Abstract
High O2 consumption, modest antioxidant defenses and a lipid-rich constitution make the brain highly vulnerable to redox imbalances. Oxidative damage in the brain causes nervous system impairment. Recently, oxidative stress has also been implicated in depression, anxiety disorders and high anxiety levels. The findings which establish a link between oxidative stress and pathological anxiety have inspired a number of other recent studies focusing on the link between oxidative status and normal anxiety and also on a possible causal relationship between cellular oxidative stress and emotional stress. This review examines the recent discoveries made on the link between oxidative status and normal anxiety levels and the putative role of oxidative stress in genesis of anxiety. We discuss the different opinions and questions that exist in the field and review the methodological approaches that are being used to determine a causal relationship between oxidative and emotional stress.
Key words: anxiety, behavior, oxidative status, oxidative stress, reactive oxygen species, antioxidants
Introduction
Anxiety is an aversive emotional state, in which the feeling of fear is disproportionate to the nature of the threat.1 In response to threatening situations, the feeling of the emotion that constitutes the subjective feature of anxiety is accompanied by emotional stress, which involves behavioral, expressive and physiological features, such as an avoidance of the source of the danger, assuming defensive postures and an increase in blood pressure, respectively.1,2 Anxiety is a normal emotional response to a threat or potential threat. When this emotion is inappropriate, extreme and persistent, it is classified as pathological.1,3 Anxiety is implicated in a number of psychiatric disorders, such as depression, panic attacks, phobias, generalized anxiety disorder, obsessive-compulsive disorder and post-traumatic stress disorder.3 Anxiety disorders affect approximately 28.8% of the US population,4 imposing both an individual and a social burden that amounts to a total cost of $42.3 billion in the US in 1990.5 Anxiety disorders are the most common class of psychiatric disorders in the US4 and many other countries.6 According to an ESEMeD study including six European countries, the 12-month prevalence of inappropriate anxiety was 6.4%.7 In the most recent systematic review of studies conducted in 16 European countries, however, this value was estimated to be 12%.8 In an obese population in the UK, 56% of patients met the minimum criteria for an anxiety disorder.9 It is estimated that one-eighth of the total population worldwide suffers from inappropriate anxiety.10 Population-based studies have shown that anxiety disorders frequently go untreated.11,12 Predominantly, the research that has been performed on anxiety has focused on the regulatory systems, including gamma-aminobutyric acidergic (GABAergic) and serotoninergic systems among others. However, Kuloglu et al.13,14 recently established a link between oxidative stress and certain anxiety disorders (obsessive-compulsive disorder and panic disorder), demonstrating that other systems, such as oxidative metabolism, can affect the regulation of anxiety. These findings, which establish a link between oxidative stress and pathological anxiety, inspired a number of other recent studies focusing on the link between oxidative status and normal anxiety (Table 1) and also on a possible causal relationship between cellular oxidative stress and emotional stress.
Table 1.
Data establishing the link between pathological/normal anxiety and oxidative cell pathways and mechanisms
| Subjects | Expression of antioxidant genes | Indirect evaluation of oxygen-derived species Activity of antioxidant proteins | Lipid peroxidation markers | Direct evaluation of oxygen-derived species Intracellular ROS levels assessed by using the 2′,7′-dichlorofluorescin diacetate (DCFH-DA) sensor | |
| Pathological anxiety | Patients with obsessive-compulsive disorder and panic disorder Vs. healthy controls (Kuloglu et al.13,14) | - | In erthrocytes: Superoxide dismutase*, catalase, glutathione peroxidase* | In plasma: Malondialdehyde* | - |
| Normal trait-anxiety | Mouse strains with high- Vs. low-anxiety-related phenotypes (Hovatta et al.25) | In brain:Glyoxalase 1* and glutathione reductase 1* | In brain: Glyoxalase 1* and glutathione reductase 1* | - | - |
| Anxious Vs. non-anxious Swiss albino mice (Rammal et al.41,42) | - | - | - | In brain and peripheral cells: Neurones*, glial cells*, granulocytes*, monocytes* and lymphocytes* | |
significantly different from controls (healthy humans, strains with low-anxiety-related phenotypes, non anxious mice).
