Abstract
Astrocytes, one of the predominant types of glial cells, function as both supportive and metabolic cells for the brain. Under cerebral ischemia/reperfusion-induced oxidative conditions, astrocytes accumulate and activate in the ischemic region. DJ-1 has recently been shown to be a sensor of oxidative stress in living cells. However, the function of astrocytic DJ-1 is still unknown. In the present study, to clarify the effect of astrocytic DJ-1 protein under massive oxidative insult, we used a focal ischemic rat model that had been subjected to middle cerebral artery occlusion (MCAO) and reperfusion. We then investigated changes in the distribution of DJ-1 in astrocytes, DJ-1 release from cultured astrocytes, and the effects of recombinant DJ-1 protein on hydrogen peroxide (H2O2)-induced death in normal and DJ-1-knockdown SH-SY5Y cells and on in vitro scavenging of hydroxyl radicals (•OH) by electron spin resonance spectrometry. At 24 h after 2-h MCAO and reperfusion, an infarct lesion was markedly observed using magnetic resonance imaging and 2,3,5-triphenyltetrazolium chloride staining. In addition, reactive astrocytes enhanced DJ-1 expression in the penumbral zone of the ischemic core and that DJ-1 protein was extracellularly released from astrocytes by H2O2 in in vitro primary cultures. Although DJ-1-knockdown SH-SY5Y cells were markedly vulnerable to oxidative stress, treatment with glutathione S-transferase-tagged recombinant human DJ-1 protein (GST-DJ-1) significantly inhibited H2O2-induced cell death. In addition, GST-DJ-1 protein directly scavenged •OH. These results suggest that oxidative stress induces the release of astrocytic DJ-1 protein, which may contribute to astrocyte-mediated neuroprotection.
Key words: DJ-1, release, astrocytes, focal ischemia, oxidative stress sensor, neuroprotection
Introduction
DJ-1, which was discovered as a novel oncogene product in collaboration with activated small GTP-binding protein ras,1 has recently been identified as the causative gene of familial Parkinson's disease PARK7.2 DJ-1 has many functions, such as transcriptional factor, molecular chaperone, etc.3–6 X-ray crystallographic and biological analyses have shown that wild-type DJ-1 protein forms a homodimer. In addition, these analyses have also shown that L166P-mutation in DJ-1 protein, which was first found in a PARK7 patient, disrupts dimer formation, resulting in a loss of function.7–11 Furthermore, the cysteine residues in DJ-1 protein are oxidized, and the isoelectric point (pI) of DJ-1 is shifted to be more acidic.12 Thus, DJ-1 protein may play an important role in oxidative stress-mediated disorders.
Cerebral ischemia, one of the leading causes of death and long-lasting disability, results from a transient or permanent reduction in cerebral blood flow in a major brain artery.13,14 The production of reactive oxygen species (ROS) has been implicated in reperfusion injury after cerebral ischemia, and antioxidant enzymes are considered to be among the major mechanisms by which cells counteract the deleterious effect of ROS after cerebral ischemia.15,16 Previous studies have reported that activated astrocytes were accumulated in the boundary zone (penumbra) of the ischemic core in the striatum.17,18 However, the function of DJ-1 protein in astrocytes remains unclear.
In the present study, to clarify the effect of astrocytic DJ-1 protein under massive oxidative insult, we examined changes in the distribution of DJ-1 in astrocytes in the in vivo ischemic brain, DJ-1 release from cultured astrocytes, and the effects of recombinant DJ-1 protein on hydrogen peroxide (H2O2)-induced death in normal and DJ-1-knockdown SH-SY5Y cells and on in vitro scavenging of hydroxyl radicals (•OH).
Results
Alteration of DJ-1 distribution in ischemic rat brains.
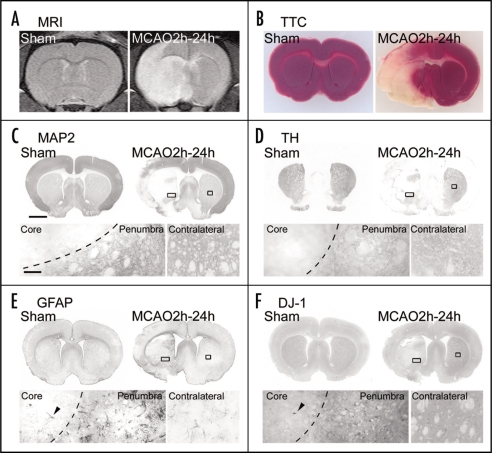
To analyze changes in DJ-1 in the brain under oxidative stress, we used an ischemic rat model that had been subjected to middle cerebral artery occlusion (MCAO) and reperfusion. First, we investigated MCAO-induced injury using magnetic resonance (MR) imaging. In sham-operated rats, an infarct lesion was not detected as MR signal on T2-weighted MR images. At 24 h after 2-h MCAO and reperfusion, an infarct lesion, as represented by an area of hyperintensity (white), was markedly observed in the ipsilateral cortex and striatum (Fig. 1A). Similar neurodegeneration was observed on staining with 2,3,5-triphenyltetrazolium chloride (TTC) (Fig. 1B). Also, microtubule-associated protein-2 (MAP2)- and tyrosine hydroxylase (TH)-immunoreactivities of the ipsilateral striatum were markedly decreased in ischemic rats (Fig. 1C and D).
