Summary
We review the evolution of domestic animals, emphasizing the effect of the earliest steps of domestication on its course. Using the first domesticated species, the dog (Canis familiaris) as an illustration, we describe the evolutionary specificities of the historical domestication, such as the high level and wide range of diversity. We suggest that the process of earliest domestication via unconscious and later conscious selection of human-defined behavioral traits may accelerate phenotypic variations. The review is based on the results of the long-term experiment designed to reproduce early mammalian domestication in the silver fox (Vulpes vulpes) selected for tameability, or amenability to domestication. We describe changes in behavior, morphology and physiology that appeared in the fox during its selection for tameability and that were similar to those observed in the domestic dog. Based on the experimental fox data and survey of relevant data, we discuss the developmental, genetic and possible molecular-genetic mechanisms of these changes. We assign the causative role in evolutionary transformation of domestic animals to selection for behavior and to the neurospecific regulatory genes it affects.
Domestication in evolutionary terms – from Darwin to the present day
It is well-known that Darwin has focused much attention on domestication as the process during which striking variation arises. Although believing that the range of changes in any direction may be different, he admitted that the “tendency to general variability is unlimited”(1) (pg. 411). He repeatedly raised the question why domestic animals are so variable. In his analysis of the causes of variation under domestication, Darwin has thought them to be exclusively due to environmental influences. He has maintained that the state of the parent organism during fertilization or embryonic development has profound effects on offspring characters. Darwin did emphasized that the organismal constitution will largely determine the kind of changes induced by the environment and acknowledged the occurrence of peculiarities due to unknown laws acting on individual constitution.
Besides environmental influences on variation, Darwin has noted the effects of crosses and inbreeding known in his time from the experience of animal breeders. Relying on their results, Darwin has provided vivid examples of the results of breeding selection apparent in a comparatively short time. However, he has also assigned a great role to unconscious selection acting for thousands of years on animals. Because all our domestic animals have been first exposed to domestication in very remote periods, it is unknown when animals started to change and at what rates. Darwin has maintained that animals continued to be variable for long periods after their early domestication, suggesting that the early domesticates were even more variable than the now existing ones and proposed that the capacity to become more variable under domestication is common to all species.
Darwin has also raised the problem of similarity of the changes observed in different domestic animals. He has regarded certain features shared by many domestic species as the result of their domestication.
Darwin's observations and conclusions on variation under domestication hold true today. The evolutionary specificity of domestication, such as, the wide phenotypic diversity of domesticates, remain puzzling. Indeed, the variation range of certain traits within a domestic species occasionally exceeds that within whole families or even orders.(2-5) Morphological variation in dogs, our earliest domesticates, is most illustrative in this respect (Fig. 1).
Fig. 1.

Variability range in dogs for:
A: body size (Chinese crested, pekingese and bullmastiff);
B: body shape (greyhound and English bulldog);
C: coat types (hairless terrier and komondor).
According to conventional genetic theory, rare (10-5 – 10-6 per gene per generation) random mutations are the most common mechanisms of phenotypic changes.(6) The divergence of the dog from the wolf might have happened some 12,000 – 15,000 years ago (3,7,8), which is a short span of time on the evolutionary scale. Nevertheless, variability has accumulated at immense rates incommensurate with random mutations. Therefore the nature and sources of the variation under domestication are intriguing.
Another hallmark feature of variation under domestication is its similar pattern in different domestic mammalian species.(9,10) When subjected to domestication, animals, whose evolutionary pathways did not cross, started to evolve in the same direction. They all lost the species-specific wild-type behavioral response to human. The activity of their reproductive system became enhanced and relatively uncoupled from the environmental photoperiod and they all, unlike their wild ancestors, acquired the capacity to breed in any season and more often than once a year.(10,11) In contrast, the activity of the hypothalamic-pituitary-adrenal (HPA) axis, the key hormonal regulator of stress and adaptation, became attenuated in the very few domesticates studied in this respect.(12-14) The same morphological changes, first in terms of overall body size and its proportions and also coat colour, length and texture appeared in many domesticates.(2,3-5,7,8,11) Some of these attributes (white spotting, floppy ears, curly tails) have been aptly called the morphological markers of domestication (Fig. 2). It seems unlikely that these similar trends of morphological and physiological transformation of different domestic animals depend on homologous independent mutations of structural homologous genes. The Russian evolutionary biologist Belyaev has suggested more than 50 years ago that domestication might involve other mechanisms contributing to phenotypic variation, mainly regulatory changes in gene activity during development.(2,15,16)
Fig. 2.

Representatives of different families and orders show the most specific morphological markers of domestication, white spotting on the head (top row) and floppy ears (bottom row):
A: Horse (Equus caballus): Order Perissodactyla, Equidae family, breed, the Soviet heavy draught-horse;
B: Cow (Bos taurus): Order Artiodactyla, Bovidae family, Aberdin-Angus breed (top), banteng breed (bottom);
C: Pig (Sus scrofa domestica): Order Artiodactyla, Suidae family, hybrid of the Vietnamese breed (top), Landrace breed (bottom);
D: Sheep (Ovis): Order Artiodactyla, Bovidae family, Romanov breed (top), balbas breed (bottom);
E: Dog (Canis familiaris): Order Carnivora, The Canidae family, a Boston terrier (top), a pug (bottom);
F: Rabbit (Oryctolagus cunticulus): Order Lagomorpha, Leporidae family, a Holland white-black (top), a German ram (bottom).
During evolution the same phenotypic results can be achieved through different developmental pathways underlain by different genes. However, there may be developmental processes, underlain by key genes with many regulatory functions which under certain recurring selective conditions may most likely be targeted by selection. According to Belyaev, the crucial selective factor during early domestication was the new social environment, the first encounter of a wild species with humans. This extremely stressful setting rendered behavior – tolerance, docility toward human and the correlated stress resistance – the main target of selection. In his opinion, the genes that control behavioral variation play a key regulatory role during development. Belyaev therefore suggested that behavioral variation was the causative variation under domestication. According to his line of thought, relationship between behavioral variation and transformation of domestic animals would be more intelligible when domestication would be traced from the beginning, i.e., when this process would be modeled experimentally. This sophisticated model was commenced with the silver fox (Vulpes vulpes) at the Institute of Cytology and Genetics, Novosibirsk, Russia, about 50 years ago.(16-19) Belyaev was the initiator of this experiment. There were two reasons why silver foxes were given preference in experimental domestication. One was the close taxonomic relationship between the fox and the dog; the other was that cage breeding of the fox started at the beginning of the 20th century. Thus, by the onset of the experiment, the captive fox has been already subjected to rigorous selection for adaptation to new social environment. This considerably facilitated the long-life experiment and reduced its duration.
Development of the experimental model of domestication
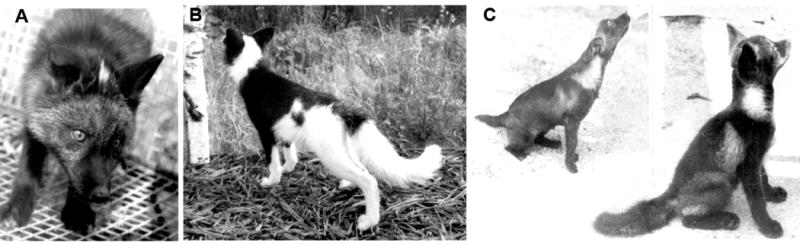
Although silver foxes had been farm-bred for about 50 years before our experiment, they retained the standard morphological phenotype, seasonal pattern of breeding specific to the foxes of natural populations and the wild-type aggressive (growing and biting) or avoidance response to human.(20) We have shown the polymorphism of the expression of these behavioral responses in foxes of large farm-bred populations (Fig. 3 ABC). Altogether several thousands foxes were tested at these fur farms. In the behavioral test, the experimenter approached the home cage, tried to open it and observed the expression of the response. This observational test was highly reproducible. According to its results about 10% of farm foxes displayed the responses of wild type very weakly or not at all (Fig. 3C). Such foxes (100 females and 30 males) were chosen from different farm bred populations as the initial parental generation for selection for tolerance of human or docility, then for tameability. The fox population was outbreeding.(20)
Fig. 3.