It is well known that low/moderate concentrations of reactive oxygen species (ROS) affect a great number of physiological functions.15 However, when ROS concentration exceeds the anti-oxidative capacity of an organism, animal cells enter a state termed oxidative stress, in which the excess ROS induces oxidative damage on cellular components.15,16 As a result, oxidative stress has been implicated in a large range of diseases, including cancer, diabetes, male infertility, autoimmune diseases, atherosclerosis and cardiovascular disorders.15–17
The brain is highly vulnerable to oxidative stress due to its high O2 consumption, its modest antioxidant defenses and its lipid-rich constitution.18,19 Human brain utilizes 20% of oxygen consumed by the body even though this organ constitutes only about 2% of the body weight.19,20 When the production of oxygen-derived metabolites prevails over the brain defense systems, however, oxidative damage to nucleic acids, proteins and neuronal membrane lipids, which are rich in highly polyunsaturated fatty acids, can occur.15,16,19 In presence of oxidative stress, the lipid-rich constitution of brain favors lipid peroxidation that results in decrease in membrane fluidity and damage in membrane proteins inactivating receptors, enzymes and ion channels.15,16,19 As a result, oxidative stress can alter neurotransmission, neuronal function and overall brain activity.16,21,22 Oxidative stress has been associated with several diseases which are specific for nervous system impairment including neurodegenerative diseases and neuropsychiatric diseases, such as schizophrenia and major depressive disorder.15,16,23,24 The intrinsic oxidative vulnerability of the brain has led some authors to suggest that oxidative damage may be a plausible pathogenic factor for certain neurological diseases including neuropsychiatric disorders.18
In this review, we discuss the relationship between oxidative stress and normal anxiety by presenting the recent advances in the field and the different views that exist. We also review the methodological approach for determining the causal relationship between oxidative stress and emotional stress.
A Link Between Oxidative Stress Metabolic Pathways and Anxiety-Related Phenotypes
In 2005, Hovatta et al.25 identified a close relationship between antioxidative defense mechanisms and anxiety-related phenotypes in six inbred mouse strains. They found that, in the brain, the expression of glutathione reductase 1 and glyoxalase 1, which are genes involved in antioxidative metabolism, is highly correlated with anxiety-related phenotypes. Furthermore, they also found that the activity of these enzymes is highest in the most anxious mice and lowest in the least anxious strains. These authors were the first to demonstrate a link between oxidative stress metabolic pathways and normal anxiety.
A link between oxidative stress and emotional stress is not surprising, since it is well accepted that oxidative damage in the brain causes an impairment of the nervous system. In living organisms, an imbalance between oxidant production and antioxidant protection that favors oxidants causes a state termed oxidative stress. In this state, there can be differences gene expression, protein conformation and cellular signaling. This state may also alter neurotransmission, neuronal function and overall brain activity, as well as disrupting membrane integrity; even neuronal cell death may result.15,16,21,22 Most studies have shown that anxiety is controlled by the nervous system and that GABAergic and serotoninergic systems play important roles in the regulation of anxiety.1,26 Abnormalities in these regulatory systems in rodents, which are used as translational models for human anxiety,25,27,28 can result in anxious behavior.26 Altering the function of the hypothalamic-pituitary-adrenal (HPA) axis, which is implicated in stress responses29 and anxiety disorders, could also impact the emotional response.29,30