Figure 1.
Changes in neurons and astrocytes in the focal ischemic rat brain. Representative photographs showing a coronal brain section 1.0 mm anterior from the bregma with T2-weighted MR imaging (A), TTC staining (a living cell marker, B), immunostaining using antibodies to MAP2 (a neural marker, C), TH (a dopaminergic neural marker, D), GFAP (an astrocytic marker, E) and DJ-1 (F). Brains were prepared at 24 h after 2-h MCAO and reperfusion (left brain). Sham-operated rats were used as the vehicle control (right brain). In the upper panel of the right brain of ischemic rats, ipsilateral rectangular boxes show the ischemic core and penumbral region, which are separated by a dotted line (left lower panel, in C—F). Contralateral boxes show a non-ischemic normal region. Arrowhead indicates the same astrocyte in the ischemic core (lower panels in E and F). Scale bars: 1 mm (upper panel in C) and 100 µm (lower panel in C).
In response to MCAO, glial fibrillary acidic protein (GFAP)-immunopositive astrocytes changed to the reactive form in the border zone (penumbral region) where MAP2-immunoreactivity was lost in the striatum and cerebral cortex (Fig. 1E). On the other hand, although DJ-1-immunoreactivity was hardly detected in the ischemic core region, this immunoreactivity was markedly increased in the penumbral region (Fig. 1F).
Intensive DJ-1 immunoreactive astrocytes after MCAO and reperfusion.
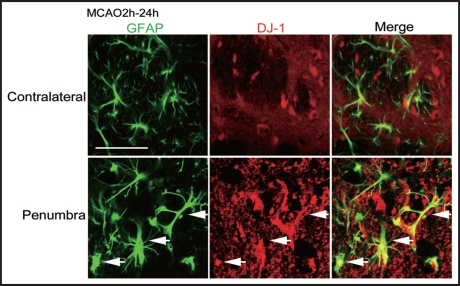
To examine the change in DJ-1 in astrocytes in ischemic rats, we performed double-staining using antibodies against GFAP and DJ-1. At 24 h after reperfusion, GFAP-immunopositive astrocytes were activated and DJ-1-immunoreactivity was markedly increased in the boundary zone of the ischemic core in the striatum, compared to the contralateral side (Fig. 2).
Figure 2.
Double-immunofluorescence staining for GFAP and DJ-1 in ischemic rat brain. Representative photographs showing brain sections, which were double-immunostained with antibodies against GFAP (green, left panels) and DJ-1 (red, middle panels) in ischemic rats at 24 h after 2-h MCAO and reperfusion. The merged images are shown in the right panels. Arrows: Intensive DJ-1 immunoreactivity was detected in many reactive astrocytes in the penumbral region, but not in the contralateral normal region. Scale bar: 100 µm.
Evaluation of H2O2-induced cell death.
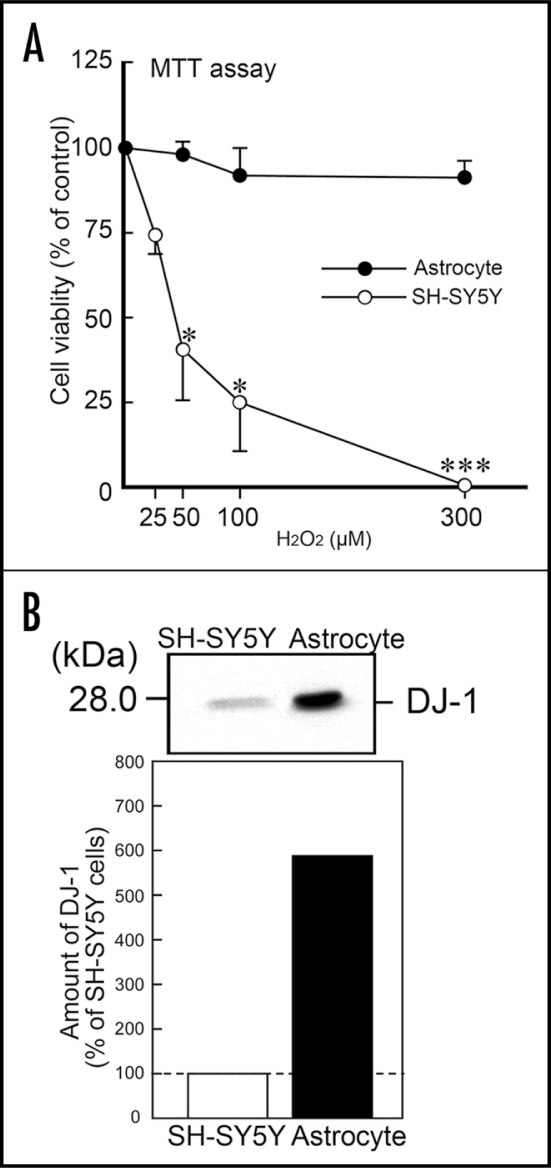
To analyze the effects of DJ-1, we used in vitro primary cultured astrocytes and neuroblastoma SH-SY5Y cells. In the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay, although massive cell death of SH-SY5Y cells was caused by H2O2 in a concentration-dependent manner, astrocytes were relatively resistant to H2O2-induced cell death (Fig. 3A). To determine the endogenous DJ-1 protein level in astrocytes and SH-SY5Y cells, we performed western blotting using anti-DJ-1 antibody. The amount of DJ-1 protein in astrocytes was about six-fold greater than that in SH-SY5Y cells (Fig. 3B).