Behavior of foxes of the farm-bred populations (top row) and of the population selected for tameability (bottom row):
A: A fox showing the aggressive response to humans;
B: A fox showing the avoidance response to humans;
C: A fox weakly expressing the wild-type aggressive-avoidance response to humans;
D-F: Domesticated foxes referred to the behavioral elite. F: from left to right: Prof. K.G. Lark (University of Utah, Salt Lake City), Prof. L.N. Trut (Institute of Cytology and Genetics, Novosibirsk), Prof. G.M. Acland (Cornell University, Ithaca) with domestic foxes.
The rationale of the experiment was that subjection of an experimental fox population to selection for a single behavioral trait, tameability, is sufficient for domestication to occur, and the experimental design followed this rationale.(20,21) The researcher tested the tameability during the time-dosed contacts with pups on the basis of their responses to hand-feeding and to attempts to touch or pet them. All the pups underwent time-dosed contacts for 2.0-2.5 months. Those that continued to show the aggressive-avoidance responses despite the contacts were discarded from the experimental population. The pressure of selection was very rigorous: less than 10% of the most tame individuals of every generation were used as parents of the next.(17-21) As a result of such a rigorous selection, the offspring exhibiting the aggressive and fear avoidance responses were eliminated from the experimental population in just two-three generations of selection.
In the fourth selected generation, there appeared pups that responded to human by dog-like tail wagging. The offspring of the next selected generations displayed more and more dog-like behavior. In the sixth generation, there appeared pups that eagerly sought contacts with human, not only wagging, also whining, whimpering, and licking in a dog-like manner. Such foxes were assigned to the elite of domestication (Fig. 3 DEF). Elite pups constituted only 1.8% (4 of the total 213 foxes) in the sixth generation of selection; 17.9% (66 of 379) in the tenth and 35% (503 of 1438) in the twentieth generations. In the thirtieth generation, the proportion of elite pups was as high as 49%, (804 of 1641).(19) By 2005-2006 almost all the foxes of the domesticated population were assigned to the behavioral elite. Quantitative differences between the behavior of elite foxes are now estimated by using video records of behavioral tests. From these records fifty most informative behavioral parameters (for example, frequency of wagging, specific vocalizations, position of body and its communicative parts, such as tail, ears and others) were analyzed by the Principal Component Analysis (PCA).(22,23) This statistical method identifies the quantitative phenotypic variation in tameness as linear combinations of correlated parameters or Principal Components (PCs).(24) These new integrated independent traits are genetically analyzable phenotypes.(25,26)
Throughout the experiment, altogether 10,500 of foxes were used as parents. In all, about 50,000 offspring were obtained and tested for their amenability to domestication.(19) The result of this directional selection is impressive: a unique domestic fox with behavior very similar to another species, the domestic dog, has been developed through methodically applied selection.
Multifaceted results
The rigorous selection of the silver fox solely for tameability brought about correlated changes in certain features of behavior, physiology and morphology.
Thus, the rise of the dog-like ability of foxes to “read” human social cues, for example, gestures and glances, was a noteworthy change in their behavior. Domestic foxes became more skilful than farm-bred in using human cues for coping with the man-made environment not only during brief experimental tests and selection procedures, but also during daily routine care.(27)
The most important physiological consequence of fox domestication is the transformation of the seasonal reproductive pattern. The mating season of foxes in nature as well as at fur farms occurs only once a year during increasing of daylight (January–February). In some genealogical groups of foxes more advanced with regard to tameness, sexual activity and mating were observed outside the limits of the breeding season (Table 1) (20,28) which is normally strongly stabilized by natural selection.(11) Moreover, a few vixens have even matted twice in a year. The total number of such animals throughout the years of the experiment was small. However, their emergence among the tame foxes is of crucial importance. Reproduction seasonality is under strong selective constraint.(11) Variations in the mating dates of different animals within this period are mainly due to environmental factors. As consequences, direct selection for mating timing during the season is ineffective and doesn't change the limits of breading period. However, the data in Table 1 suggest that the capacity to breed at any time throughout the year, characteristic of domestic animals, might have occurred as a correlated consequence of selection for tameability.
Table 1.
The time course of mating in the tame foxes showing extraseasonal sexual activity. The circles next to the order number of males indicate their matings in the years when they mated outside the breeding season. White circles, sterile mating; black circles, fertile mating.
| Male | 10 Oct |
10 Nov |
15 Nov |
20 Nov |
25 Nov |
30 Nov |
10 Dec |
15 Dec |
25 Dec |
30 Dec |
10 Jan |
20 Jan |
30 Jan |
10 Feb |
20 Feb |
28 Feb |
10 Mar |
30 Mar |
5 Apr |
30 Apr |
5 May |
10 May |
15 May |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | o • | • | • | • • | • • | • | |||||||||||||||||
| 2 | • | • • | • • | o | |||||||||||||||||||
| 3 | o | • | • • | • | |||||||||||||||||||
| 4 | o | • | • | • | • | ||||||||||||||||||
| 5 | • | o | |||||||||||||||||||||
| 6 | • | • | • • | • | • | ||||||||||||||||||
| 7 | • | • | |||||||||||||||||||||
| 8 | o | • | • • | ||||||||||||||||||||
| 9 | o | o | o | • • | • | ||||||||||||||||||
| ooo | ooo | o | • | • • | • | ••• | • | ||||||||||||||||
| 10 | o | •• | • | • | |||||||||||||||||||
| 11 | o | o | ••• | • | o | ooo | o | ||||||||||||||||
| 12 | o | o | oo o | o | • | ••• | |||||||||||||||||
| 13 | o | o | o | ooo | • • | ••• | |||||||||||||||||
| 14 | oo | o | •• | ||||||||||||||||||||
| 15 | • | • | oo | oo | |||||||||||||||||||
| 16 | o | •• o | •• o | ••• | • • | ||||||||||||||||||
| o | o | o | •• | ••• | |||||||||||||||||||
| 17 | o | • | • • | • | |||||||||||||||||||
| o | •••• | • | o | o | |||||||||||||||||||
| 18 | • | ••• | o | • | |||||||||||||||||||
| 19 | oo | o | • | •• | • | o | |||||||||||||||||
| 20 | • | ••• | |||||||||||||||||||||
| 21 | • | •• | o | ||||||||||||||||||||
| 22 | • • | •• | • | o | |||||||||||||||||||
| 23 | o | o | • • | • | |||||||||||||||||||
| 24 | o | • | |||||||||||||||||||||
| 25 | o | • | ••• | • | • | ||||||||||||||||||
| 26 | o | o• | • • | • | • | • | |||||||||||||||||
| o | • | • | • | ||||||||||||||||||||
| 27 | o | •• | • | • • | • | • | |||||||||||||||||
| 28 | • | ••• | • | ||||||||||||||||||||
| 29 | • | • | • | ||||||||||||||||||||
| Mating season | |||||||||||||||||||||||
The consequences of selection for behavior are thought-provoking (Fig. 4). In the foxes of the domesticated population, several morphological traits that mirrored dog morphological features began to appear at different frequencies (Table 2). The changes in standard coat color pattern appeared earlier than the others, in the 8 – 10 selected generations. Specific piebald Star spotting (Figs. 4F and 5A,B) and brown mottling on the background of standard silver-black color (Fig. 5C) are the most typical. The Star spotting is determined by the incompletely dominant autosomal Star mutation.(29) The gene control of brown mottling in foxes is still unclear. In dogs, it is controlled by one of the mutations at the Agouti locus. According to cultural historical evidence, similar changes in coat colour occurred in early dogs.(8)
Fig. 4.
