Abstract
In order to demonstrate whether the known biological effects of Aloe vera (L.) Burm. fil. could correlate with the antioxidant activity of the plant, the antioxidant activity of the aqueous leaf extract was investigated. The present study demonstrated that the aqueous extract from A. vera leaves contained naturally occuring antioxidant components, including total phenols, flavonoids, ascorbic acid, β-carotene and α-tocopherol. The extract exhibited inhibitory capacity against Fe3+/ascorbic acid induced phosphatidylcholine liposome oxidation, scavenged stable DPPH•, ABTS•+ and superoxide anion radicals, and acted as reductant. In contrast, the leaf inner gel did not show any antioxidant activity. It was concluded that the known beneficial effects of Aloe vera could be attributed to its antioxidant activity and could be related to the presence of phenolic compounds and antioxidant vitamins.
Key words: Aloe vera, aqueous extract, antioxidant activity, antioxidant vitamins
Introduction
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and antioxidant defense and may lead to oxidative damage. Excess production of ROS, and other radicals have been implicated as inducers of tissue injury in several pathological conditions including cancer, cardiovascular diseases, atherosclerosis, neurodegenerative diseases such as Parkinson disease and Alzheimer dementias, diabetes, ischemic reperfusion injuries, rheumatoid arthritis and even the process of aging.1 However, humans have evolved highly complex antioxidant protection mechanisms that function interactively and synergically to neutralize free radicals. These include antioxidant enzymes that catalyse free radical quenching reactions, metal binding proteins that sequester free iron and copper ions, capable of catalysing oxidative reactions and diet-derived antioxidants like ascorbic acid, vitamin E, carotenoids, polyphenols and other low molecular weight compounds such as α-lipoic acid.2
Many different plant material have recently become a major interest of scientific research as a result of naturally occuring antioxidants, which may protect cell constituents against oxidative damage and therefore, limit the risk of various degenerative diseases associated to oxidative stress.3 Referred to as a miracoulous plant, Aloe vera (L.) Burm. fil., member of Aloaceae family (formerly member of Liliaceae), possesses many pharmacological activities, including antiinflammatory,4–6 immunostimulant,7,8 wound healing,9–11 antiulcer,12 antidiabetic13–17 and antitumor,18,19 in which the mediation of ROS levels could be involved. The use of and research on this plant have been described in well-referenced reviews20,21 and books.22,23 The multiplicity of the biological activities of A. vera has been attributed to the variety of its chemical components including anthraquinones, glycoproteins, polysaccharides, vitamins and enzymes.24,25 The substances responsible for the biological and antioxidant activity of the plant are under investigation although some resarchers claim that the synergistic relationship between the elements could be useful for maintaining the integrity of the antioxidant status26 or the antitumor effect.27 The extracts used in most studies constituted the gel portion of the leaves.
In order to continue on investigating the therapeutic properties of the leaves of A. vera, and assuming that its therapeutic benefit can be attributed to the antioxidant activity, we have decided to determine the antioxidant potential of the aqueous extract prepared from the leaves devoid of the inner gel part, in commonly accepted assays including TBA test using the lipid peroxidation of soybean phosphatidylcholine liposomes, Trolox equivalence antioxidant capacity, DPPH• and superoxide radical scavenging activities, and ferric ion reducing antioxidant power. Total phenolics, flavonoids, ascorbic acid, β-carotene and α-tocopherol contents were also determined. The antioxidant activity of the separated leaf gel was also estimated.
Phenolic substances known to be responsible for the antioxidant activity of plant extracts are mostly extracted by organic solvents, but as plants are commonly consumed as water extracts by people, the aqueous extract of A.vera leaves was preferably used in our investigation.
Results
Extract yield (amount of total extractable compounds) and contents of total phenolics, flavonoids, ascorbic acid, β-carotene and α-tocopherol.