Figure 3.
H2O2-induced cell death in SH-SY5Y cells and astrocytes. (A) Primary cultured astrocytes and SH-SY5Y cells were exposed to H2O2 at 0–300 µM for 24 h. Subsequently, cell viability was measured by the MTT assay. Each value is the mean±SEM of three determinations, based on the vehicle control as 100%. Significance (Bonferroni/Dunn post hoc comparisons after ANOVA): *p < 0.05, ***p < 0.001 vs. SH-SY5Y cells. (B) Immunoblotting assay for DJ-1 protein in cell lysates of SH-SY5Y cells and astrocytes.
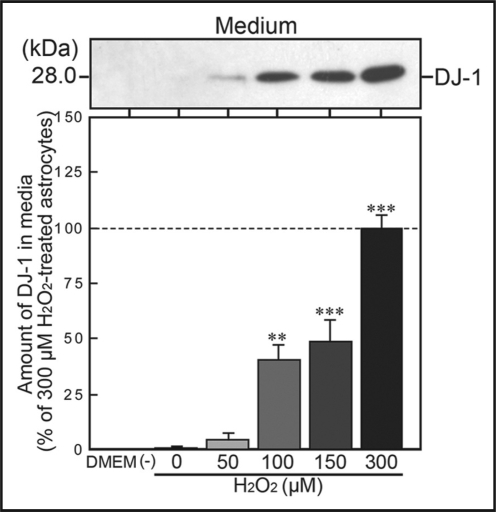
In addition, we measured the amount of DJ-1 protein, which was secreted under oxidative conditions from astrocytes into cultured media. In normal cultured media, released DJ-1 protein was undetectable. In contrast, DJ-1 protein was gradually and significantly released by exposure to H2O2 in a concentration-dependent manner (Fig. 4).
Figure 4.
H2O2-induced release of DJ-1 protein from astrocytes. Astrocytes were cultured with DMEM in the presence or absence of H2O2 (at 0–300 µM) for 24 h. Subsequently, when media, prepared from H2O2-treated astrocytic cultures, were assessed by immunoblotting, the amount of extracellularly released DJ-1 protein was increased in a concentration-dependent manner. In the media without H2O2, however, extracellular DJ-1 protein was undetectable.
Protective effects of recombinant DJ-1 protein in SH-SY5Y cells.
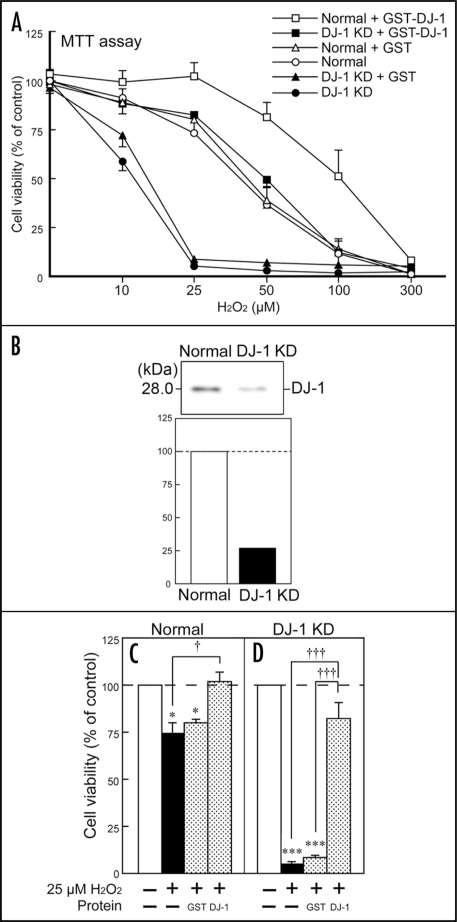
To clarify the function of released DJ-1 protein, we further examined the effects of recombinant human DJ-1 protein on H2O2-treated SH-SY5Y cells (Fig. 5). In the present study, we generated DJ-1-knockdown SH-SY5Y cells, in which the endogenous expression of human DJ-1 was suppressed by approximately 74% (Fig. 5B). In normal SH-SY5Y cells, cell death slightly occurred with 25 µM H2O2 (Figure 5A and C). In contrast, the same concentration (25 µM) of H2O2 caused marked and significant cell death in DJ-1-knockdown cells. Simultaneous treatment with 5 µM glutathione S-transferase-tagged recombinant human DJ-1 protein (GST-DJ-1), but not glutathione S-transferase (GST), rescued DJ-1-knockdown cells to the level in normal cells (Fig. 5A and D). In addition, a concentration-dependent curve of H2O2-induced cell death was further shifted to the right by treatment with GST-DJ-1, but not GST (Fig. 5A).