Nevertheless, Hovatta et al.25 obtained surprising results in a genetic manipulation using lentivirus-mediated gene transfer: local overexpression of glutathione reductase 1 and glyoxalase 1 in the cingulated cortex of the murine brain results in an increase of anxiety-like behavior, while inhibition of glyoxalase 1 expression produces low-anxiety mice. Thus, Hovatta et al.25 were able to make a causal link between the antioxidative status of the brain and anxiety-related behavior and hypothesize that glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. It is worth mentioning that in vivo, antioxidant genes are overexpressed in response to an uncontrolled production of ROS. Indeed, in vivo, excessive ROS accumulation induces the overexpression of the glutathione redox system, including glutathione reductase, and a general overexpression of endogenous antioxidants.31,32 In the lentivirus experiments of Hovatta et al.25, however, the overexpression of the transgenes (glyoxalase 1 and glutathione reductase 1) was induced in vivo with a lentiviral vector and not an excessive production of toxic oxygen metabolites. Clearly, the mechanism by which these enzymes regulate anxiety is of great interest.26
During the same period in which the Hovatta et al.25 study was being conducted, other studies were performed on two Swiss CD1 mouse lines with contrasting anxiety-like behavioral phenotypes. These mice were generated from wild type mice after >15 generations of selection. Results from these studies have led researchers to propose that glyoxalase 1 might be a biological marker for trait anxiety.33,34 These results are discordant with those of Hovatta et al.25 however, and complicate the understanding of the relationship between oxidative stress and trait anxiety. Krömer et al.33 and Ditzen et al.34 examined the expression of glyoxalase 1 in several areas of the brain areas and in red blood cells and found that this protein is expressed more in a line with a low-anxiety-related behavioral phenotype than in a line with a high-anxiety-related behavioral phenotype. It is worth noting that Hovatta et al.25 suggested that there is a link between glyoxalase 1 and oxidative stress but that this link is indirect.35 Indeed, glyoxalase 1 is an enzyme of the glyoxalase system, which protects against carbonyl stress; glutathione is a determinant cofactor for the enzymatic reaction that is catalyzed by glyoxalase 1.35–37
A Link Between Oxidative Stress Metabolic Pathways and Anxiety-Related Behavior
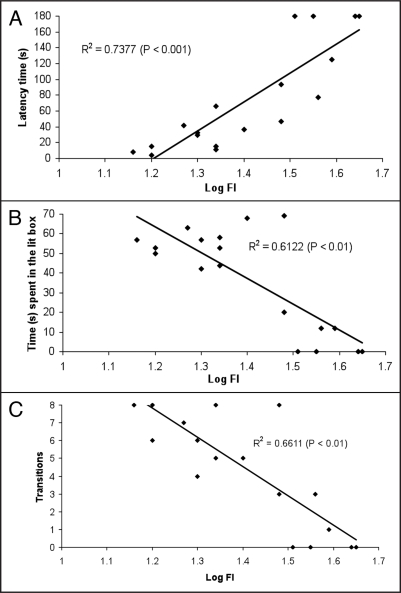
The disagreements in the data of Hovatta et al.25, Krömer et al.33 and Ditzen et al.34 can be partly explained by the differences in the genotypes of the strains.35 Thus, it would be interesting to compare in the same strain the oxidative status of mice with contrasting levels of anxiety, rather than compare the oxidative status of strains that differ in their anxiety-related phenotypes. We have observed that naive Swiss albino male mice have a large heterogeneity in their anxiety levels.38 In first order regression analyses of the performances of mice in the light/dark choice test, which are used as a behavioral indices of anxiety, and the intracellular ROS accumulation in blood granulocytes, we found correlation coefficients (R2) ranging from 0.61 (p < 0.01) to 0.73 (p < 0.001) (Fig. 1). Our results suggest that there is a positive correlation between peripheral blood cell oxidative status and anxiety-related behavior in the light/dark box in Swiss albino male mice.38
Figure 1.
Linear correlation between intracellular redox status and different parameters used for assessment of anxiety-related behavior in mice (n = 18). Correlation of peripheral blood granulocyte oxidative status with latency of first crossing from dark to lit box (A), time spent in lit box (B) and number of transitions between two boxes (C), respectively. Fluorescence intensity (FI) expressed as a decimal logarithm. FI emitted by granulocytes is due to oxidation of intracellular DCFH-DA by ROS (data published by Bouayed et al.38 in Eur J Pharmacol).