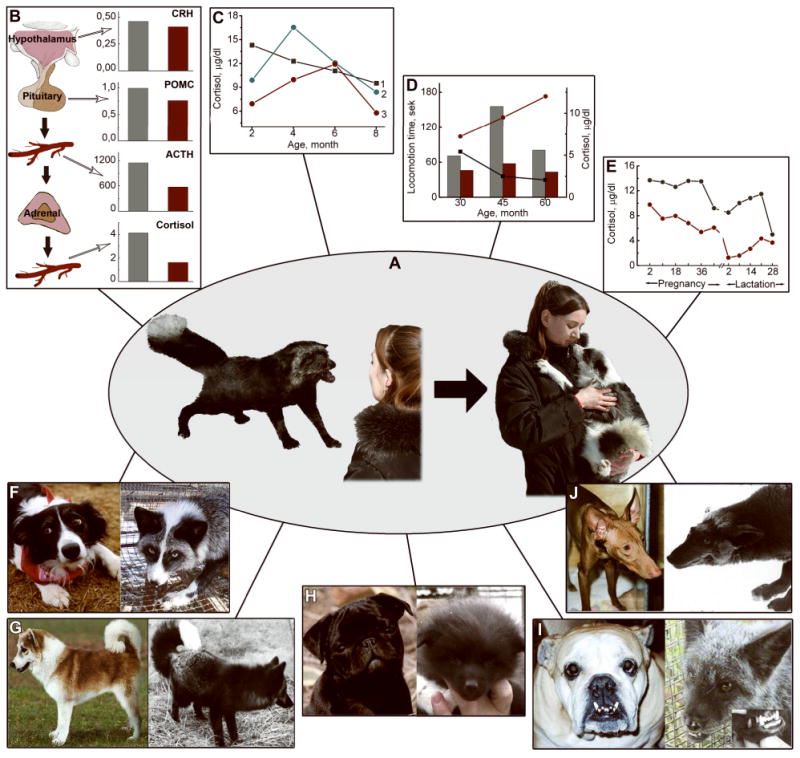
A schematic representation of the results of the fox domestication experiment.
- Left: a fox of the farm-bred population unselected for behavior. Foxes of this population show the typical aggressive response to human.
- Right: a fox of the experimental domesticated population. The dog-like behavior of foxes of this population is the result of many years of selection for tameability.
B – E: Activity of the HPA axis in farm-bred and domesticated foxes. Farm-bred foxes are shown in grey, the domesticated are in red.
B: Hypothalamic CRF (CRFmRNA/18SmRNA) and pituitary POMC (POMCmRNA/18SmRNA) gene expression, ACTH (pg/ml) and cortisol (μg/dl) level in farm-bred and domesticated foxes.
C: Age-related changes in plasma cortisol level in farm-bred and domesticated foxes: 1) farm-bred foxes with aggressive response to human, 2, 3) foxes of the domesticated population with low (2), and high (3) domestication scores.
D: Total time of locomotion, an indicator of exploratory behavior, and plasma cortisol level in farm-bred and domesticated foxes at the age of 1-2 months: locomotion is plotted on the graph; plasma cortisol level is represented as bars.
E: Plasma cortisol in silver foxes during pregnancy and lactation.
F – J: Dog-like morphological changes arisen in foxes of the domesticated population:
F: Similarity of coat depigmentation between dogs and foxes: Left, a border collie; Right, a tame fox.
G: Tail carriage, curly tail: Left, an Islandsk Farehund; Right, a tame fox.
H: Ears are floppy and face skull is widened in some pups of tame foxes: Left, a pug; Right, a tame fox pup.
I: Long jaw (elongation of the lower jaw) in the English bulldog occurs among tame foxes: Left, an English bulldog; Right, a tame fox.
J: Elongation of face skull in certain dog breeds and tame foxes: Left, a Pharaoh hound, Right, a tame fox.
Table 2.
Frequencies of phenotypic changes newly arisen in fox populations.
| Traits | Frequency in populations | |
|---|---|---|
| Domesticated | Farm-bred | |
| Star | 1.24 × 10-1 | 0.71 × 10-2 |
| Brown mottling | 0.45 × 10-2 | 0.9 × 10-3 |
| Floppy ears | 0.23 × 10-2 | 0.17 × 10-3 |
| Shortened tails | 0.14 × 10-2 | 0.2 × 10-3 |
| Curly tails | 0.94 × 10-1 | 0.8 × 10-2 |
Fig. 5.
Specific markers of domestication.
A: Two different phenotypic changes in a Ss heterozygote : the Star depigmentation and a floppy right ear.
B: Homozygote for the Star mutation (SS).
C: Phenotypic similarity between brown mottling in fox and dog.
In the domesticated foxes, morphological aberrations such as floppy ears and curly tails occurred in addition to changes in standard coat colour. These morphological traits are also characteristic of many domesticates, mainly dogs (Fig. 4 F-J). At the more advanced steps of selection, changes in the parameters of the skeletal system began to arise. They included shortened legs, tail, snout, upper jaw and widened skull (Fig. 4).
Some of the phenotypic changes appeared not only in the domesticated foxes, but also in those of the farm-bred populations, not subjected to selection for tameability (Table 2). The above observations suggest a relation between selection for tameability and the appearance of a subset of phenotypic changes marking domestic animals. The appearance of some phenotypic changes in the foxes of the nonselected populations is not at variance with this suggestion. These populations have been bred in captivity for about a hundred years, during which period they have been inevitably subjected to selection for adaptation to captivity or amenability to domestication. Consideration of this subset in terms of traditional quantitative genetics of correlated variability is difficult.(30) In fact, strong selection pressure on any quantitative trait, especially of adaptive significance, breaks down the genetic system kept in balance by preceding evolution, and as a consequence inevitably leads to the appearance of deviants from the phenotypic means, whose reproductive capacity is reduced. Certainly, the correlated responses of any population to any selection, depend on its genetic pool structure, and therefore they are, in a sense, unique and irreproducible. However, domestication of different populations of one species, different species, or even orders as well as selection of foxes for tameability is consistently associated with the same morphological and physiological changes. This remarkable parallelism can be hardly regarded as usual correlated responses to selection for any quantitative character. In addition, the reproductive performance of all animals under domestication improved, in contrast to what happened in the case of the correlated responses in terms of traditional quantitative genetics.(30)
The inbreeding coefficients of the population were estimated on the basis of its reproductive part(19,20) and of a set of randomly chosen polymorphic microsatellite markers(31) at different stages of selection; the coefficient values were not smaller than 0.02 and never greater then 0.07 in both the domesticated and farm populations. From these estimates it follows that the probability of appearance of changes due to stochastic fixation of the alleles that pre-existed in the initial population and that control each and every observed phenotypic change was very low.