Figure 5.
Protective effects of recombinant GST-DJ-1 against H2O2-induced oxidative stress in normal and DJ-1-knockdown SH-SY5Y cells. (A) Normal wild-type SH-SY5Y cells (Normal) and DJ-1-knockdown cells (DJ-1KD) were treated with H2O2, in the presence or absence of recombinant GST-tagged human DJ-1 protein (GST-DJ-1, at 1 µM) or GST-tag protein (GST, at 1 µM). After 24 h, cell viability was assessed by the MTT assay. (B) In DJ-1 KD cells, the expression of endogenous human DJ-1 was suppressed by approximately 74% in comparison with normal cells. (C) Cell survival under exposure to 25 µM H2O2, in the presence or absence of GST-DJ-1 or GST (each at 1 µM). Data are the mean±SEM of four determinations, based on untreated cultures as 100%. Significance (Bonferroni/Dunn post hoc comparisons after ANOVA): *p < 0.05, ***p < 0.001 vs. treatment with the vehicle control. †p < 0.05, †††p < 0.001 vs. 25 µM H2O2 alone.
Effects of recombinant DJ-1 protein on •OH trapping in vitro.
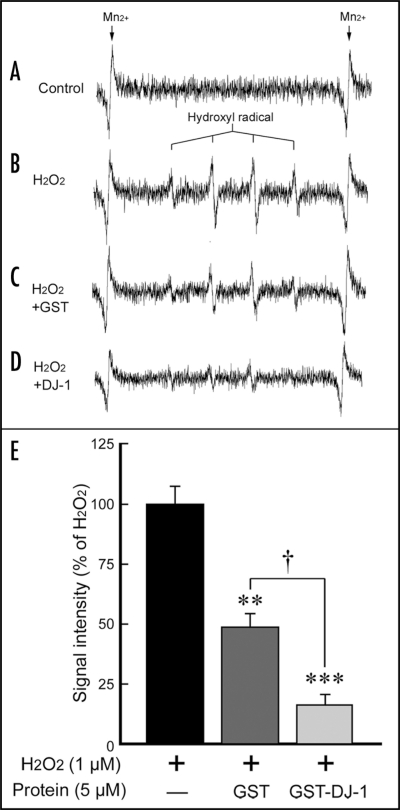
To clarify the direct scavenging effect of recombinant DJ-1 protein against ROS, such as •OH, we further performed electron spin resonance (ESR) analysis using the spin-trapping agent 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (Fig. 6). As an internal reference, Mn2+ signal was detected as two peaks at both edges (Fig. 6A–D). Although no marked signal was detected in the absence of Fe2+, major four peaks with an intensity ratio of 1:2:2:1 appeared at the mid-section between the Mn2+ signal, in the presence of H2O2 and Fe2+ (Fig. 6B). This characteristic quartet signal was almost completely suppressed by thiourea (data not shown), a specific •OH scavenger,19,20 suggesting that the quartet signal indicates a DMPO-OH spin adduct. On the other hand, H2O2-induced DMPO-OH signal was slightly reduced by GST, and markedly reduced by GST-DJ-1 (Fig. 6).
Figure 6.
Direct •OH scavenging of recombinant GST-DJ-1 protein. Traces show typical spectra of DMPO-OH spin adducts generated from the vehicle control (Control, A) or H2O2/Fe2+, in the absence (H2O2, B) or presence of GST (GST, C) and GST-DJ-1 (DJ-1, D). (E) The amount of •OH was semi-quantitatively measured as the formation of DMPO-OH spin adducts by ESR spectrometry, after the reaction for exactly 1 min. Each value is the mean±SEM of three determinations, based on H2O2/Fe2+ as 100% (B). Significance (Bonferroni/Dunn post hoc comparisons after ANOVA): **p < 0.01, ***p < 0.001 vs. signal intensity of H2O2/Fe2+, and †p < 0.05 vs. signal intensity of H2O2/Fe2+ with GST.
Discussion
Oxidative stress is defined as an imbalance between ROS generation and the anti-oxidant capacity of a cell. The production of ROS, which occurs immediately and massively after focal ischemia and reperfusion, is followed by delayed post-ischemic inflammation and apoptosis, and these events are involved in the progression and expansion of neurodegeneration.21,22 We observed severe neuronal damage in the striatum and cerebral cortex at 24 h after 2-h MCAO and reperfusion (Fig. 1). We then performed a noninvasive evaluation of the infarct area, using a 7T Unity Inova MR scanner, and detected the infarct lesion, as represented by an area of hyperintensity (white), in the ipsilateral cortex and striatum. Subsequently, we examined MAP2- and TH-immunoreactivities and confirmed that these immunoreactivities corresponded to the results of MR imaging. It has been shown that severe neuronal damage leads to a strong and delayed activation of astrocytes and produces neurotrophic factors.17 In this study, we also observed the accumulation of reactive astrocytes in the penumbral region of the ischemic core in the ipsilateral striatum and cerebral cortex (Figs. 1 and 2). Recently, it has been reported that DJ-1 protein was increased in the serum or plasma of patients with breast cancer, melanoma, familial amyloidotic polyneuropathy, stroke and Parkinson's disease. Therefore, oxidative stress may be related to the onset of these disorders.23–27 In the present study, we found that DJ-1 protein was actually released from astrocytes into the culture medium without cell death (Fig. 4). In addition, the amount of secreted DJ-1 protein was increased by exposure to H2O2 in a concentration-dependent manner. Thus, oxidative stress activates astrocytes and induces DJ-1 secretion.
