipping cream. The size (dietary energy) of the milkshake was dependent on each woman's body mass, and equal to 1.2 grams of fat and carbohydrate, and 0.25 grams of protein per kilogram. The milkshake provided approximately 17 kilocalories (71 kilojoules) per kilogram. This was based on our previous work noting significant increases in our chosen biomarkers following consumption of such a meal,7 as well as other studies including fat content between 1–1.4 grams per kilogram of body mass, carbohydrate content between 0.9–1.3 grams per kilogram of body mass and protein content between 0.2–0.4 grams per kilogram of body mass. The postprandial blood collection period lasted six hours from the start of meal intake, during which time four additional blood samples were collected (1, 2, 4 and 6 hours post meal). The women remained in the laboratory during this period and expended little energy (i.e., watched movies, worked on the computer; completed class work). No additional meals or calorie containing beverages were allowed during this period. However, water was allowed ad libitum, with an average intake between 16 and 24 ounces during the six hour postprandial period.
Blood sampling and biochemistry.
Venous blood samples (∼20 mL per draw) were taken from each woman's forearm veins via needle and vacutainer pre-meal (resting and fasting; 0 hour), and at 1, 2, 4 and 6 hours post meal. Blood was immediately processed and stored in several aliquots at −80°C until analyzed. Assays were performed in duplicate on first thaw. Antioxidant capacity was measured in serum using the Trolox-equivalent antioxidant capacity (TEAC) assay using procedures outlined by the reagent provider (Sigma Chemical, St. Louis, MO) and as previously described.35 The coefficient of variation (CV) for this assay was 5.6% Both xanthine oxidase activity (a radical generating enzyme) and hydrogen peroxide were measured in plasma using the Amplex Red reagent method as described by the manufacturer (Molecular Probes, Invitrogen Detection Technologies, Eugene, OR). The CV was 4.6% and 4.3%, respectively. Malondiadehyde was measured in plasma using commercially available reagents (Northwest Life Science Specialties, Vancouver, WA) using the method described by Jentzsch et al.19 The CV was 5.1%. Assays for TAG, glucose, total cholesterol and HDL cholesterol were performed using serum following standard enzymatic procedures as described by the reagent manufacturer (Thermo Electron Clinical Chemistry). For HDL cholesterol analysis, precipitation of apolipoprotein B containing lipoproteins [VLDL, LDL and Lp(a)] was performed using phosphotungstic acid, coupled with centrifugation and HDL cholesterol was measured in the supernatant. LDL cholesterol was calculated using the Friedwald equation as follows: LDL cholesterol = TC − HDL cholesterol − (TAG/5). Total, HDL and LDL cholesterol was measured in pre-meal samples only, and used simply for descriptive purposes. The CV for the lipid and glucose assays was <5%.
Dietary and physical activity records.
The women were asked to maintain their normal diet and activity, and to record these variables on data forms during the seven days prior to the test meal. Dietary records were analyzed for total dietary energy, protein, carbohydrate, fat and a variety of antioxidant micronutrients (Food Processor SQL, version 9.9, ESHA Research, Salem, OR). Activity records were reviewed to determine the amount of activity done during the week prior to the test day. Activity was classified as light, moderate or hard. Light activity (e.g., walking the dog) required some effort but maintained normal breathing. Moderate activity (e.g., housework or gardening) was somewhat difficult with an increase in breathing and heart rate. Hard activity (e.g., strenuous manual labor) involved labored breathing and difficulty holding a conversation during the activity. Both diet and activity records were reviewed in detail with women upon acceptance, in an attempt to ensure accuracy. The women were given specific instructions to avoid physically stressful tasks (including exercise) during the 24 hour period preceding the test meal. This was important in order to control for any acute effects of physical activity on postprandial oxidative stress, as a recent study has demonstrated that acute strenuous exercise may attenuate the rise in oxidative stress observed following feeding.24
Statistical analysis.
Outcome measures were analyzed using a 2 (obesity status) × 5 (time) repeated measures analysis of variance (ANOVA). Tukey's post hoc test was used for analysis of the time main effect. Contrasts were performed for comparison of obese and non-obese women within the interaction. Dietary, physical activity and descriptive data were analyzed using a one way ANOVA. Data are presented as mean ± standard error of the mean. All analyses were performed using JMP statistical software (version 4.0.3, SAS Institute, Cary, NC). Statistical significance was set at p ≤ 0.05.
