Abstract
Aging hearts are known to have diminished capacity to be protected against reoxygenation ischemia/reperfusion (IR) injury provided by various cardioprotective regimens. In search of a more successful regimen, we have studied the response of aged hearts to preconditioning (PC) and postconditioning (POST) elicited by sphingosine or sphingosine 1-phosphate treatment.
An ex vivo rat heart model was used to study the ability of PC and POST to protect old hearts (27 month) against I/R injury generated by 40 minutes (min) of index ischemia followed by 40 min of reperfusion. The response to ischemic PC was reduced in 27 month old hearts relative to 3–6 month (young) hearts as noted by a poor recovery of left ventricular developed pressure (LVDP) upon reperfusion (45% vs. 74% in young hearts) and a large infarct size after 40 min of reperfusion (37% versus 8% in young hearts). PC with sphingosine 1-phosphate (S1P) was also poor in old hearts yielding only 49% recovery of LVDP and a 27% infarct size. In contrast, PC with sphingosine was unaffected by aging; the 78% recovery of LVDP and 8% infarct size were not different from young hearts. Ischemic POST was less affected by aging than ischemic PC, but the old hearts still experienced infarct sizes of 28%. POST of old hearts with S1P was also associated with a substantial infarct size (24%). However, POST of old hearts with sphingosine was superior to the other forms of POST in that it reduced the infarct size to 12%. S1P levels were found to be lower in old hearts which may contribute to the decreased effectiveness of ischemic PC and POST. Further, phospho-Akt levels and distribution were altered in response to cardioprotection in the old hearts. In conclusion, POST was less affected by aging than PC; and sphingosine is a uniquely effective agent for both PC and POST of aging hearts.
Key words: aging, Akt, cardioprotection, ischemia/reperfusion injury, preconditioning, postconditioning, sphingosine, sphingosine 1-phosphate
Introduction
The loss of coronary blood flow for an extended period of time leads to ischemic damage and cardiomyocyte death. However, ischemic tissues must be exposed to molecular oxygen on reperfusion to manifest tissue injury.1,2 This strongly implicates reactive oxygen species in the injury process.1,2 This effect has been termed IR injury. Reoxygenation IR injury is a major complication of acute ischemia/infarction, cardiopulmonary bypass, angioplasty and heart transplantation.3 However, hearts can be treated (“conditioned”) in such a way that the damage associated with ischemia and subsequent reoxygenation is greatly diminished.4,5 Ischemic preconditioning consists of one or more cycles of a short-term non-damaging period of ischemia followed by reperfusion initiated prior to the extended index ischemia and subsequent reperfusion. This prevents loss of myocardial function and reduces subsequent infarct size.4,5 This same treatment can also be initiated after the index ischemia just prior to full reperfusion with equal effectiveness.6 This is referred to as postconditioning. A variety of pharmacologic agents can also serve as pre- and post-conditioning agents.
Studies in animals and humans have shown that aging hearts have reduced resistance to IR injury7–9 and a reduced capacity to be preconditioned.9 This includes both ischemic preconditioning as well as pharmacologic preconditioning.9 It should be noted that the cardioprotective effect of preconditioning has been shown to be impaired with aging in humans.9 Recent studies have indicated that post-conditioning regimens are also compromised with aging.10,11
We were interested in examining the effectiveness of sphingolipid signaling pathways during pre- and post-conditioning of aging hearts. The lipid mediator S1P is known to be a potent cardiac pre- and post-conditioning agent.12–16 Further, the enzyme SKase that catalyzes its synthesis has also been shown to be important in pre- and post-conditioning.17–19 Recently, we found that sphingosine also is a potent pre- and post-conditioning agent and that it accomplishes this via a different and complementary pathway from that of S1P.15,16 In this paper we have examined the effectiveness of both S1P and sphingosine in protecting the aging heart from reoxygenation injury.
Results
For these studies, we used the Langendorff isolated perfused heart model. We compared hearts from young rats (3–6 months) with hearts from approximately 27-month-old rats (range 26–28 months). The baseline heart rates and coronary flow rates for isolated perfused hearts were more variable in older rats but not different on average from young hearts. Contractile function was slightly elevated for aged hearts (LVDP 128 ± 9 versus 115 ± 4 mm Hg in young hearts).