In addition, astrocytes were more resistant to H2O2-induced cell death than SH-SY5Y cells, due to the higher expression of DJ-1 protein (Fig. 3), while DJ-1-knockdown SH-SY5Y cells were more susceptible to exposure to H2O2. H2O2-induced cell death was significantly inhibited by simultaneous treatment with recombinant GST-DJ-1 protein, but not by GST-tag protein alone (Fig. 5). From these observations, we consider that astrocytes, which are rich in DJ-1 protein, are resistant to oxidative stress; and that DJ-1 protein released from astrocytes may contribute to this neuroprotection. However, since cerebral ischemia/reperfusion causes neuronal death, astrocytic release of DJ-1 may be a compensatory neuroprotective response.
Human and rat DJ-1 protein has three cysteine residues at amino acid numbers 46, 53 and 106 (C46, C53 and C106, respectively), and C106 is the most sensitive to oxidative stress.12 On the other hand, GST-tag protein involves four cysteine residues. Recent studies have suggested that H2O2 is produced by mitochondrial dysfunction, and •OH is easily generated in the presence of Fe2+ (ref. 28). •OH is one of most potent neurotoxic factors under ischemic insult and also causes protein oxidation. In the present study, •OH, which was generated in vitro by H2O2 and Fe2+, was directly scavenged partially by GST alone and markedly by GST-DJ-1 protein. A cysteine residue is oxidized from a reduced form (−SH) to sulphenation (−SOH), sulphination (-SO2H) and sulphonation (−SO3H), in order of oxidative development. Thus, the oxidation of cysteine residues contributes to the direct scavenging of •OH. However, since GST alone did not inhibit the H2O2-induced cell death of SH-SY5Y cells, the scavenging of •OH by cysteine residues (cysteine oxidation) by itself may be insufficient to give a neuroprotective effect.
We previously found that recombinant GST-DJ-1 protein was taken up into dopaminergic neurons after intranigral microinjection and inhibited neural death in the in vivo brain of Parkinson's disease-model rats.9 Recent studies have reported that DJ-1 inhibits various apoptotic pathways, such as pyrimidine tract-binding protein-associated splicing factor (PSF),29 apoptosis signal regulating kinase 1 (ASK1), death-domain-associated protein (Daxx),30 and/or the reduction of homeodomain-interacting protein kinase 1 (HIPK1).31 In addition, DJ-1 induces anti-oxidative episodes mediated by the stabilizing nuclear factor erythroid 2-related factor (Nrf2), which is a master regulator of antioxidant gene responses.32 Interestingly, DJ-1 oxidation at C106 is necessary for DJ-1 to exert anti-apoptotic and molecular chaperoning responses, and massive oxidation may cause its inactivation.33,34 In brief, SOH- and SO2H-oxidation at C106 in DJ-1 protein may be active forms, while SO3H-peroxidation causes loss-of-function and an inactive form. Based on these observations, we consider that oxidized DJ-1 protein after MCAO and reperfusion may induce anti-apoptotic responses and result in compensatory neuroprotection.
In conclusion, since astrocytes are endogenously rich in DJ-1 protein, these glial cells may be relatively resistant to oxidative stress compared to neurons. In addition, astrocytic DJ-1 protein was secreted by exposure to H2O2. Furthermore, recombinant GST-DJ-1 protein directly trapped •OH and inhibited H2O2-induced cell death. These results suggest that astrocytic DJ-1 protein may be useful for the development of novel therapeutic targets in various oxidative stress-mediated disorders, including cerebral ischemia.
Materials and Methods
Preparation of recombinant human DJ-1 protein and anti-DJ-1 antibody.
Recombinant proteins, such as GST and GST-DJ-1, were expressed in and purified from Escherichia coli as described previously.1,6 The preparation and specificity of a rabbit polyclonal DJ-1 antibody have been described previously.1,6,9
Rat model of focal cerebral ischemia.
Wistar rats were purchased from Japan SLC, Inc. (Hamamatsu, Japan). The animals were acclimated to and maintained at 23°C under a 12-h light/dark cycle (lights on 08:00–20:00 hours). Rats were housed in standard laboratory cages and had free access to food and water throughout the study period. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Committee for Animal Research at Kyoto Pharmaceutical University.