Levels of ROS can be evaluated directly with sensors, such as 2′,7′-dichlorofluorescin diacetate (DCFH-DA),39,40 or indirectly by measuring the levels of certain antioxidant enzymes, byproducts of lipid peroxidation or some transition metals, such as copper, zinc and iron.13,14 In our study, the intracellular redox status of the cells was evaluated using the tracer DCFH-DA, a well known sensor of ROS.39,40 To confirm the relationship between oxidative stress and emotional stress, we comparatively evaluated the peripheral oxidative status of mice with contrasting levels of anxiety (anxious and non-anxious). Mice with intermediate behaviors were eliminated.41 We found that high anxiety in mice in the light-dark box is accompanied by significantly high levels of intracellular ROS in lymphocytes, granulocytes and monocytes (Table 2). Our results confirm that there is the relationship between the level of intracellular ROS in peripheral blood cells and anxiety-related behavior in mice.41
Table 2.
Intracellular ROS accumulation in peripheral immune cells of anxious and non-anxious mice
| Mean fluorescent intensity (arbitrary units) | ||
| Type of cells | Anxious mice | Non-anxious mice |
| Lymphocytes | 215 ± 31* | 135 ± 12 |
| Monocytes | 46 ± 8* | 25 ± 4 |
| Granulocytes | 1635 ± 375** | 418 ± 87 |
The mean fluorescent intensity corresponds to fluorescence resulting from intracellular DCFH-DA oxidation by intracellular ROS. Data are expressed as mean ± SEM. (n = 10). *p < 0.05; **p < 0.01. (data published by Rammal et al.41 in Eur J Pharmacol).
These results prompted us to study the oxidative status of the brain in mice with contrasting levels of anxiety. Using the same behavioral approach to distinguish between anxious and non-anxious mice, we found that anxiety in mice is accompanied by markedly elevated levels of ROS in neuronal and glial cells within the cerebellum and hippocampus, as well as in neurons of the cerebral cortex and in blood monocytes, granulocytes and lymphocytes (Table 3). As a consequence, we suggest that an imbalance in the redox system of anxious mice plays a role in neuroinflammation and neurodegeneration, predisposing them to recurrent infection and chronic inflammation.42 The results of our studies38,41,42 are in good concordance with the initial findings of Hovatta et al.25 and show a clear link between oxidative stress and trait anxiety, but our findings do not permit us to declare a causal relationship between these stresses.
Table 3.
Intracellular ROS accumulation in cerebral and peripheral cells of anxious and non-anxious mice
| Mean fluorescent intensity (arbitrary units) | ||
| Type of cells | Anxious mice | Non-anxious mice |
| Neurons of cerebral cortex | 1223 ± 232** | 177 ± 76 |
| Neurons of cerebellum | 546 ± 128* | 227 ± 41 |
| Neurons of hippocampus | 1749 ± 566* | 227 ± 51 |
| Glial cells of cerebral cortex | 429 ± 48 | 310 ± 72 |
| Glial cells of cerebellum | 343 ± 36* | 195 ± 31 |
| Glial cells of hippocampus | 468 ± 43* | 319 ± 29 |
| Lymphocytes | 251 ± 46* | 152 ± 11 |
| Monocytes | 56 ± 9* | 35 ± 2.5 |
| Granulocytes | 1979 ± 405** | 448 ± 77 |
The mean fluorescent intensity corresponds to fluorescence resulting from intracellular DCFH-DA oxidation by intracellular ROS. Data are expressed as mean ± SEM. (n = 10). *p < 0.05; **p < 0.01. (data published by Rammal et al.42 in Brain Behav Immun).