We wanted to verify that in our system aged rat hearts have a deficient response to ischemic pre- and post-conditioning. We first investigated ischemic preconditioning (IPC). We attempted to protect the 27-month hearts from ischemia/reperfusion injury using the standard IPC regimen19 of two cycles of 4 min ischemia/6 min reperfusion. The data in Table 1 reveal that the recovery of LVDP was only 45 ± 10% and the infarct size was large (37 ± 12%). While this recovery of LVDP was highly significantly better (p < 0.001) than untreated old hearts (10 ± 5% LVDP), the infarct size was not statistically different (p > 0.1) from that of untreated hearts (44 ± 2). IPC was not completely effective in old rats. In contrast, hearts from young rats (3- to 6-months-old) responded to IPC with 74 ± 5% recovery of LVDP and only a 8 ± 2% infarct size. The differences in response to IPC between young and old rats was highly significant (p < 0.02) for both LVDP and infarct size.
Table 1.
Effect of age on pre- and post-conditioning
| 3 months | 28 months | |||
| Treatment | LVDP | Infarct size | LVDP | Infarct size |
| No Treatment | 8 ± 3 | 45 ± 1 | 10 ± 5 | 44 ± 2 |
| Isc Pre-C | 74 ± 5 | 8 ± 2 | 45 ± 10 | 37 ± 12 |
| Isc Post-C | 70 ± 7 | 11 ± 5 | 57 ± 15 | 28 ± 7 |
| S1P Pre-C | 72 ± 8 | 6 ± 1 | 49 ± 13 | 27 ± 11 |
| S1P Post-C | 80 ± 9 | 8 ± 2 | 65 ± 7 | 24 ± 2 |
| Sph Pre-C | 76 ± 7 | 7 ± 3 | 78 ± 11 | 8 ± 1 |
| Sph Post-C | 75 ± 8 | 11 ± 7 | 68 ± 8 | 12 ± 4 |
Ex vivo hearts from 3 month and 28 month old rats were exposed to 20 min of equilibration or preconditioning, 40 min of ischemia, and then 40 min of reperfusion. The recovery of left ventricular developed pressure (LVDP) upon reperfusion is expressed as % of the preischemic value. Infarct size (as % of the risk area) was determined at the end of 40 min of reperfusion. The data are presented as the mean ± standard deviation (n ≥ 4).
The response of old hearts to pharmacologic preconditioning with S1P or sphingosine has not previously been investigated. Preconditioning of 27 month hearts with 0.2 µM S1P (Table 1) was marginally effective. There was a 49 ± 13% recovery of LVDP but a sizeable infarct size (27 ± 11%). Using S1P at a higher concentration (0.4 µM) was less successful (48 ± 10% recovery of LVDP and 39 ± 6% infarct size). However, by comparison, for young hearts S1P gave rise to 72 ± 8% recovery of LVDP with almost no areas of infarction (6 ± 1% infarct size). Thus, aged hearts are significantly (p < 0.05) less responsive to S1P than young hearts. The most effective preconditioning agent was D-erythro-sphingosine (sphingosine). Preconditioning 27-month-old hearts with 0.2 µM sphingosine (the optimum concentration) resulted in 78 ± 11% recovery of LVDP with only an 8 ± 1% infarct size. This is equivalent to the protection obtained in young hearts (Table 1).
We next examined ischemic postconditioning (IPOST). This was accomplished by instituting four cycles of 15 sec ischemia/15 sec reperfusion immediately after the index ischemia just prior to full reperfusion. The level of protection achieved by IPOST was poor in the old rats. The recovery of LVDP was lower in old hearts (57 ± 15% with old hearts versus 70 ± 7% with young hearts) though not significantly so (p = 0.1). Old hearts had significantly (p = 0.02) larger infarct sizes (28 ± 7% with old hearts versus 11 ± 5% with young hearts).
Postconditioning with 0.2 µM S1P resulted in significantly reduced (p = 0.036) recovery of LVDP relative to young hearts (65 ± 7% in old hearts as opposed to 80 ± 9% in young hearts), and highly significantly larger (p < 0.001) infarct sizes (24 ± 2% versus 8 ± 2% in young hearts). As with preconditioning, 0.2 µM sphingosine (the optimum concentration) proved to be the most effective treatment for postconditioning old hearts. It provided good recovery of hemodynamic function (68 ± 8% recovery of LVDP) and had a very significantly (p < 0.004) smaller infarct size (12 ± 4%) compared to IPOST (28 ± 7%) and S1P (24 ± 2%). In fact, both the LVDP and the infarct sizes with sphingosine postconditioning of aged hearts are almost as good as the response in young hearts (75 ± 8% recovery of LVDP and 11 ± 7% infarct size).