Male Wistar rats weighing 260–300 g were used in the present study. Focal cerebral ischemia was induced by the intraluminal introduction of a nylon thread35 as described previously.18 Briefly, animals were anesthetized with 4% halothane (Takeda Pharmaceutical, Osaka, Japan) and maintained on 1.5% halothane using a facemask. After a midline neck incision was made, 20 mm of a 4-0 nylon thread with its tip rounded by heating and coated with silicon (Xantopren M; Heraeus Kulzer, Hanau, Germany) was inserted into the left internal carotid artery (ICA) as far as the proximal end using a globular stopper. The origin of MCA was then occluded by a silicon-coated embolus. Anesthesia was discontinued, and the development of right hemiparesis with upper limb dominance was used as the criterion for ischemic insult. After 120 min of MCAO, the embolus was withdrawn to allow reperfusion of the ischemic region via the anterior and posterior communicating arteries. Body temperature was maintained at 37–37.5°C with a heating pad and lamp during surgery. In the sham operation, a midline neck incision was made to expose the arteries, but the nylon thread was not inserted into the carotid artery.
MR imaging.
Rats were subjected to a sham-operation or an operation on the MCAO. After 24 h, MR images of the rat heads were acquired with a 7 T Unity Inova MR scanner (Varian, Palo Alto, CA). A T2-weighted MR sequence was used with an acquisition repetition time of 2,000 ms, echo time of 40 ms, 35 × 35-mm2 field of view, and slice thickness of 1 mm. During the MR sessions, spontaneously breathing animals were anesthetized with 1.5% isoflurane in 50% O2 and 50% air.
TTC staining.
At 24 h after MCAO, brains were removed and cut into 2-mm-thick coronal sections. These sections were immersed in a 2% solution of TTC (Wako Pure Chemical Industries, Osaka, Japan) in saline at 37°C for 20 min, and then fixed in 4% paraformaldehyde in 100 mM phosphate buffer (PB) at 4°C.
Immunohistochemistry.
Rats were deeply anesthetized with pentobarbital (100 mg/kg, i.p.) and perfused through the aorta with 150 mL of 10 mM phosphate-buffered saline (PBS), followed by 300 mL of a cold fixative consisting of 4% paraformaldehyde in 100 mM PB. After perfusion, brains were quickly removed and post-fixed for two days with 4% paraformaldehyde in 100 mM PB and then transferred to 15% sucrose solution in 100 mM PB containing 0.1% sodium azide at 4°C. The brain samples were cut into 20-µm thick slices using a cryostat and collected in 100 mM PBS containing 0.3% Triton X-100 (PBS-T). The free-floating sections were treated with 0.3% H2O2 in PBS-T to eliminate endogenous peroxidase activity. After several washes, sections were incubated for three days at 4 °C with the following primary antibodies: MAP2 (dilution, 1:3,000; Sigma, St. Louis, MO) and TH (1:3,000; Sigma); mouse monoclonal antibodies against GFAP (1:3,000; Chemicon International, Temecula, CA); rabbit polyclonal antibodies against DJ-1 (1:10,000). After several washes, sections were incubated with biotinylated antibodies against mouse or rabbit IgG (1:2,000; Vector Laboratories, Burlingame, CA) for 2 h at room temperature. The sections were then incubated with 1:4,000 avidin peroxidase (ABC Elite kit, Vector Laboratories) for 1 h at room temperature. All of the sections were washed several times with PBS-T after each incubation, and labeling was then revealed by 3,3′-diaminobenzidine (DAB; Dojindo Laboratories, Kumamoto, Japan), with nickel ammonium, which yielded a dark blue color.
Double-immunofluorescence staining.
For double-immunofluorescence staining, sections were simultaneously incubated for three days at 4 °C with the following primary antibodies: mouse monoclonal antibody against GFAP (1:1,000; Chemicon Int.) and rabbit polyclonal anti-DJ-1 antibody (1:5,000). Reactivity with primary antibody was detected with anti-mouse IgG antibody conjugated with Alexa Fluor 488 or anti-rabbit IgG antibody conjugated with Alexa Fluor 546 (each diluted 1:500; Molecular Probes, Eugene, OR) for 2 h at room temperature. Fluorescence was then detected using a laser scanning confocal microscope (LSM410, Carl Zeiss, Jena, Germany).
Cell culture and detection of extracellular DJ-1 protein.
The human neuroblastoma cell line SH-SY5Y36 and rat primary astrocytes37 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum (FCS), 50 µg/mL penicillin and 100 µg/mL streptomycin and kept at 37 °C in humidified 5% CO2/95% air. Astrocytes were incubated in DMEM without FCS for 12 h and then in the presence or absence of H2O2 (at 0–300 µM) for an additional 24 h. After incubation, the media were centrifuged at 15,000 rpm at 4 °C for 15 min to remove cells and debris, and transferred to a new tube. For the detection of extra-cellular DJ-1 protein, the collected media were subjected to western blotting with anti-DJ-1 antibody.