Acknowledgements
Funding for this work was provided byThe University of Memphis. The authors declare that they have no competing interests.
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: http://www.landesbioscience.com/journals/oximed/article/7860
References
- 1.Alessio HM. Lipid peroxidation in healthy and diseased models: Influence of different types of exercise. In: Sen CK, Packer L, Hanninen O, editors. Handbook of Oxidants and and Antioxidants in Exercise. Vol. 3. Amsterdam, The Netherlands: Elsevier; 2000. pp. 3–54. [Google Scholar]
- 2.Anderson RA, Evans ML, Ellis GR, Graham J, Morris K, Jackson SK, et al. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis. 2001;154:475–483. doi: 10.1016/s0021-9150(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 3.Bae JH, Bassenge E, Kim KB, Kim YN, Kim KS, Lee HJ, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155:517–523. doi: 10.1016/s0021-9150(00)00601-8. [DOI] [PubMed] [Google Scholar]
- 4.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 5.Bloomer RJ, Fisher-Wellman KH. Blood oxidative stress biomarkers: Influence of sex, exercise training status and dietary intake. Gend Med. 2008;5:218–228. doi: 10.1016/j.genm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29:245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 7.Bloomer RJ, Solis AD, Fisher-Wellman KH, Smith WA. Postprandial oxidative stress is exacerbated in cigarette smokers. Br J Nutr. 2008;99:1055–1060. doi: 10.1017/S0007114507844370. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 9.Bullo M, Garcia-Lorda P, Peinado-Onsurbe J, Hernandez M, Del Castillo D, Argiles JM, et al. TNFalpha expression of subcutaneous adipose tissue in obese and morbid obese females: relationship to adipocyte LPL activity and leptin synthesis. Int J Obes Relat Metab Disord. 2002;26:652–658. doi: 10.1038/sj.ijo.0801977. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: Effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211–1218. doi: 10.1161/01.cir.0000027569.76671.a8. [DOI] [PubMed] [Google Scholar]
- 11.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 12.Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41:599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Galaris D, Mantzaris M, Amorgianiotis C. Oxidative stress and aging: The potential role of iron. Hormones (Athens) 2008;7:114–122. doi: 10.1007/BF03401502. [DOI] [PubMed] [Google Scholar]
- 14.Goralski KB, Sinal CJ. Type 2 diabetes and cardiovascular disease: Getting to the fat of the matter. Can J Physiol Pharmacol. 2007;85:113–132. doi: 10.1139/y06-092. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Oxygen radicals: A commonsense look at their nature and medical importance. Med Biol. 1984;62:71–77. [PubMed] [Google Scholar]
- 16.Halliwell B, Cross CE. Oxygen-derived species: Their relation to human disease and environmental stress. Environ Health Perspect. 1994;102:5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNFalpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 19.Jentzsch AM, Bachmann H, Furst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 20.Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender and ethnicity. Hypertension. 2000;36:878–884. doi: 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- 21.Liochev SI, Fridovich I. The Haber-Weiss cycle—70 years later: An alternative view. Redox Rep. 2002;7:55–57. doi: 10.1179/135100002125000190. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98:458–473. doi: 10.1017/S000711450774268X. [DOI] [PubMed] [Google Scholar]
- 23.Mangat R, Su J, Scott PG, Russell JC, Vine DF, Proctor SD. Chylomicron and apoB48 metabolism in the °CR:LA corpulent rat, a model for the metabolic syndrome. Biochem Soc Trans. 2007;35:477–481. doi: 10.1042/BST0350477. [DOI] [PubMed] [Google Scholar]
- 24.Mc Clean CM, Mc Laughlin J, Burke G, Murphy MH, Trinick T, Duly E, et al. The effect of acute aerobic exercise on pulse wave velocity and oxidative stress following postprandial hypertriglyceridemia in healthy men. Eur J Appl Physiol. 2007;100:225–234. doi: 10.1007/s00421-007-0422-y. [DOI] [PubMed] [Google Scholar]