We sought to determine the basis for the diminished response of aged hearts to ischemic pre- and post-conditioning. Because S1P has proved to be an important endogenous cardioprotectant (see Introduction), we compared S1P levels between 27-month-old rats and 6-month-old rats (Table 2). There was a trend (p = 0.07) toward reduction in S1P levels in old hearts (82 ± 32 versus 117 ± 6 ng/g dry wt), though there was great variability in the S1P levels for old hearts. These data suggest that hearts from most aged rats have reduced levels of S1P.
Table 2.
Level of S1P and SKase in hearts from harlan F-344 rats
| Heart | S1P (ng/g wet wt. heart) | SKase (pmol/min/g wet wt.) |
| 27 Month | 82 ± 32 | 155 ± 6 |
| 6 Month | 117 ± 6 | 152 ± 20 |
Rat heart homogenates were analyzed for S1P content and SKase activity as indicated in Methods. There was an n of 4 for each.
We next measured SKase activity in these hearts. SKase is not only the enzyme responsible for S1P synthesis but its activity has been shown to be critical to both pre- and post-conditioning.17,18 Surprisingly the SKase activity was not significantly different in the 27 month hearts (155 ± 6 pmol/min/mg) as compared with 6 month hearts (152 ± 20 pmol/min/mg).
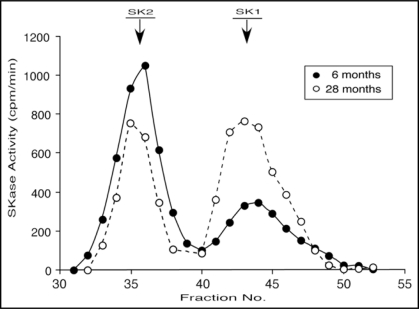
Although total SKase activity didn't change with aging, a surprising finding was a very dramatic change with age in the ratio of SK1 to SK2 in the cytosolic fraction (the predominant location of SKase in rat heart19). In 6 month old hearts (as with 3 month old hearts21), SK2 is the predominant form comprising 63 ± 2% (n = 4) of the total activity (measured either by the area under the curve for each peak or in pooled fractions). In 28 month old hearts this ratio is reversed with SK1 comprising 60 ± 4% (n = 4) of the total activity. Thus, SK1 activity nearly doubles with aging while SK2 activity is halved. A representative example of the separation of SK1 and SK2 form young and old rats is shown in Figure 1.
Figure 1.
Gel Filtration Profile of Cytoslic SKase Activity from Young and Old Rat Hearts. The cytosolic fractions isolated from 6-month-old (-●-) and 27-month-old (-○-) rat hearts were chromatographed on a Sephacryl S200 gel filtration column. The fractions were assayed for SKase activity as described in Methods. The profile is representative of four separate experiments. The identification of the SK1 and SK2 peaks was described previously (Vessey et al. 2007).
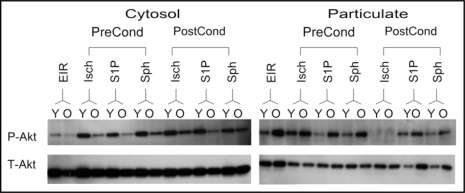
We also examined the responsiveness of signaling pathways to pre- and post-conditioning in old versus young hearts. We previously demonstrated that pre- and post-conditioning in young hearts by both S1P and sphingosine was associated with high levels of phospho-Akt in the cytosolic fraction during reperfusion.15,16 Therefore, we compared the levels of phospho-Akt during reperfusion for old versus young hearts. Preconditioning with ischemia, S1P or sphingosine supported relatively higher levels of phospho-Akt in the cytosol of young hearts after 40 min of reperfusion as compared to old hearts (Fig. 2). For pharmacologic preconditioning with S1P, this appeared to be in part due to retention of phospho-Akt in the particulate fraction (Fig. 2). For pharmacologic postconditioning, both S1P and sphingosine supported recovery of phospho-Akt levels during reperfusion of young hearts (Fig. 2). In 27 month old hearts, a reduced recovery of phospho-Akt in the cytosol was noted for both S1P and sphingosine postconditioning, and this was accompanied by an increase in phospho-Akt in the particulate fraction (Fig. 2). However, ischemic postconditioning was not associated with phospho-Akt in the particulate fractions of either young or old hearts, which is clearly different from sphingosine and S1P postconditioning.