Finally, it is difficult to interpret the changes in the domesticated foxes as a result of randomly arisen new mutations. Thus, in the same litter of phenotypically standard parents, even in the same offspring of such parents, referred, as a rule, to the tame elite, there appeared several different changes in the standard phenotype (Fig. 5A). This is incompatible with the mutational nature of their appearance. The results of the genetic analysis of morphological changes are also incompatible with the view that each phenotypic alteration is due to a single independent gene; the offspring of parents with one or another morphological alteration, contrary to expectation, showed quite different morphological changes. Only the Star depigmentation phenotype showed an independent genetic basis. The results of genetic analysis of the other phenotypic changes demonstrated that a common genetic basis may underlie the set of different morphological aberrations. All this strongly suggests that the phenotypic variation in the domesticated fox population may result from changes caused by selection for tameability in the regulation of development by the key genes.(18-20)
Association between phenotypic changes and developmental rate
The association between the newly generated traits and changes in developmental rates in the domestic foxes is noteworthy. Certain behavioral and morphological changes are correlates of delayed development of normal traits. The emotional expression of positive responses to human, widened skulls, shortened snouts, floppy ears, curly tails, are all juvenile traits certain domesticated individuals retain to adulthood.(3,32,33) Delay in developmental rates was observed as early as during embryonic morphogenesis. As already noted, the specifically located Star depigmented spots are the specific markers of domestication (Figs. 2 and 5). It was shown that retardation of the development (proliferation and migration from the neural crest) of the embryonic precursors of melanocytes, or primary melanoblasts, is the mechanism underlying depigmentation.(34) This retardation leads to the absence of melanocytes from specific areas of the coat and, hence, to their depigmentation.
The shifts in the timing of development brought about by selection of foxes for tameability have a neotenic-like tendency: the development of individual somatic traits is decelerated, while sexual maturation is accelarated. Higher levels of sexual hormones in plasma and heavier gonads during the prepubertal period are indicators of earlier sexual maturation of tame foxes. Judging by these parameters, tame foxes reach sexual maturity, on the average, one month earlier than their farm-bred counterparts.(35) Many researchers regard the neotenic tendency of development as a mechanism of evolutionary transformation of domestic animals. However, the causes of the emergence of this tendency during domestication remain unclear.(8,32,33,36) Our fox experiment demonstrated that neotenic shifts in developmental rate may arise as a correlated consequence of selection for tameability, i.e., social adaptation to humans. The point is that there is a sensitive socialization period in mammals when they explore their environment and get used to particular social factors.(37,38) This period starts early with the functioning of the sense organs and the formation of locomotion and enables the animal to learn about its surroundings and to form attachments. The maturation of the neurophysiological substrate of the fear response makes socialization difficult. Increasingly fearful pups start to avoid the impact of social factors, thus limiting their further socialization. It was found that selection of foxes for tameability slowed down the development of the fear response and shifted it to later dates. In foxes that were not selected for tameability, the exploratory activity (estimated on the basis of locomotion in unknown setting) became sharply reduced by the age of 45 days or so when fear was first manifested.(39) This reduction was lacking in the tame foxes to the age of four months.(19) Quite plausibly, retardation of the development of the neurophysiological substrate of fear is just one of the numerous manifestations of the regulatory effects of genes that affect developmental rates and are targeted by selection for domestication. It cannot be excluded that these genes perform a broader function by regulating the developmental rates of other traits.
Neuroendocrine changes under domestication
What putative genes for developmental rate may possibly be involved in selection for domestication? We assumed that some of these genes may perhaps control the glucocorticoid (GC) status in animals. This assumption is based on the close association between social behavior and the formation of the GC status: the reduction in exploratory activity, which, as noted above, sets an end to the sensitive socialization period, was found to be correlated with a rise in plasma cortisol level during early postnatal development.(40) In the tame pups, whose socialization period was longer, this rise occurred later (Fig. 4D). Not only the rate of social development, but also of the embryonic precursors of melanocytes were found to be associated with the GC status.(41,42) These observations suggested that GCs may function as coordinators of the parameters of development timing. There are data indicating that during embryogenesis GCs may have an inhibitory effect on cell proliferation and thereby promote cell transition to differentiation, the next step of early development. (43 - 46)
Only few studies were concerned with the glucocorticoid function under domestication. It has been reported that domestication of grey rats is associated with a decrease in the weight of adrenals, changes in their morphology and glucocorticoid synthesis under basal conditions and stress, or under the effect of exogenous adrenocorticotropic hormone (ACTH).(12,13) Comparison of domestic guinea pigs with their wild ancestors revealed a decrease in the stress response of the HPA axis in domesticates.(14) It is of importance that the function of the HPA axis decreased in foxes with advancing selection for tameness. We started the comparative analysis of the HPA axis from generation 10 of the selection for tame behavior. In this generation, the levels of plasma glucocorticoids in the tame foxes were significantly lower in all seasons of the year than in the farm-bred.(47) The levels of the hormones decreased with advancing selection. The basal cortisol levels in the blood of the domesticated foxes in generation 20 were almost twofold lower than of the non-domesticated, and it was about 30% lower under stress. In generation 45, basal and stress-induced blood cortisol levels in foxes of the tame population were already three- and fivefold lower than in the farm-bred foxes.(48) These data suggest that genes controlling plasma glucocorticoids were possibly the targets during selection for tameability.
In the foxes selected for domestication, the activity of the HPA axis is reduced at all levels (Fig. 4B-E). This includes the total GCs pool, the in vitro GC production by the adrenals, the basal ACTH level in plasma, and the adrenal response to stress.(49,50) The expression of the proopiomelanocortin (POMC) gene, whose protein product includes ACTH and β-endorphin in the pituitary, was also reduced, and the expression of the corticotropin-releasing hormone (CRH), the releasing factor for ACTH, tended to decrease in the hypothalamus.(51) It is also of importance that the total GCs pool in blood was considerably decreased during pregnancy and lactation in the tame foxes.(52,53) As a consequence, the entire embryonic and early postnatal development of the tame offspring proceeded on the background of lower maternal GCs. With the multifarious effects of GCs on development,(54) the impact of their changes on developmental processes is evident. They are directly associated with the expression of the GC receptor (GCR) gene, whose promoter region has a complex structure. The multiple and tissue-specific promoters provide fine regulation of changes in GCR gene expression in different early environments.(55,56) Most importantly, the GCR gene expression in the hippocampus, a brain structure modulating the regulation of behavior and activity of the HPA axis and in the frontal cortex is higher in the domestic animals.(57) Thus, the effects of selection for tame behavior on the HPA axis are manifest at all levels, from phenotypic parameters to the expression of the CRH, POMC, and the GCR genes.
Domestication also affects the developmental neurotransmitter systems. The role of neurotransmitters in development regulation has been discussed(58) and recently revaluated.(59) The brain serotonin system deserves special attention with reference to domestication. Its contribution to the inhibition of aggressiveness in animals, including foxes, has been recently discussed in Bioessays.(60) The studies of the brain serotonin system showed that the domesticated foxes differed from farm-bred in higher levels of serotonin and its main metabolite 5-hydroxyindol acetic acid in a number of brain structures. Differences in the activities of monoamine oxidase, the principal enzyme in serotonin degradation, and of tryptophan hydroxylase, the key enzyme in serotonin synthesis, were also demonstrated. It was shown that the activity of tryptophan hydroxylase was higher in the tame than in the non-tame foxes. The higher activities of this key enzyme and the higher level of serotonin in the brains of domesticated foxes agree well with the data concerning the inhibitory influence of serotonin on a number of aggressive types of behavior.(49, 60) Thus, it is important to emphasize the role of serotonin in development. Previous(58) and recent(61) publications have shown that serotonin acts as multifarious signal molecules important during development and capable of eliciting a cascade of gene activations.