- 25.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 26.Miyazaki Y, Kawano H, Yoshida T, Miyamoto S, Hokamaki J, Nagayoshi Y, et al. Pancreatic B-cell function is altered by oxidative stress induced by acute hyperglycaemia. Diabet Med. 2007;24:154–160. doi: 10.1111/j.1464-5491.2007.02058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002;75:767–772. doi: 10.1093/ajcn/75.4.767. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 29.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 30.Neri S, Signorelli SS, Torrisi B, Pulvirenti D, Mauceri B, Abate G, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: A single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance and healthy controls. Clin Ther. 2005;27:1764–1773. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Palanivel R, Maida A, Liu Y, Sweeney G. Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia. 2006;49:183–190. doi: 10.1007/s00125-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 32.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate and peroxynitrite. Ann N Y Acad Sci. 1993;686:27–28. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 33.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 34.Rebolledo OR, Actis Dato SM. Postprandial hyperglycemia and hyperlipidemia-generated glycoxidative stress: Its contribution to the pathogenesis of diabetes complications. Eur Rev Med Pharmacol Sci. 2005;9:191–208. [PubMed] [Google Scholar]
- 35.Rice-Evans CA. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic Res. 2000;33:59–66. [PubMed] [Google Scholar]
- 36.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 37.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et al. Guidelines on diabetes, pre-diabetes and cardiovascular diseases: Executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 38.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 39.Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ. Plasma F2 isoprostanes: Direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi: 10.2337/diacare.25.3.537. [DOI] [PubMed] [Google Scholar]
- 40.Schinkovitz A, Dittrich P, Wascher TC. Effects of a high-fat meal on resistance vessel reactivity and on indicators of oxidative stress in healthy volunteers. Clin Physiol. 2001;21:404–410. doi: 10.1046/j.1365-2281.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- 41.Serin O, Konukoglu D, Firtina S, Mavis O. Serum oxidized low density lipoprotein, paraoxonase 1 and lipid peroxidation levels during oral glucose tolerance test. Horm Metab Res. 2007;39:207–211. doi: 10.1055/s-2007-970419. [DOI] [PubMed] [Google Scholar]
- 42.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- 43.Tsai WC, Li YH, Lin CC, Chao TH, Chen JH. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci (Lond) 2004;106:315–319. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- 44.Tucker PS, Fisher-Wellman K, Bloomer RJ. Can exercise minimize postprandial oxidative stress in patients with type 2 diabetes? Curr Diabetes Rev. 2008;4:309–319. doi: 10.2174/157339908786241160. [DOI] [PubMed] [Google Scholar]
- 45.Tushuizen ME, Nieuwland R, Scheffer PG, Sturk A, Heine RJ, Diamant M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J Thromb Haemost. 2006;4:1003–1010. doi: 10.1111/j.1538-7836.2006.01914.x. [DOI] [PubMed] [Google Scholar]
- 46.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 48.Vincent HK, Morgan JW, Vincent KR. Obesity exacerbates oxidative stress levels after acute exercise. Med Sci Sports Exerc. 2004;36:772–779. doi: 10.1249/01.mss.0000126576.53038.e9. [DOI] [PubMed] [Google Scholar]
- 49.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 50.Vincent HK, Vincent KR, Bourguignon C, Braith RW. Obesity and postexercise oxidative stress in older women. Med Sci Sports Exerc. 2005;37:213–219. doi: 10.1249/01.mss.0000152705.77073.b3. [DOI] [PubMed] [Google Scholar]
- 51.Vollaard NB, Shearman JP, Cooper CE. Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Med. 2005;35:1045–1062. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 52.Wang CC, Chu CY, Chu KO, Choy KW, Khaw KS, Rogers MS, et al. Trolox-equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clin Chem. 2004;50:952–954. doi: 10.1373/clinchem.2004.031526. [DOI] [PubMed] [Google Scholar]
- 53.Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat Res. 1996;145:523–531. [PubMed] [Google Scholar]
- 54.Whaley MH. ACSM's Guidelines for Exercise Testing and Prescription. 7th edition 2005. [Google Scholar]