Figure 2.
Effect of Preconditioning with Ischemia, S1P or Sphingosine on the Phosphorylation of Akt. Ex vivo hearts from young (Y) and old (O) rats were ischemically preconditioned (Isch-PreCond) or ischemically postconditioned (Isch-PostCond) as described in Methods; or pharmacologically pre- or post-conditioned with either 0.2 µM S1P (S1P-PreCond and S1P-PostCond) or 0.2 µM sphingosine (Sph-PreCond and Sph-PostCond). The index ischemia was for 40 min and full reperfusion was for 40 min. Hearts were collected after the 40 min of reperfusion and analyzed for phospho-Akt (P-Akt) levels by western analysis as described in Methods. Total Akt levels are also shown (T-Akt). Controls consisted of non-pre- or post-conditioned hearts that underwent 30 min of equilibration, 40 min of ischemia and 40 min of reperfusion (EIR).
Discussion
Previous work revealed that aging results in a loss of responsiveness to a number of cardioprotectants.7–11,22 Using an ex vivo rat heart model, we have also found that ischemic pre- and post-conditioning is less effective in 27-month-old rats relative to 3- to 6-month-old rats. This was particularly true for ischemic preconditioning of old hearts which did not prevent the development of large infarct sizes (37 ± 12%). It was surprising that the LVDP recovered as well as it did in the old hearts (45 ± 10%) considering the magnitude of the infarcts. Ischemic postconditioned hearts recover somewhat better than ischemic preconditioned hearts but still had infarct sizes of 28 ± 7%, which is much greater than for young hearts (11 ± 5%). Thus, while ischemic post-conditioning is less affected by age, it still didn't prevent development of significant areas of infarction in aged hearts.
The response of old hearts to pharmacologic preconditioning with S1P was nearly the same as ischemic preconditioning. There was a fairly modest recovery of LVDP (49 ± 13%) that was much less than in young hearts, but more importantly there were notable areas of infarction (27 ± 11%). By contrast, preconditioning with sphingosine appeared to be little affected by age. Sphingosine preconditioning gave rise to 78 ± 11% recovery of LVDP with infarct sizes of only 8 ± 1%. This is equivalent protection to that obtained with young hearts. Thus, sphingosine appears to be an effective preconditioning agent in aged hearts. Preconditioning with sphingosine had previously been shown to operate via a PKA and PKG dependent pathway while S1P operates via a GPCR pathway.15 This suggests that cyclic nucleotide-dependent pathways are less affected by age than are GPCR coupled pathways. The GPCR pathways have been shown to involve the activation of PI3 kinase, Akt and protein kinase C (PKC).11,15 PKC content and activation by translocation have been shown to be diminished with age.11 This could account for the decreased effectiveness with age of GPCR linked cardioprotection pathways. By contrast, sphingosine signaling is independent of PKC,15 and this may in part account for its sustained effectiveness with age.
Postconditioning was also affected by aging but to a lesser extent than preconditioning. Ischemically postconditioned old hearts showed substantial hemodynamic recovery (57 ± 15% recovery of LVDP) but with sizable infarcts (28 ± 7% of risk area). Postconditioning with S1P gave similar results (65 ± 7% recovery of LVDP but 24 ± 2% infarct size). However, sphingosine protection again proved to be relatively unaffected by age. Postconditioning with sphingosine resulted in 68 ± 8% recovery of LVDP and only a 12 ± 4% infarct size. Since postconditioning with sphingosine, like preconditioning, involves PKA and PKG dependent pathways15 this again reinforces the hypothesis that the cyclic nucleotide-dependent pathways are less sensitive to the effects of aging than GPCR-dependent pathways. Postconditioning by sphingosine differs from GPCR-dependent pathways in being independent of PKC;15 and we hypothesize that this is in part due to lack of reliance on PKC signaling which is diminished with age.11 Importantly, these data reveal that sphingosine is the most effective cardioprotectant for aging rat heart and suggest that it might have the greater clinical relevance.