In agreement with our recent findings,41,42 Yasunari et al.43 observed a significant relationship between trait anxiety and ROS formation in monocytes of hypertensive individuals. Recently, Masood et al.29 published work that contributes to the understanding of the relationship between oxidative stress and anxiety. They examined the direct effect of oxidative stress on anxiety-like behavior and established that oxidative stress leads to anxiogenic behavior in mice. They found that treating mice with buthionine-S,R-sulfoximine (BSO), an inducer of oxidative stress, induces anxious behavior through the NADPH oxidase pathway. The anxiogenic behavior due to BSO treatment was observed in several mouse models of anxiety, including elevated plus maze, hole-board and open field tests. Masood et al.29 induced oxidative stress in mice by depleting glutathione with BSO inhibition of gamma-glutamylcysteine synthetase. Glutathione depletion causes a myriad of cellular stresses, including oxidative, nitrosative and carbonyl stresses, as glutathione is an important determinant of the oxygen, nitrogen and dicarbonyl metabolisms.15,16,35,44 Excessive production of ROS induces oxidative damage of cellular structures;15,16,19,45 production of reactive nitrogen species triggers nitrosylation reactions, which can alter the structure of proteins to inhibit their normal function;15,16,46 excessive accumulation of reactive dicarbonyl compounds leads to damage of protein and nucleotides by dicarbonyl glycation.35–37
Indirect Evidence for the Causal Link Between Oxidative Stress and Anxiety
Recent data from Desrumaux et al.47, Souza et al.48 and Berry et al.49 provide indirect evidence for the causal link between oxidative stress and anxiety-related behavior. Desrumaux et al.47 showed that vitamin E deficiency in the mouse brain significantly increases the levels of central oxidative stress markers, resulting in anxiogenic behavior without abnormalities in the locomotor performance of the mice. Souza et al.48 demonstrated in rats that the consumption of a highly palatable diet enriched with sucrose leads to an obese phenotype, increases protein oxidation in the frontal cortex and induces anxiety-like behavior in the dark/light choice test without altering locomotion in an open field test. Berry et al.49 showed that mice developed anxious behavior during aging, likely due to the accumulation of oxidative damage, which is a characteristic of the aging process in animals.50,51 In addition, Berry et al.49 showed that a deletion of the p66Shc longevity gene in mice, which results in lower levels of oxidative stress and an extended life span, decreases anxiety-related behavior. Overall, the data presented in these reports suggest that oxidative stress can provoke anxious behavior in rodents.
Conclusion
This review summarizes the data to support a link between oxidative stress and anxiety. While all of the data demonstrate that there is a link between oxidative stress and high-anxiety-related behavior, a cause-effect relationship has yet to be completely established. Some of these studies suggest that oxidative stress causes anxiety-related behaviors but do not explain the underlying mechanisms. While there are some limits in the approach to establish the anxiogenic effect of oxidative stress, the available data are consistent this causal relationship. The potential causal role of oxidative stress on anxiety may generate interest in antioxidants. Masood et al.29 were able to show that oxidative stress-related anxiety can be reversed in mice upon inhibition of NADPH oxidase or phosphodiesterase-2, enzyme that is indirectly implicated in oxidative stress mechanisms. Surprisingly, they found that diazepam, which is a well known anxiolytic, does not fully reverse the oxidative stress-related anxiety. These results point to a possible use for antioxidants in the prevention or reduction of high anxiety. Further research will be necessary to show whether anxious subjects need more antioxidants than non-anxious subjects. Recent work52,53 has shown that some dietary polyphenols have both anxiolytic and antioxidant effects, which may be beneficial to anxious subjects.