Destabilizing selection as a possible accelerator of evolutionary transformation of domestic animals
Surveying the observations made in the course of experimental fox domestication, Belyaev found no satisfactory explanation within the traditional genetic framework and came to the idea of destabilizing selection as a factor of domestication.(16) By destabilizing selection Belyaev meant selection causing destabilization of regulatory systems controlling development and, hence, destabilization of the morphological and physiological organization stabilized by previous natural selection.(62) Under domestication, selection for particular behavioral features accomplishes the destabilization function. Belyaev regarded the selection for just these features as a major accelerator of the evolutionary transformation of domestic animals. It appears likely that destabilizing selection can affect animals at any organizational level, whose populations experience selection pressure on behavioral traits. Illustrative examples of multifaceted consequences of selection for behavior(63,64) were reported even in Drosophila.(65,66,67) The evolutionary role of selection for behavior would increase greatly in the case of highly organized mammals, because their phenotypic manifestation of behavioral traits involves more numerous regulatory neurotransmitter and neurohormonal relationships.(62)
The evolutionary function of destabilizing selection is the reverse to that of stabilizing selection.(68) Stabilizing selection acts always in environment with which a species has coped well, this leads to stabilization of development to form a phenotype that is optimal under this condition. Stabilizing selection acts by suppressing the effects of mutations that disrupt development and alter the normal phenotype or even by eliminating them. It maintains variability within the optimum range of the evolutionary established norm, but it does not produce variability beyond the phenotype of the species. Analysis of the effects of selection for behavior on development and variability in our fox model demonstrated that it sharply destabilized the correlated developmental systems and led to an increase in phenotypic variation. What mechanisms can be involved in this process? And what is the nature of the changes in the specific genes that are induced by destabilizing selection?
It appears plausible that the phenotypic novelties in the experimental fox population could be due to changes in gene activity, largely in its epigenetic modification. Hormonal changes under domestication may result in either gene silencing or gene activation. In this context, of importance is the mentioned above Star phenotype (Fig. 5) appearing repeatedly at high frequencies (10-1 - 10-2) in the population of tame foxes and determined by the incompletely dominant S gene.(29) Segregation analysis of offspring from crosses between Ss heterozygotes for this gene demonstrated a shortage of SS homozygotes. This shortage cannot be accounted for by selective embryonic, zygotic and gametic mortality. Some foxes, proven SS homozygotes, have the heterozygous phenotype (Ss) and inheritance pattern characteristic of heterozygotes. It was assumed that one of the two homologous mutant Star alleles became functionally inactive. Also, cases of birth of homozygotes in crosses between a heterozygous and a standard parent have been documented (unpublished data). This suggests the reverse process of activation of a wild-type “silent” allele in rare heterozygotes for the Star gene. A similar phenomenon of heritable gene inactivation-reactivation has been described for the kinked tail character in the mouse controlled by the dominant Axin-fused gene, the phenotypic expression of the gene is extremely variable, to the extent that some of its carriers have a normal phenotype.(69) Subsequent studies showed that the absence of the kinked tail phenotype correlates with the level of DNA methylation in a retrotransposon within AxinFu.(70) Moreover, the epigenetic modification of the mutant kinked allele of both maternal and paternal derivation passes meiosis, i.e. it does not undergo reprogramming during gametogenesis.(70)
In the mouse, another example of inheritance of epigenetic modification in the Agouti locus, which controls coat colour of wild type, has been described. The Avy allele bears a transposon insertion. The number of viable yellow phenotypes was related to the methylation pattern of this insertion.(71-74) The Agouti locus is implicated to be involved in brown mottling in the dog. It prompts study of Agouti at the molecular level in the domesticated fox to reveal whether the locus possess structural components that can act as effective carriers of methylation marks.
Methylation of the promoter regions of DNA is implicated as a mechanism of the epigenetic alteration in gene expression; methylation is related to the transcriptional silencing of regulated genes, while hypomethylation is related to their transcriptional activity. As to the Star mutation in foxes, it is unknown whether its activity changes at the transcriptional level or at subsequent steps of gene product conversion. It may be speculated only that the selection-related genetic changes in regulatory neurohormonal system in the domesticated foxes could induce changes in expression of many other downstream genes, thereby causing phenotypic destabilization. However, only limited changes in gene expression in fox brain have been shown so far.(51, 75) As indicated above, these changes involve also genes of the HPA axis.(48,51) This suggests that behavioral and morphological and physiological changes in the domestic foxes may depend on changes in the expression of a very small number of brain genes with many regulatory down stream effects.
It is of interest that the expression profiles of the brain-specific genes showed remarkable differences between domestic dogs and their closest wild relatives wolves (Canis lupus) and coyotes (Canis latrans).(76) The most prominent gene expression changes in dogs occurred in the hypothalamus, a biologically important, evolutionary conserved brain structure, as well as a modulator of behavioral and neuroendocrine responses to environmental agents. Comparisons of the differences in gene expression between wolves and coyotes, which have been separated for 2 Myr BP,(77) have demonstrated that the differences are minimal and that the expression profiles of the hypothalamic genes in the wild canids are conserved. This is in striking contrast with the variable expression of these genes in dogs and with the differences between them and wild canids. It could not be ruled out that selection for tame behavior during 12,000 – 15,000 years of dog domestication accelerated divergence of gene expression in the brain, as postulated by the authors.(76)
Molecular-genetic implications for phenotypic changes under domestication
As yet, little is known about the correlation between the expression levels of specific genes and phenotypic variability in particular traits. However, some data indirectly indicate that this relation exists. For example, it is known that coding regions abound with presumably noncoding simple sequence repeats (SSRs). It is curious that half of these repeats cluster in regions containing one or several genes with key role in development.(78) This suggests that they are important for regulation of the expression of these key developmental genes. Their altered regulation may be implicated as the major source of changes in many genes under their control. Thus, it has been shown that variation in the number of tandem repeats in two such genes, Runx-2 and Alx-4, may be related to the variation in skeletal traits in dogs.(79) Variation rates in the number of tandem repeats in genes for neurotransmitters and their receptors may be involved in determining variations in behavior.(80) The variation in repeat number can exceed by far that of point mutations. This suggested that variation in the length of tandem repeats may be a predominant source of rapid morphological evolution and that the repeats associated with the developmental genes increase the pace of evolution.(79)
In addition to SSRs, phenotypic variation may be influenced by canid-specific small interspersed nuclear elements (SINEs).(81) Both seem to be often present at positions influencing gene expression.(82,83) Bsp repeats similar to canine SINEs have been detected in the fox genome and they may potentially perform regulatory functions in it.(84,85) The presence of SSR, SINE or Bsp elements in the genes of the hormonal and neurotransmitter systems may have important consequences. Changes in their expression may be implicated in changes in the expression of many genes under their control.
The molecular-genetic mechanisms of behavioral and morphological traits of the now existing dog breeds may not, however, reflect the genetic nature of the early changes brought about by domestication. The domestic fox is therefore a unique model of the genetic events of early domestication. At the IC&G fur farm, foxes are methodically selected not only for domestication, i.e. elimination of the aggressive genes, but also in the opposite direction – for retention and enhancement of the expression of aggressive behavior. As a result, a population of aggressive foxes has also been set up. This population, together with the domesticated serves is valuable resources for genetic study of early domestication.