To examine other factors that could potentially contribute to the diminished cardioprotective capacity of GPCR pathways with aging, we investigated the S1P metabolic pathway. S1P levels were found to be generally reduced in 27 month old hearts. In view of the importance of S1P as an endogenous cardioprotectant,12,14–16 this might be a contributing factor in the diminished response of aged hearts to ischemic pre- and postconditioning. Not only is the level of S1P low in aged hearts, but as discussed above, the response to it is also diminished. We measured the activity of SKase, the enzyme catalyzing the synthesis of S1P, and were surprised to find that the total SKase activity was not significantly different in the 28 -month hearts as compared to 6-month hearts. These data suggest that any reduction in S1P levels in older hearts is not due to SKase activity but rather to other factors; perhaps increased S1Pase activity or an age-dependent decline in sphingosine levels.
It has been proposed that the SK1 form of SKase mediates prosurvival effects while SK2 is proapoptotic.23,24 This provided the rationale for our investigation of the ratio of SK1 to SK2 in old versus young hearts. We found that was even though total SKase activity did not change, there was a dramatic shift in the ratio of SK1 to SK2. SK1 activity nearly doubles with aging while SK2 activity is halved. These are substantial changes. The data suggest that the activity of the two forms can be differentially regulated. Whether this shift impacts S1P levels and/or cardioprotective mechanisms is not yet clear. It may be that the upregulation of SK1 with age is an attempt to protect against enhanced mitochondrial ROS formation in aged myocardium or age-related declines in other cell signaling components.11 Alternatively, it may in part reflect an age-dependent increase in fibroblasts which have very high levels of SKase.25 If true, this could be masking a decrease in myocyte SKase.
Phospho-Akt is a known pro-survival signaling molecule26 which has been shown to be a key feature of cardioprotective signaling pathways.15,17,27,28 While phospho-Akt is known to have anti-apoptotic effects, it also has other pro-survival effects that are more important for protection during the early period of reperfusion. Signs of apoptosis do not appear until after several hours of reperfusion.17 Protection by phospho-Akt during the early phase of reperfusion is likely due to stabilization of mitochondria29 with a resultant decrease in the formation of reactive oxygen species.
When phospho-Akt levels were measured during reperfusion, it was found for both ischemic preconditioning as well as pharmacologic preconditioning with S1P or sphingosine that the phospho-Akt level was lower in the cytosolic fraction of old hearts relative to young hearts. This indicates clearly that the Akt signaling pathway is altered with aging. However, in contrast to changes in the cytosolic fraction, for S1P pre- and post-conditioning, the phospho-Akt levels in the particulate fraction of old hearts were increased relative to that for young hearts. The same was true for sphingosine postconditioning. Whether these shifts of phospho-Akt from the cytosolic to the particulate fraction impacts protection is not yet known. Interestingly, for IPOST there was no phospho-Akt detectable in the particulate fraction for either young or old hearts. This suggests that retention of phospho-Akt in the particulate fraction is not essential for cardioprotection.
Alterations in receptor sensitivity and Akt phosphorylation with aging have been reported for adenosine mediated signaling pathways22 but not such a cytosol to particulate shift in phospho-Akt. Studies in ex vivo mouse heart suggested that a deficit in ERK phosphorylation contributed to a loss of post-conditioning in old mouse hearts,11 but we have not found ERK phosphorylation to be a key factor in cardioprotection in rat heart. Clearly though there are alterations in the signaling mechanism with aging.
In conclusion, we have found that ischemic preconditioning is not effective in ex vivo aged hearts. Ischemic postconditioning is also diminished in effectiveness in aged hearts but not to the same extent as preconditioning. Pre- and post-conditioning with S1P are similarly affected in aging rat hearts. However, we have discovered that aged hearts respond well to sphingosine preconditioning and can also be effectively postconditioned with sphingosine. Thus, sphingosine holds promise as an effective cardioprotectant in the most vulnerable population, the aged.
Materials and Methods
Materials.