Establishment of DJ-1-knockdown cells. We established a DJ-1-knockdown SH-SY5Y cell line by a method that was essentially similar to that in mouse Flp-In3T3 cells.38 In brief, the nucleotide sequence of the upper strand of the oligonucleotide used for construction of an siRNA vector targeting the human DJ-1 gene is 5′-GGATCCCGTCAAGGCTGGCATCAGGACAATTGATATCCGTTGTCCTGATGCCAGCCTTGATTTTTTCCAAAAGCTT-3′. After oligonucleotides corresponding to the upper and lower strands of DNA were annealed, they were inserted into BamHI- HindIII sites of pRNA-U6.1/Neo. These plasmids were transfected into human SH-SY5Y cells by the calcium phosphate precipitation method, and the cells were cultured in RPMI-1640 medium in the presence of 400 µg/mL G418 for 14 days. Cells that were resistant to the drug were then selected, and the intrinsic expression of DJ-1 was checked by western blotting with an anti-human DJ-1 antibody.
Measurement of cell viability.
To evaluate cell viability, we performed MTT (Dojindo Laboratories) assay as an index of surviving cells. In living cells, MTT is converted to formazan, which has a specific absorption maximum. SH-SY5Y cells, in which endogenous DJ-1 expression was normal or knocked-down by siRNA vector, were seeded at 3 × 104 cells/well in a 96-microwell plate, and treated with H2O2 (at 0–300 µM) 24 h after plating. At 24 h after treatment, the culture medium was changed to a medium containing 5 mg/mL MTT, and the cells were incubated for an additional 4 h. They were then mixed thoroughly with an equal volume of isopropanol/0.04 M HCl, and sonicated to dissolve formazan completely. Absorbance was then measured at 570 nm with a microplate reader (BioRad Laboratories, Hercules, CA).
Analysis of ESR.
•OH formation was monitored by ESR spectrometry with DMPO (Labotec Ltd., Tokyo, Japan), a spin-trapping agent. In a final volume of 200 µL of 100 mM PB, recombinant protein such as GST-DJ-1 (5 µM) or GST (5 µM) was added to the reaction mixture containing diethylene-triamine pentaacetic acid (25 µM), FeSO4 (12.5 µM), H2O2 (1 µM) and DMPO (22.5 mM). These recombinant proteins and reagents were solubilized in Milli Q water. The reaction mixture was transferred to a flat quartz cuvette and placed in the cavity of an X-band JEOL RFR-30 Radical Analyzer system (JEOL Ltd., Tokyo, Japan). •OH, which was generated by Fenton's reaction between Fe2+ and H2O2, was trapped by DMPO, and the stable adduct DMPO-OH was measured exactly 1 min after the addition of DMPO. The Mn2+-derived split signal was used as the internal standard. Typical instrument settings were as follows: incident-microwave 4 mW, modulation-amplitude 0.03 mT, time-constant 0.10 s, and sweeprate 5 mT/min.
Statistical analysis.
Results are presented as the mean ± standard error of the mean (SEM). The significance of differences was determined by an analysis of variance (ANOVA). Further post hoc comparisons were performed using Bonferroni/Dunn tests (Stat View; Abacus Concepts, Berkeley, CA).
Acknowledgements
This study was supported in part by Open Research Program and grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and by the program for promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) in Japan.
Abbreviations
- ANOVA
analysis of variance
- ASK1
apoptosis signal regulating kinase 1
- C46, C53 and C106
cysteine residues at amino acid numbers 46, 53 and 106, respectively
- DAB
3,3′-diaminobenzidine
- DMEM
Dulbecco's modified Eagle's medium
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- Daxx
death-domain-associated protein
- ESR
electron spin resonance
- FCS
fetal calf serum
- GFAP
glial fibrillary acidic protein
- GST
glutathione S-transferase
- GST-DJ-1
glutathione S-transferase-tagged recombinant human DJ-1 protein
- H2O2
hydrogen peroxide
- HIPK1
homeodomain-interacting protein kinase 1
- ICA
internal carotid artery
- MAP2
microtubule-associated protein-2
- MCAO
middle cerebral artery occlusion
- MR
magnetic resonance
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide
- Nrf2
nuclear factor erythroid 2-related factor
- •OH
hydroxyl radical
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PBS-T
phosphate-buffered saline containing 0.3% Triton X-100
- PSF
pyrimidine tract-binding protein-associated splicing factor
- ROS
reactive oxygen species
- SEM
standard error of the mean
- TH
tyrosine hydroxylase
- TTC
2,3,5-triphenyltetrazolium chloride
- pI
isoelectric point
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: www.landesbioscience.com/journals/oximed/article/7985
References
- 1.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 2.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 3.Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SM, Ariga H. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol Cancer Res. 2003;1:247–261. [PubMed] [Google Scholar]
- 4.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:1764–1773. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinbo Y, Taira T, Niki T, Iguchi-Ariga SM, Ariga H. DJ-1 restores p53 transcription activity inhibited by Topors/p53BP3. Int J Oncol. 2005;26:641–648. [PubMed] [Google Scholar]