It should be noted again that an intriguing consequence of domestication, historical of the dog and experimental of the silver fox, were the newly arisen morphological variations. This is amazing with respect to the traits of the skeletal system that define body size and conformation. We are now led to raise the important evolutionary question of whether behavioral and morphological traits are integrated at the genomic level, i.e., whether there exist loci which co-regulate morphological-behavioral traits. We started research in this direction in collaboration with the University of Utah (Prof. Lark) and the Cornell University (Prof. Ackland and Dr. Kukekova). The PCA approach was used for genetic dissection of not only complex behavioral traits as above but also of skeletal traits.(86,87) This approach revealed sets of correlated parameters (PCs) structuring variability. Differences between tame, aggressive and control populations were expressed in terms of the PCs. The PCs behaved as individual phenotypes in the segregating generations from the Tame × Aggressive cross and the subsequent backcrosses, as we reported.(22,23,86) Thus, these phenotypes were appropriate for molecular-genetic analysis. Search of the loci for behavior and morphology, along with their common loci, is of current interest, because co-segregation of some of their PCs suggests that they may share certain loci. Construction of the meiotic map of the genome of the fox was followed by genetic mapping of the loci for the PCs(88) which will be used to further study the co-regulation of morphology and behavior.
Conclusions
We proceeded on the assumption that regulatory changes in gene activity may generate the remarkable level of diversity and its similar patterns among domestic animals. These regulatory changes were presumably caused by selection animals for specific behavior, tameability as a marker of tolerance and successful adaptation to the human social environment. The experimental model of domestication, as a kind of forced evolution, was developed by systematically applying selection for tameability on silver foxes.
The founders of selected population were chosen from large farm-bred populations. They represented the genetic variants that had reproductive success and showed the weakest wild aggressive-avoidance response to human. Such foxes undoubtedly had been under natural and artificial selection for tameability through all the years of cage breeding. However, this selection did not affect the standard morphological and physiological phenotype of the fox in all populations we had studied. We succeeded in rapidly setting up a unique population of domestic foxes following the effect of rigorous selection pressure for tame behavior. The behavioral patterns of these foxes are dog-like. They also have a range of morphological and physiological traits attributable to domestic animals. The fox domestication experiment demonstrated that this selection affected the genes controlling the neurohormonal status. Some of the genes responsible for the association between tameability and hormonal and transmitter levels might have been brought together and become fixed by 8-10 selected generations. Their fixation might have modified the activity of many other downstream genes, thereby destabilizing and shifting development timing, and uncovering some of the phenotypically hidden potentialities of the genome. This means that the interactions between genetic variants altered during selection might have produced new patterns of gene expression and new phenotypes.
Different molecular mechanisms are implied in the phenotypic expression of non-expressing genes and their epigenetic modification. They include non-coding sequences and methylation-demethylation of the regulatory regions of genes. Whatever the molecular mechanisms of changes in gene activity under domestication, the results suggest that these changes could be produced by selection for tameability.
In the light of the results of the fox domestication experiment, the similar patterns of behavioral and morphological and physiological transformation in foxes, dogs and other domesticates are suggested to be the result of selection for tameability. This selection is thought to be the causative and universal mechanism for evolutionary transformation of domestic animals.
Acknowledgments
Authors thank Anna Fadeeva for translation of the manuscript from Russian into English and Yekaterina Omelchenko for technical assistance.
Funding agency: Funding agencies: NIH grants # 1R03 TW 008098-01, # R01 MH 077811-01, and the Programs of Basic Research of the RAS Presidium “Biodiversity and gene pool dynamics” and “Molecular and Cell Biology”.
References
- 1.Darwin CR. The variation of animals and plants under domestication. 2-d. II. London: John Murray; 1875. p. 495. [Google Scholar]
- 2.Belyaev DK. Domestication of animals. Sci J. 1969;5:47–52. [Google Scholar]
- 3.Herre WK. Todays status of domestication research. Naturwissenschaftlich Rundschau. 1959;12:87–94. In Germany. [Google Scholar]
- 4.Bogolyubski SH. Manual for State Universities. Moscow: Soviet Science; 1959. Origin and transformation of domestic animals. In Russian. [Google Scholar]
- 5.Postel-Vinay O. The dog, a biological enigma. La Recherche. 2004;375:30–37. In French. [Google Scholar]
- 6.Ayala FJ, Kiger JA. Modern genetics. Vol. 3 University of California. Davis; 1988. [Google Scholar]
- 7.Morey DF. The early evolution of the domestic dog. Am Sci. 1994;82:336–347. [Google Scholar]
- 8.Clutton-Brock J. Origins of the dog: domestication and early history In Serpell J ed; Domestic Dog: its Evolution, Behavior and Interactions With People. Cambridge: Cambridge University Press; 1997. pp. 2–19. [Google Scholar]
- 9.Belyaev DK, Trut LN. The convergent nature of incipient forms and the concept of destabilizing selection. In: Ovchinnikov Yu A, Rapoport IA., editors. Vavilov's heritage in modern biology. Moscow: Nauka; 1989. pp. 155–169. [Google Scholar]
- 10.Trut LN. The variable rates of evolutionary transformation and their parallelism in terms of destabilizing selection. J Anim Breed Genet. 1988;105:81–90. [Google Scholar]
- 11.Sadleir RMFS. The ecology of reproduction in wild and domestic mammals. London: Methuen; 1969. [Google Scholar]
- 12.Richter CP. Domestication of the Norway rat and its implication for the study of genetics in man. Am J Hum Genet. 1952;4:273–285. [PMC free article] [PubMed] [Google Scholar]
- 13.Woods JW. The effects of acute stress and of ACTH upon ascorbic acid and lipid content of the adrenal glands of wild rats. J Physiol. 1957;135:390–399. doi: 10.1113/jphysiol.1957.sp005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Künzl C, Sachser N. The behavioral endocrinology of domestication: A comparison between the domestic Guinea pig (Cavia aperea f. porcellus) and its wild ancestor, the cavy (Cavia aperea) Horm Behav. 1999;35:28–37. doi: 10.1006/hbeh.1998.1493. [DOI] [PubMed] [Google Scholar]
- 15.Belyaev DK. On some problems of correlative variability and their significance for the theory of evolution and selection of animals. Proc Siberian Division of SSSR Acad Sci. 1962;10:111–124. In Russian. [Google Scholar]