The aqueous extract of A. vera prepared from 50 g leaves, gave a yield of 26.4 mg EC per gram FW. The aqueous extract was shown to contain 0.241 ± 0.014 mg/g FW phenolic compounds expressed as gallic acid equivalents and 0.245 ± 0.005 mg/g FW flavonoids expressed as catechin equivalents. Scavenging of different types of reactive oxygen and nitrogen species, mostly free radicals, is thought to be one of the main mechanisms of the antioxidant action exhibited by phenolic phytochemicals.28 Flavonoids are the most widespread polyphenolic compounds that occur ubiquitously in plant foods and structurally have variations in the C ring that characterizes the different types namely, flavonols, flavones, isoflavones, flavonones, flavanols and anthocyanins. One of the prominent and medically most useful properties of many flavonoids is their excellent radical scavenging ability which is also a valuable aspect for their therapeutic and prophylactic applications, e.g., after infection, inflammation, burns or radiation injury.29
It was observed that the amount of flavonoids in the analysed plant extract showed high correlation (r2 = 0.9939) with the total amount of phenolics. The flavonoid content values found in this work were lower than those of two (3.63 ± 0.38 mg/g), three (4.70 ± 0.48 mg/g) and four-year old (4.26 ± 0.18 mg/g) A. vera30 which may be explained by climatic conditions of the region.
The ratio of total phenolic compounds to the total extractable compounds was average 0.9%. This means that average 99.1% of the total extractable compounds were other than phenolic compounds.
Our results, in agreement with literature,24 demonstrated that A. vera leaves contain not only phenolic compounds and flavonoids but are also a good source of some of components able to contribute to the antioxidant activity, such as ascorbic acid (0.422 ± 0.029 mg/g FW), β-carotene (15.51 ± 2.39 µg/g FW) and α-tocopherol (1.47 ± 0.11 µg/g FW).
Ascorbic acid, α-tocopherol and carotenoids also play important roles in preventing free radical damage. Ascorbic acid is one of the most important water-soluble antioxidants in cells, efficiently scavenging reactive oxygen species such as superoxide, hydroxyl radicals and singlet oxygen, also vitamin C was shown to act as a chain breaking scavenger for peroxy radicals and act as synergist with vitamin E. The vitamin C can regenerate α-tocopherol from the tocopherol radical, formed from reaction with other radicals.31 α-Tocopherol is the most important lipid soluble chain-breaking antioxidant in humans, localized within the phospholipid bilayer of cell membranes to protect them against biological lipid peroxidation. It is able to repair oxidizing radicals directly, preventing the chain propagation step during lipid autoxidation. Thus both vitamin C and α-tocopherol seem to contribute to minimizing the consenquences of lipid peroxidation in membranes.32 Carotenoids are very powerful antioxidant agents involved in the scavenging of two of the reactive oxygen species, singlet molecular oxygen and peroxy radicals. A number of epidemiological studies have revealed that an increased consumption of a diet rich in ascorbic acid, α-tocopherol and carotenoids is correlated with a diminished risk for several degenerative disorders, including various types of cancer, cardiovascular or ophthalmological diseases.33,34 Consequently, when relating the antioxidant activity of the extract to desease prevention and health, it is important to consider the contribution of ascorbic acid, α-tocopherol and carotenoids in addition to that of phenolic compounds with antioxidant activity.
Antioxidant activity on liposome peroxidation.
Membrane lipids are particularly susceptible to oxidation. Free radicals are known to attack the highly unsaturated fatty acids of membrane systems to induce lipid peroxidation, which is an autocatalytic process and may cause peroxidative tissue damage. Lipid peroxidation is measured as an index of ROS damage.35
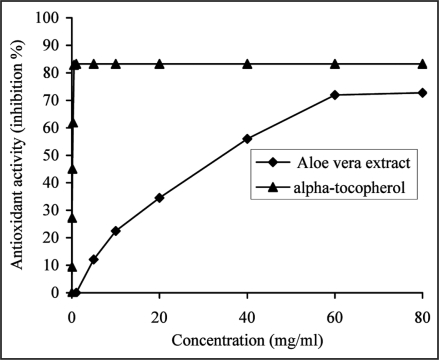
As shown in Figure 1, the antioxidant activity was concentration-dependent for A. vera extract and α-tocopherol. At 60 mg/ml, A. vera extract showed marked antioxidant activity of 71.91 ± 0.65%, however the extract was significantly less effective (p < 0.05) than α-tocopherol, which exhibited an antioxidant activity of 82.90 ± 0.56% at a concentration as low as 0.5 mg/ml.