Aprotinin, ATP, chymostatin, leupeptin, pepstatin A, ponceau S, protein phosphatase inhibitor cocktails, Sephacryl S200, trisodium EDTA and triphenyltetrazolium chloride were obtained from Sigma (St. Louis MO). D-erythro-sphingosine and D-erythro-sphingosine-1-phosphate were obtained from Biomol Research Laboratories (Plymouth Meeting PA). D-Erythro-[3-3H]-sphingosine was obtained from American Radiolabeled Chemical Inc., (St. Louis MO). The rabbit Akt and phospho-Akt (ser473) antibodies were obtained from Cell Signaling Technology.
Langendorff ex vivo perfused heart.
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academic Press, Washington DC, 1996). Rats were obtained from the Aging Rat Colony via the National Institute of Aging. The aging rats were on average 27-months-old (range 26–28 months). Young hearts were from 3- to 6-month-old rats. Hearts were removed under pentobarbital anesthesia and mounted on a Langendorff apparatus as described previously.19 Hearts were perfused with oxygenated (95/5 O2:CO2) Krebs-Henseleit solution at 37°C. Left ventricular developed pressure (LVDP) was measured using a Millar micromannometer-tipped catheter and is reported in units of mm Hg. To measure infarct size, hearts were sectioned, stained with TTC and the infarct area determined by computer analysis.19
The protocol for non-conditioned hearts consisted of continuous perfusion for 30 min after mounting the heart on the Langendorff apparatus (equilibration). Sustained ischemia (index ischemia) was then induced by halting perfusion for indicated lengths of time. During the index ischemia the heart was lowered into a thermostated chamber that maintains an ambient temperature of 37°C. This was followed by the reperfusion stage in which flow was again initiated for 40 min. For ischemic preconditioning, after 10 min of equilibration, the heart was exposed to two cycles of 4 min ischemia/6 min reperfusion just prior to the index ischemia, Pharmacologic preconditioning consisted of adding either S1P or sphingosine to the perfusion medium for the 30 min period preceding the index ischemia. For ischemic postconditioning, at the end of the index ischemia, reperfusion was first initiated with four cycles of alternating 15 sec of reperfusion/15 sec of ischemia, followed by full reperfusion. Pharmacologic postconditioning consisted of adding either S1P or sphingosine to the reperfusion medium for the 40 min of reperfusion. To administer S1P, a stock solution of 2.67 mM was prepared in DMSO and 90 µl (for 0.4 µM final S1P concentration) was added per 600 ml of perfusion buffer. To administer D-erythro-sphingosine, a stock solution of 20 mM was prepared in ethanol and added directly to the perfusion buffer at a final concentration of 0.2 or 0.4 µM. Vehicle alone was without effect. To measure infarct size, hearts were sectioned, stained with triphenyltetrazolium chloride and the infarct area determined by computer analysis as described.19
Preparation of sub-cellular fractions and assay for sphingosine kinase.
Rat hearts were immediately plunged into cold perfusion buffer (0°C), rapidly blotted dry, weighed, and homogenized in 0.13 M KCl, 20 mM HEPES pH 7.4, 1 mM EGTA, 1 µg/l leupeptin, 0.25 µg/l each of aprotinin and pepstatin A plus protein phosphatase inhibitors 1 and 2 (Sigma). The homogenate was either assayed directly, or in some cases subfractionated by ultracentrifugation into cytosolic and particulate fractions. The particulate fraction was washed once and the wash added back to the cytosolic fraction. Homogenates and cell fractions were immediately assayed for SKase activity. The standard assay for SKase activity has been described previously.20 The assay contained 5 mM ATP, 10 mM MgCl2, 1 µM [3H]sphingosine, 250 mM KCl, 0.05% Triton X100 and 50 mM Tris pH 8.0. at 30°C. Measurement of S1P levels was conducted by combined liquid chromatography/dual tandem mass spectrometry (LC/MS/MS) as described previously.19
Separation of SK1 and SK2 activity.
The cytosolic cell fraction from each individual heart, freshly prepared, was immediately loaded on a rapid flow 1.8 × 50 cm Sephacryl S200 chromatography column and eluted with 250 mM KCl, 20 mM Tris 7.5, 1 mM EGTA, 1 mg/l leupeptin, 0.2 mg/l each of aprotinin and pepstatin A. Elution fractions were monitored for protein (OD280 nm) and SKase activity. The SK1 and SK2 fractions were identified by western analysis as described previously.21 Pooled fractions were assayed using multiple time point measurements to obtain true initial rates of activity.
Western analysis.