- 16.Belyaev DK. Destabilizing selection as a factor in domestication. J Hered. 1979;70:301–308. doi: 10.1093/oxfordjournals.jhered.a109263. [DOI] [PubMed] [Google Scholar]
- 17.Trut LN. Early canid domestication: farm-fox experiment. Am Sci. 1999;87:160–169. [Google Scholar]
- 18.Trut LN. Experimental Studies of Early Canid Domestication. In: Ruvinsky A, Sampson J, editors. The Genetics of the Dog. Wallingford, UK: CABI Publishing; 2001. pp. 15–42. [Google Scholar]
- 19.Trut LN, Plyusnina IZ, Oskina IN. An experiment on fox domestication and debatable issues of evolution of the dog. Rus J Genetics. 2004;40:644–655. [PubMed] [Google Scholar]
- 20.Trut LN. Doctoral (Biol) Dissertation. Novosibirsk: Inst Cytol Genet; 1980. The role of behavior in domestication-associated changes in animals as revealed with the example of silver fox. In Russian. [Google Scholar]
- 21.Trut LN. The genetics and phenogenetics of domestic behavior. In: Belyaev DK, editor. Problems in general genetics. Proceedings XIY Int Cong Genet. book 2. Vol. 2. Moscow: Mir; 1980. pp. 123–137. [Google Scholar]
- 22.Kukekova AV, Oskina IN, Kharlamova AV, Chase K, Erb HN, et al. The genetics of domesticated behavior in canids: What dogs and silver foxes can tell us about each other? In: Ostrander EA, Giger U, Lindblad-Toh K, editors. The Dog and Its Genome. New York: Cold Spring Harbor Laboratory Press; 2006. pp. 515–537. [Google Scholar]
- 23.Kukekova AV, Trut LN, Chase K, Shepeleva DV, Vladimirova AV, et al. Measurement of segregating behaviors in experimental silver fox pedigrees. Behav Genet. 2008;38:185–194. doi: 10.1007/s10519-007-9180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson JE. A User's Guide to Principal Components. New York: Wiley; 1991. [Google Scholar]
- 25.Chase K, Adler FR, Miller-Stebbings K, Lark KG. Teaching a new dog old tricks: identifying quantitative trait loci using lessons from plants. J Hered. 1999;90:43–51. doi: 10.1093/jhered/90.1.43. [DOI] [PubMed] [Google Scholar]
- 26.Chase K, Carrier DR, Adler FR, Jarvik T, Ostrander EA, et al. Genetic basis for systems of skeletal quantitative traits: Principal component analysis of the canid skeleton. Proc Natl Acad Sci USA. 2002;99:9930–9935. doi: 10.1073/pnas.152333099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare B, Plyusnina I, Ignacio N, Schepina O, Stepika A, et al. Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Curr Biol. 2005;15:226–230. doi: 10.1016/j.cub.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Belyaev DK, Trut LN. Reorganization of the seasonal rhythm of reproduction in the silver black foxes during their selection for the capacity to domestication. Zh Obshch Biol. 1983;42:739–752. In Russian. [Google Scholar]
- 29.Belyaev DK, Ruvinsky AO, Trut LN. Inherited activation-inactivation of the star gene in foxes: its bearing on the problem of domestication. J Hered. 1981;72:267–274. doi: 10.1093/oxfordjournals.jhered.a109494. [DOI] [PubMed] [Google Scholar]
- 30.Falconer DS. Introduction to quantitative genetics. London: Londgman; 1981. p. 365. [Google Scholar]
- 31.Kukekova AV, Trut LN, Oskina IN, Kharlamova AV, Shikhevich SG, et al. A marker set for construction of a genetic map of the silver fox (Vulpes vulpes) J Hered. 2004;95:185–194. doi: 10.1093/jhered/esh033. [DOI] [PubMed] [Google Scholar]
- 32.Wayne RK. Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution. 1986;40:243–259. doi: 10.1111/j.1558-5646.1986.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Coppinger RP, Glendinning J, Torop E. Degree of behavioral neoteny differentiates canid polymorphs. Ethology. 1987;75:89–108. [Google Scholar]
- 34.Prasolova LA, Trut LN. Effect of the “Star” gene on the rate of melanoblast migration in silver fox (Vulpes vulpes) embryos. Dokl Akad Nauk. 1993;329:787–789. In Russian. [PubMed] [Google Scholar]
- 35.Oskina IN. Ontogenesis of endocrine function in silver foxes under domestication. Proceedings of the 28th Intern Congr ISAE; Denmark. 1994. [Google Scholar]
- 36.Wayne RK, Ostrander E. Origin diversity, and genome structure of the domestic dog. BioEssays. 1999;21:247–257. doi: 10.1002/(SICI)1521-1878(199903)21:3<247::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 37.Scott JP. Critical period in behavioral development. Science. 1962;138:949–958. doi: 10.1126/science.138.3544.949. [DOI] [PubMed] [Google Scholar]
- 38.Serpelle J, Jagoe J. Early experience and the development of behavior. In: Serpell J, editor. The Domestic Dog: its Evolution, Behavior and Interactions With People. Cambridge: Cambridge University Press; 1997. pp. 79–102. [Google Scholar]
- 39.Belyaev DK, Plyusnina IZ, Trut LN. Domestication in the silver fox (Vulpes fulvus Desm): Changes in physiological boundaries of the sensitive period of primary socialization. Appl Anim Behav Sci. 1985;13:359–370. [Google Scholar]
- 40.Plyusnina IZ, Oskina IN, Trut LN. An analysis of fear and aggression during early development of behavior in silver foxes (Vulpes vulpes) Appl Anim Behav Sci. 1991;32:253–268. [Google Scholar]
- 41.Trut LN, Oskina IN, Prasolova LA, Pliusnina IZ. Activity of pituitary-adrenal system and development of melanoblasts in embryos of gray rats (Rattus norvegicus) Dokl Akad Nauk. 1998;360:428–432. In Russian. [PubMed] [Google Scholar]
- 42.Oskina IN, Trut LN, Prasolova LA, Plyusnina IZ. Pituitary-adrenal axis and problems of phenotypic diversity under animal domestication. Biodiversity and dynamics of ecosystems in North Eurasia. 2000;1:82–85. [Google Scholar]
- 43.Yu IT, Lee SH, Lee YS, Son H. Differential effects of corticosterone and dexametasone on hippocampal neurogenesis in vitro. Biochem Biophys Res Commun. 2004;317:484–490. doi: 10.1016/j.bbrc.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 44.Hakeda Y. Action of glucocorticoid on bone-forming and bone-resorbing cells. Clin Calcium. 2006;16:1817–1822. [PubMed] [Google Scholar]
- 45.Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci. 2006;26:5402–5410. doi: 10.1523/JNEUROSCI.4906-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inukai Y, Ikeda R, Aiyama S. Effect of glucocorticoid on the differentiation and development of terminal tubules in the fetal rat submandibular gland. Cells Tissues Organs. 2008;187:233–242. doi: 10.1159/000110806. [DOI] [PubMed] [Google Scholar]
- 47.Trut LN, Naumenko EV, Belayev DK. Change in pituitary-adrenal function in silver foxes under selection for domestication. Genetika. 1972;5:35–43. In Russian. [PubMed] [Google Scholar]
- 48.Oskina IN, Herbeck YuE, Shikhevich SG, Plyusnina IZ, Gulevich RG. Alterations in the hypothalamus-pituitary-adrenal and immune systems during selection of animals for tame behavior. VOGiS Herald. 2008;12:39–49. In Russian. [Google Scholar]
- 49.Naumenko EV, Belyaev DK. Neuroendocrine mechanisms in animal domestication. In: Belyaev DK, editor. Problems in general genetics. Proceedings XIY Int Cong Genet. book 2. Vol. 2. Moscow: Mir; 1980. pp. 12–24. [Google Scholar]
- 50.Oskina IN. Analysis of the functional state of the pituitary-adrenal axis during posnatal development of domesticated silver foxes (Vulpes vulpes) Scientifur. 1996;20:159–167. [Google Scholar]