Figure 1.
The ability of the aqueous extract from Aloe vera leaf and α-tocopherol, used as a reference antioxidant to inhibit Fe3+/ascorbic acid induced peroxidation in liposomes was estimated as a function of increasing extract (from 5 to 60 mg/ml) and α-tocopherol (from 0.031 to 0.5 mg/ml) concentrations and by recording the absorbance at 532 nm. The results were expressed as a mean percent inhibiton on MDA formation by extract (from 12.0 ± 1.38 to 71.9 ± 0.65%) and α-tocopherol (from 9.4 ± 0.76 to 82.9 ± 0.56%), derived from three separate assays. The extract (at 60 mg/ml) was significantly less effective (p < 0.05) than α-tocopherol (at 0.5 mg/ml).
Based on the EC50 values, the antioxidant activity of A. vera extract was significantly lower (p < 0.05) with an EC50 value of 41.74 ± 0.18 mg/ml in comparison with α-tocopherol (0.30 ± 0.01 mg/ml). Although the inhibitory effect of the extract on MDA formation was less than that of α-tocopherol, A. vera may also be expected to protect against damage to cell membranes, because it also reduces the level of peroxides.
MDA formed from the breakdown of polyunsaturated fatty acids, serves as a convenient index to determine the extent of lipid peroxidation. At 0.5 mg/ml α-tocopherol showed 0.91 ± 0.07 nmol/g lecithin MDA, whereas the effect of the extract was not so strong (1.98 ± 0.03 nmol/g lecithin MDA at 60 mg/ml). However, the formation of MDA for the control was 6.99 ± 0.18 nmol/g lecithin. It is evident that addition of the extract or α-tocopherol strongly inhibits lipid peroxidation as shown by the low accumulation of oxidative products.
Antioxidant activity increased proportionally to the polyphenol and flavonoid contents: a linear relationship between antioxidant activity values and total polyphenol (r2 = 0.9998) and flavonoid (r2 = 0.9956) contents was established. This indicated that the antioxidant activity of A. vera extract on Fe3+/ascorbate induced lipid peroxidation might be dependent on the chemical composition of the extract containing mainly the phenolics and flavonoids.
Total radical antioxidant potential (TRAP).
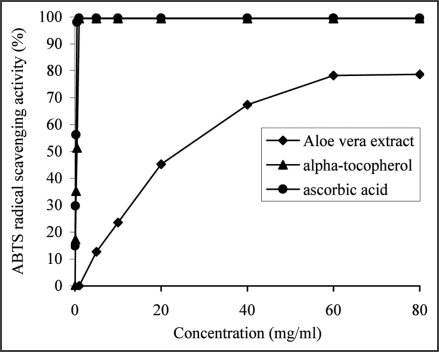
It was found that the extract was able to scavenge ABTS•+ radical cation in a dose dependent manner (Fig. 2) and its antioxidant activitity (78.22 ± 0.85%) at 60 mg/ml was significantly lower than that of ascorbic acid (98.03 ± 0.64%) at 0.5 mg/ml and α-tocopherol (99.41 ± 0.45%) at 1 mg/ml.
Figure 2.
The ability of the aqueous extract from Aloe vera leaf or α-tocopherol and ascorbic acid, used as reference antioxidants to scavenge ABTS*+ was estimated as a function of increasing extract (from 5 to 60 mg/ml) or α-tocopherol (from 0.125 to 1 mg/ml) and ascorbic acid (from 0.0625 to 0.5 mg/ml) concentrations and by recording the decrease in absorbance at 734 nm. The results were expressed as a mean percent scavenging activity of the extract (from 12.6 ± 0.96 to 78.2 ± 0.85%), α-tocopherol (from 17.2 ± 0.43 to 99.4 ± 0.45%) and ascorbic acid (from 14.8 ± 0.78 to 98.03 ± 0.64%), derived from three separate assays. The extract (at 60 mg/ml) was significantly less effective (p < 0.05) than α-tocopherol (at 1 mg/ml) and ascorbic acid (at 0.5 mg/ml).