Hearts were homogenized in 0.13 M KCl, 20 mM HEPES pH 7.4, 1 mM EGTA, 1 µg/l leupeptin, 0.25 µg/l each of aprotinin and pepstatin A, and 1% (v/v) each of phosphatase inhibitor cocktails 1 and 2 (Sigma). The homogenate was filtered through two layers of cheesecloth to remove debris. Homogenates were centrifuged at 100,000 xg to generate a particulate and a cytosolic fraction. Fresh cytosol and rinsed particulate fractions were made 0.5% in SDS. The protein concentrations of these preparations were determined using the Bio-Rad DC (detergent-compatible) protein assay against a BSA standard, diluting to an apparent concentration of 2 mg/ml, and then reassaying. The fractions were then diluted to a final concentration of 1 mg/ml in Laemmli buffer for SDS-polyacrylamide gel electrophoresis. Each lane was loaded with an equivalent amount of protein (generally 5 or 10 µg). Following electroblotting, the gel was stained with 0.1% ponceau S to visualize total protein to ensure that all lanes were equivalently loaded. Western analysis was then conducted using a rabbit antibody as described previously.15
Statistical analysis.
Statistical data are expressed as mean ± standard deviation (SD). Numerical data (n ≥ 4) were compared using unpaired Students's t test for a 95% confidence interval. p values are reported for statistical comparisons.
Acknowledgements
The authors thank Dr. Guan-Ying Wang for careful review of this manuscript.
This work was supported by a grant from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (DAV).
Abbreviations
- GPCR
G-protein coupled receptor
- PKC
protein kinase C
- PC
preconditioning
- POST
postconditioning
- IR
ischemia reperfusion
- LVDP
left ventricular developed pressure
- SKase
sphingosine kinase
- S1P
sphingosine 1-phosphate
- SD
standard deviation
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: http://www.landesbioscience.com/journals/oximed/article/8622
References
- 1.Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 2.Siemionow M, Arslan E. Ischemia/reperfusion injury: A review in relation to free tissue transfers. Microsurgery. 2004;24:468–475. doi: 10.1002/micr.20060. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell SR, Lip GY. Reperfusion injury: A review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Int J Cardiol. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 5.Das M, Das DK. Molecular mechanism of preconditioning. IUBMB Life. 2008;60:199–203. doi: 10.1002/iub.31. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cerebral Blood Flow Metab. 2009:1–13. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakatta EG, Yin FC. Myocardial aging: Functional alterations and related cellular mechanisms. Am J Physiol. 1982;242:927–941. doi: 10.1152/ajpheart.1982.242.6.H927. [DOI] [PubMed] [Google Scholar]
- 8.Tani M, Suganuma Y, Hasegawa H, Shinmura K, Ebihara Y, Hayashi Y. Decrease in ischemic tolerance with aging in isolated perfused Fischer 344 rat hearts: Relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol. 1997;29:3081–3089. doi: 10.1006/jmcc.1997.0533. [DOI] [PubMed] [Google Scholar]
- 9.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp033. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.JJin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKCε knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:1970–1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z-Q, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine hearts. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 14.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNFa and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 15.Vessey DA, Li L, Kelley M, Karliner JS. Sphingosine can pre- and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Molec Toxicol. 2008;22:113–118. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 16.Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Z-Q, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Jin Z-Q, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–140. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 19.Vessey DA, Kelley M, Li L, Huang Y, Zhou H, Zhu BQ, et al. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monitor. 2006;12:318–324. [PubMed] [Google Scholar]
- 20.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Analytical Biochem. 2005;337:136–142. doi: 10.1016/j.ab.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 Inhibit the SK1 Form but Activate the SK2 Form of Sphingosine Kinase from Rat Heart. J Biochem Molec Toxicol. 2007;22:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 22.Willems L, Ashton KJ, Headrick JP. Adenosine-mediated cardioprotection in the aging myocardium. Cardiovasc Res. 2005;66:245–255. doi: 10.1016/j.cardiores.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi N, Okada T, Hayashi Y, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 25.Kacimi R, Vessey DA, Honbo N, Karliner JS. Adult cardiac fibroblasts null for sphingosine kinase-1 exhibit growth dysregualtion and an enhanced proinflammatory response. J Molec Cell Cardiol. 2007;43:85–91. doi: 10.1016/j.yjmcc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. AKT promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:3150–3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 29.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]