- 51.Gulevich RG, Oskina IN, Shikhevich SG, Fedorova EV, Trut LN. Effect of selection for behavior on pituitary-adrenal axis and proopiomelanocortin gene expression in silver foxes (Vulpes vulpes) Physiol Behav. 2004;82:513–518. doi: 10.1016/j.physbeh.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 52.Oskina IN, Tinnikov AA. Interaction between cortisol and cortisol-binding protein in silver foxes (Vulpes fulvus) Comp Biochem Phisiol. 1992;101A:665–668. doi: 10.1016/0300-9629(92)90341-m. [DOI] [PubMed] [Google Scholar]
- 53.Trut LN, Plyusnina IZ, Oskina IN, Prasolova LA. Phenotypic diversity of domestic animals and temporal developmental parameters. Biodiversity and dynamics of ecosystems in North Eurasia. 2000;1:119–123. [Google Scholar]
- 54.Demir N, Demir R. Effect of maternal bilateral adrenalectomy on fetal rat cerebral cortex. Int J Neurosci. 2001;111:21–38. doi: 10.3109/00207450108986550. [DOI] [PubMed] [Google Scholar]
- 55.McCormick JA, Lyons V, Jacobson MD, Noble J, Diorio J, et al. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Mol Endocrinol. 2000;14:506–517. doi: 10.1210/mend.14.4.0438. [DOI] [PubMed] [Google Scholar]
- 56.Weaver I, Diorio J, Seckl JR, Szyf M, Meaney M. Early environmental regulation of hippocampal glucocorticoid receptor gene expression characterization of intracellular mediators and potential genomic target sites. Ann NY Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 57.Oskina IN, Herbeck YuE, Il'ina OV, Gulevich RG. Expression of the glucocorticoid receptor genes in grey rats under selection for behavior. Proceedings of the 20th Congress of the Pavlov's Physiological Society; Moscow: Russian physician Publishing House; 2007. p. 364. In Russian. [Google Scholar]
- 58.Buznikov GA. Neurotransmitters in ontogenesis. Moscow: Nauka; 1987. In Russian. [Google Scholar]
- 59.Levine M, Buznikov GA, Lauder JM. Of minds and embryos: Left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- 60.Popova NK. From genes to aggressive behavior: the role of serotonergic system. BioEssays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- 61.Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trut LN. Is selection an alternative or complementary to variation? Genetika. 1993;29:1941–1952. In Russian. [PubMed] [Google Scholar]
- 63.Kaidanov LZ. Animal population genetics. Sov Sci Rev F Physiol Biol. 1989;3:201–256. [Google Scholar]
- 64.Ricker JP, Hirsch J. Genetic changes occurring over 500 generations in lines of Drosophila melanogaster selected divergently for geotaxis. Behav Genet. 1988;18:13–25. doi: 10.1007/BF01067072. [DOI] [PubMed] [Google Scholar]
- 65.Spieth HT. Mating behavior and evolution of the Hawaiian Drosophila. In: White MJD, editor. Genetic Mechanisms of speciation in insect. Sydney: A.N.Z. Book. Co.; 1974. pp. 94–101. [Google Scholar]
- 66.Ringe IM. Why 300 species of Hawaiian Drosophila: The sexual selection hypothesis. Evolution. 1977;31:694–696. doi: 10.1111/j.1558-5646.1977.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 67.Val FC. Genetic analysis of the morphological differences between two interfertile species of Hawaiian Drosophila. Evolution. 1977;31:611–629. doi: 10.1111/j.1558-5646.1977.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 68.Schmalhausen I. Factory of evolution. The theory of stabilizing selection. With a preface to the English ed by Th Dobzhansky. Blakiston Company; Philadelphie et Toronto: 1949. [Google Scholar]
- 69.Belyaev DK, Ruvinsky AO, Borodin PM. Inheritance of alternative states of the fused gene in mice. J Hered. 1981;72:107–112. doi: 10.1093/oxfordjournals.jhered.a109436. [DOI] [PubMed] [Google Scholar]
- 70.Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, et al. Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. PNAS. 2003;100:2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michaud EJ, van Vugt MJ, Bultman SL, Sweet HO, Davisson MT, et al. Differential expression of the new dominant agouti allele (Aiapy) is correlated with methylation state and is influenced by parental lineage. Genes Dev. 1994;8:1463–1472. doi: 10.1101/gad.8.12.1463. [DOI] [PubMed] [Google Scholar]
- 72.Argeson AC, Nelson KK, Siracusa LD. Molecular basis of the pleiotropic phenotype of mice carrying the hypervariable yellow (Ahvy) mutation at the agouti locus. Genetics. 1996;142:557–567. doi: 10.1093/genetics/142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wollf GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 74.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 75.Lindberg, Bjornerfeldt S, Saetre P, Svartberg K, Seehuus B, et al. Selection for tameness has changed brain gene expression in silver foxes. Curr Biol. 2005;15:R915–916. doi: 10.1016/j.cub.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Saetre P, Lindberg J, Leonard JA, Olsson K, Pettersson U, et al. From wild wolf to domestic dog: gene expression changes in the brain. Brain Res Mol Brain Res. 2004;126:198–206. doi: 10.1016/j.molbrainres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Wayne RK, Van Valkenburgh B, O'Brein SJ. Molecular distance and divergence time in carnivores and primates. Mol Biol Evol. 1991;8:297–319. doi: 10.1093/oxfordjournals.molbev.a040651. [DOI] [PubMed] [Google Scholar]
- 78.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–821. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 79.Fondon JW, 3rd, Garner HR. Molecular origins of rapid and continuous morphological. Proc Natl Acad Sci USA. 2004;101:18058–18063. doi: 10.1073/pnas.0408118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- 81.Lin L, Faraco J, Li R, Kadotani H, Rogers W. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 82.Kirkness EF. SINEs of Canine Genomic Evolution. In: Ostrander EA, Giger U, Lindblad-Toh K, editors. The Dog and Its Genome. New York: Cold Spring Harbor Laboratory Press; 2006. pp. 209–221. [Google Scholar]
- 83.Spady TC, Ostrander EA. Canine behavioral genetics: pointing out the phenotypes and herding up the genes. Am J Hum Genet. 2008;82:10–18. doi: 10.1016/j.ajhg.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belyaeva TA, Vishnivetsky PN, Potapov VA, Zhelezova AI, Romashchenko AG. Species-and tissue-specific transcription of complex, highly repeated satellite-like Bsp elements in the fox genome. Mamm Genome. 1992;3:233–236. doi: 10.1007/BF00355724. [DOI] [PubMed] [Google Scholar]
- 85.Romashchenko AG, Yudin NS. Molecular evolution of Canidae Bsp-repeats: structure formation and the mechanisms of informational saturation of DNA molecules. In: Shumny VK, Markel AL, editors. Modern concepts of evolutionary genetics. Novosibirsk: ICG SB RAS; 2000. pp. 177–192. [Google Scholar]
- 86.Trut LN, Kharlamova AV, Kukekova AA, Acland DR, Carrier DR, et al. Morphology and Behavior: Are they cupled at the Genome Level? In: Ostrander EA, Giger U, Lindblad-Toh K, editors. The Dog and Its Genome. New York: Cold Spring Harbor Laboratory Press; 2006. pp. 81–93. [Google Scholar]
- 87.Kharlamova AV, Trut LN, Carrier DR, Chyase K, Lark KG. Genetic regulation of canine skeletal traits: trade-offs between the hind limbs and forelimbs in the fox and dog. Int Comp Biol. 2007;47:373–381. doi: 10.1093/icb/icm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kukekova AV, Trut LN, Oskina IN, Johnson JL, Temnykh SV, et al. A meiotic linkage map of the silver fox, aligned and compared to the canine genome. Genome Res. 2007;17:387–399. doi: 10.1101/gr.5893307. [DOI] [PMC free article] [PubMed] [Google Scholar]