- 6.Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J Biol Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 7.Honbou K, Suzuki NN, Horiuchi M, Niki T, Taira T, Ariga H, Inagaki F. The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease. J Biol Chem. 2003;278:31380–31384. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 8.Huai Q, Sun Y, Wang H, Chin LS, Li L, Robinson H, Ke H. Crystal structure of DJ-1/RS and implication on familial Parkinson's disease. FEBS Lett. 2003;549:171–175. doi: 10.1016/s0014-5793(03)00764-6. [DOI] [PubMed] [Google Scholar]
- 9.Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Tao X, Tong L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson's disease. J Biol Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- 11.Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA. The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci USA. 2003;100:9256–9261. doi: 10.1073/pnas.1133288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 13.De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing? Trends Neurosci. 1999;22:535–540. doi: 10.1016/s0166-2236(99)01463-0. [DOI] [PubMed] [Google Scholar]
- 14.Dirnagl U, Isadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 15.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 16.Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39:429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura Y, Nomura Y. Stress proteins and glial functions: Possible therapeutic targets for neurodegenerative disorders. Pharmacol Ther. 2003;97:35–53. doi: 10.1016/s0163-7258(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura Y, Yanagisawa D, Inden M, Takata K, Tsuchiya D, Kawasaki T, et al. Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J Pharmacol Sci. 2005;97:289–293. doi: 10.1254/jphs.sc0040129. [DOI] [PubMed] [Google Scholar]
- 19.Fox RB, Harada RN, Tate RM, Repine JE. Prevention of thiourea-induced pulmonary edema by hydroxyl-radical scavengers. J Appl Physiol. 1983;55:1456–1459. doi: 10.1152/jappl.1983.55.5.1456. [DOI] [PubMed] [Google Scholar]
- 20.Araújo MC, Antunes LM, Takahashi CS. Protective effect of thiourea, a hydroxyl-radical scavenger, on curcumin-induced chromosomal aberrations in an in vitro mammalian cell system. Teratog Carcinog Mutagen. 2001;2:175–180. doi: 10.1002/1520-6866(2001)21:2<175::aid-tcm6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:306–315. doi: 10.1097/00004647-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, Dirnagl U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Allard L, Burkhard PR, Lescuyer P, Burgess JA, Walter N, Hochstrasser DF, Sanchez JC. PARK7 and nucleoside diphosphate kinase A as plasma markers for the early diagnosis of stroke. Clin Chem. 2005;51:2043–2051. doi: 10.1373/clinchem.2005.053942. [DOI] [PubMed] [Google Scholar]
- 24.Koide-Yoshida S, Niki T, Ueda M, Himeno S, Taira T, Iguchi-Ariga SM, Ando Y, Ariga H. DJ-1 degrades transthyretin and an inactive form of DJ-1 is secreted in familial amyloidotic polyneuropathy. Int J Mol Med. 2007;19:885–893. [PubMed] [Google Scholar]
- 25.Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, Hanash SM. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328–3335. [PubMed] [Google Scholar]
- 26.Pardo M, García A, Thomas B, Piñeiro A, Akoulitchev A, Dwek RA, Zitzmann N. The characterization of the invasion phenotype of uveal melanoma tumour cells shows the presence of MUC18 and HMG-1 metastasis markers and leads to the identification of DJ-1 as a potential serum biomarker. Int J Cancer. 2006;119:1014–1022. doi: 10.1002/ijc.21942. [DOI] [PubMed] [Google Scholar]
- 27.Maita C, Tsuji S, Yabe I, Hamada S, Ogata A, Maita H, Iguchi-Ariga SM, Sasaki H, Ariga H. Secretion of DJ-1 into the serum of patients with Parkinson's disease. Neurosci Lett. 2008;431:86–89. doi: 10.1016/j.neulet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Sheng J, Tang Z, Wu X, Yu Y, Guo H, Shen Y, Zhou C, Paraoan L, Zhou J. Cystatin C prevents degeneration of rat nigral dopaminergic neurons: In vitro and in vivo studies. Neurobiol Dis. 2005;18:152–165. doi: 10.1016/j.nbd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci USA. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekito A, Koide-Yoshida S, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. DJ-1 interacts with HIPK1 and affects H2O2-induced cell death. Free Radic Res. 2006;40:155–165. doi: 10.1080/10715760500456847. [DOI] [PubMed] [Google Scholar]
- 32.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989;20:1037–1043. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura Y, Kosaka T, Kakimura JI, Matsuoka Y, Kohno Y, Nomura Y, Taniguchi T. Protective effects of the antiparkinsonian drugs talipexole and pramipexole against 1-methyl-4-phenylpyridinium-induced apoptotic death in human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1998;54:1046–1054. [PubMed] [Google Scholar]
- 37.Yanagida T, Takata K, Inden M, Kitamura Y, Taniguchi T, Yoshimoto K, et al. Distribution of DJ-1, Parkinson's disease-related protein PARK7, and its alteration in 6-hydroxydopamine-treated hemiparkinsonian rat brain. J Pharmacol Sci. 2006;102:243–247. doi: 10.1254/jphs.sc0060098. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi-Niki K, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson's disease patients. Biochem Biophys Res Commun. 2004;320:389–397. doi: 10.1016/j.bbrc.2004.05.187. [DOI] [PubMed] [Google Scholar]