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: http://www.landesbioscience.com/journals/oximed/article/7944
References
- 1.Weinberger DR. Anxiety at the frontier of molecular medicine. N Engl J Med. 2001;344:1247–1249. doi: 10.1056/NEJM200104193441612. [DOI] [PubMed] [Google Scholar]
- 2.Leman S, Le Guisquet A, Belzung C. Liens anxiété-mémoire: Études expérimentales. In: Ferreri M, editor. Dans : “Anxiété, anxiolytiques et troubles cognitifs”. Paris: Elsevier; 2004. pp. 71–79. (Fre). [Google Scholar]
- 3.Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Lépine JP. The epidemiology of anxiety disorders: prevalence and societal costs. J Clin Psychiatry. 2002;63:4–8. [PubMed] [Google Scholar]
- 6.Alonso J, Lépine JP. Overview of key data from the European Study of Epidemiology of Mental Disorders (ESEMeD) J Clin Psychiatry. 2007;68:3–9. [PubMed] [Google Scholar]
- 7.ESEMed/MHEDEA 2000 investigators, author. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiat Scand. 2004;109:21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 8.Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe-a critical review and appraisal of 27 studies. Eur J Neuropsychopharmacol. 2005;15:357–376. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Tuthill A, Slawik H, O'Rahilly S, Finer N. Psychiatric co-morbidities in patients attending specialist obesity services in the UK. QJM: Monthly Journal of the Association of Physicians. 2006;99:317–325. doi: 10.1093/qjmed/hcl041. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 11.de Graaf R, Bijl RV, Smit F, Vollebergh WAM, Spiker J. Risk factors for 12-month comorbidity of mood, anxiety and substance use disorders: Findings from the Netherlands Mental Health Survey and Incidence Study. Am J Psychiatry. 2002;159:620–629. doi: 10.1176/appi.ajp.159.4.620. [DOI] [PubMed] [Google Scholar]
- 12.Issakidis C, Andrews G. Service utilisation for anxiety in an Australian community sample. Social Psychiatry and Psychiatric Epidemiology. 2002;37:153–163. doi: 10.1007/s001270200009. [DOI] [PubMed] [Google Scholar]
- 13.Kuloglu M, Atmaca M, Tezcan E, Gecici O, Ustundag B, Bulut S. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology. 2002;46:186–189. doi: 10.1159/000067810. [DOI] [PubMed] [Google Scholar]
- 14.Kuloglu M, Atmaca M, Tezcan E, Gecici O, Tunckol H, Ustundag B. Antioxidant enzyme activities and malondialdehyde levels in patients with obsessive-compulsive disorder. Neuropsychobiology. 2002;46:27–32. doi: 10.1159/000063573. [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Delattre J, Beaudeux JL, Bonnefont-Rousselot D. Radicaux libres et stress oxydant. Aspects biologiques et pathologiques. Éditions Médicales internationales. 2005:1–492. (Fre). [Google Scholar]
- 17.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxidative Medicine and Cellular Longevity. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008:1–26. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 20.Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Sigel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic neurochemistry: molecular, cellular and medical aspects. Philadelphia: Lippincott-Raven; 1999. pp. 637–669. [Google Scholar]
- 21.Lebel C. Oxygen radicals: Common mediators of neurotoxicity. Neurotox Teratol. 1991;13:341–346. doi: 10.1016/0892-0362(91)90081-7. [DOI] [PubMed] [Google Scholar]
- 22.Cardozo PF, Song S, Parthasarathy A, Hazzi C, Naidu K, Ramos RJ. Oxidative DNA damage in the aging mouse brain. Mov Disord. 1999;14:972–980. doi: 10.1002/1531-8257(199911)14:6<972::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 24.Yao JK, Reddy RD, Kammen DP. Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs. 2001;15:287–310. doi: 10.2165/00023210-200115040-00004. [DOI] [PubMed] [Google Scholar]
- 25.Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 26.Gingrich JA. Oxidative stress is the new stress. Nature medicine. 2005;11:1281–1282. doi: 10.1038/nm1205-1281. [DOI] [PubMed] [Google Scholar]
- 27.Belzung C, Berton F. Further pharmacological validation of the BALB/c ByJ neophobia in the free exploratory paradigm as an animal model of trait anxiety. Behav Pharmacol. 1997;8:541–548. doi: 10.1097/00008877-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 29.Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: Implications for novel therapeutics. Am J Med Genet C. 2008;148:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- 31.Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MNVR. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Splettstoesser WD, Schuff-Werner P. Oxidative stress in phagocytes-“The enemy within”. Microsc Res Techniq. 2002;57:441–445. doi: 10.1002/jemt.10098. [DOI] [PubMed] [Google Scholar]
- 33.Krömer SA, Keßler MS, Milfay D, Birg IN, Bunck M, Czibere L, Panhuysen M, Pütz B, Deussing JM, Holsboer F, Landgraf R, Turck CW. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci. 2005;25:4375–4384. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ditzen C, Jastorff AM, Keßler MS, Bunck M, Teplytska L, Erhardt A, Krömer SA, Varadarajulu J, Targosz BS, Sayan-Ayata EF, Holsboer F, Landgraf R, Rainer, Turck CW. Protein biomarkers in a mouse model of extremes in trait anxiety. Mol Cell Proteomics. 2006;5:1914–1920. doi: 10.1074/mcp.M600088-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Thornalley PJ. Unease on the role of glyoxalase 1 in high-anxiety-related behaviour. Trends Mol Med. 2006;12:195–199. doi: 10.1016/j.molmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Thornalley PJ. Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification—A role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 37.Thornalley PJ. The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1996;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol. 2007;564:146–149. doi: 10.1016/j.ejphar.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 39.Carini M, Giancarlo A, Piccone M, Facino RM. Fluorescent probes as markers of oxidative stress in keratinocyte cell lines following UVB exposure. Il Farmaco. 2000;55:526–534. doi: 10.1016/s0014-827x(00)00037-9. [DOI] [PubMed] [Google Scholar]
- 40.Rota C, Chignell CF, Mason R. Evidence for free radical formation during the oxidation of 2′,7′-dichlorofluorescin to the fluorescent dye 2′,7′-dichlorofluorescein by horoserdish peroxidase: Possible implication for oxidative stress measurements. Free Radical Biol Med. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 41.Rammal H, Bouayed J, Younos C, Soulimani R. The impact of high anxiety levels on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharmacol. 2008;589:173–175. doi: 10.1016/j.ejphar.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 42.Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008;22:1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Yasunari K, Matsui T, Maeda K, Nakamura M, Watanabe T, Kiriike N. Anxiety-induced plasma norepinephrine augmentation increases reactive oxygen species formation by monocytes in essential hypertension. Am J Hypertens. 2006;19:573–578. doi: 10.1016/j.amjhyper.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 44.Amicarelli F, Colafarina S, Cattani F, Cimini A, Di Ilio C, Ceru MP, et al. Scavenging system efficiency is crucial for cell resistance to ROS-mediated methylglyoxal injury. Free Radical Bio Med. 2003;35:856–871. doi: 10.1016/s0891-5849(03)00438-6. [DOI] [PubMed] [Google Scholar]
- 45.Halliwell B. Phagocyte-derived reactive species: Salvation or suicide? Trends Biochem Sci. 2006;31:509–515. doi: 10.1016/j.tibs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Lohinai Z, Benedek P, Fehér F, Györfi A, Rosivall L, Fazekas A, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Brit J Pharmacol. 1998;123:353–360. doi: 10.1038/sj.bjp.0701604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desrumaux C, Risold PY, Schroeder H, Deckert V, Masson D, Athias A, et al. Phospholipid transfer protein (PLTP) deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J. 2005;19:296–297. doi: 10.1096/fj.04-2400fje. [DOI] [PubMed] [Google Scholar]
- 48.Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sciences. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Berry A, Capone F, Giorgio M, Pelicci PG, de Kloet ER, Alleva E, et al. Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp Gerontol. 2007;42:37–45. doi: 10.1016/j.exger.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann NY Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 51.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 52.Vignes M, Maurice T, Lanté F, Nedjar M, Thethi K, Guiramand J, Récasens M. Anxiolytic properties of green tea polyphenol (-)-epigallocatechin gallate (EGCG) Brain Res. 2006;1110:102–115. doi: 10.1016/j.brainres.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 53.Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci. 2007;262:77–84. doi: 10.1016/j.jns.2007.06.028. [DOI] [PubMed] [Google Scholar]