As compared by the EC50 values, A. vera extract (34.77 ± 0.41 mg/ml) showed significantly less (p < 0.05) ABTS cation radical scavenging activity than ascorbic acid (0.276 ± 0.0028 mg/ml) and α-tocopherol (0.533 ± 0.0021 mg/ml).
The antioxidant activity of the extract was expressed as Trolox equivalent, the concentration of Trolox having an antioxidant capacity equivalent to 1 mM of the substances under investigation. The extract exhibited an ABTS cation radical scavenging activity of 1.66 ± 0.017 mM/L Trolox equivalents at a concentration of 60 mg/ml or 1.21 ± 0.020 mM of Trolox per gram of FW, which corresponded to a high phenolic (r2 = 0.9904) and flavonoid (r2 = 0.9691) contents. This value was significantly lower (p < 0.05) than that of ascorbic acid (2.09 ± 0.011 mM/L) at 0.5 mg/ml and α-tocopherol (2.12 ± 0.010 mM/L) at 1 mg/ml.
DPPH radical scavenging activity.
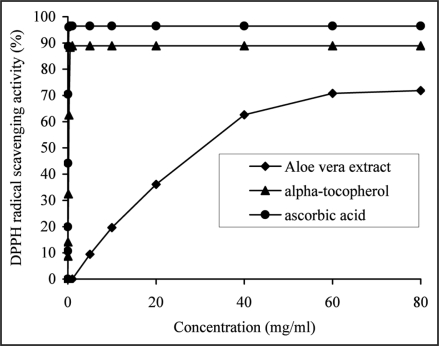
The scavenging activity of the extract and reference antioxidants (ascorbic acid or α-tocopherol) against DPPH• increased in a dose dependent manner (Fig. 3). The extract showed high DPPH radical scavenging activity of 70.81 ± 0.27% at 60 mg/ml. This value was comparable with the results reported for two (62.70 ± 0.44%), three (72.19 ± 0.98%) and four-year-old (67.64 ± 2.99%) A. vera.30 However, when compared to reference antioxidants, ascorbic acid (96.01 ± 0.37%) at 0.25 mg/ml and α-tocopherol (88.31 ± 1.81%) at 0.5 mg/ml, the extract showed significantly lower (p < 0.05) DPPH radical scavenging activity.
Figure 3.
The ability of the aqueous extract from Aloe vera leaf or α-tocopherol and ascorbic acid, used as reference antioxidants to scavenge DPPH• was estimated as a function of increasing extract (from 5 to 60 mg/ml) or α-tocopherol (from 0.031 to 0.5 mg/ml) and ascorbic acid (from 0.008 to 0.25 mg/ml) concentrations and by recording the decrease in absorbance at 517 nm. The results were expressed as a mean percent DPPH scavenging activity of the extract (from 6.4 ± 0.84 to 70.8 ± 0.27%), α-tocopherol (from 8.7 ± 1.06 to 88.3 ± 1.81%) and ascorbic acid (from 10.6 ± 1.74 to 96.01 ± 0.37%), derived from three separate assays. The extract (at 60 mg/ml) was significantly less effective (p < 0.05) than α-tocopherol (at 0.5 mg/ml) and ascorbic acid (at 0.25 mg/ml).
The DPPH radical scavenging activity of the extract, expressed in the term of EC50 was 41.81 ± 0.55 mg/ml and significantly different (p < 0.05) from the EC50 values obtained for the reference antioxidants, ascorbic acid (69.22 ± 2.65 µg/ml) and α-tocopherol (341.02 ± 1.05 µg/ml).
The antioxidant activity in DPPH• assay was highly correlated with phenolic (r2 = 0.9999) and flavonoid (r2 = 0.9927) contents. This suggested that scavenging effect of A. vera extract may depend on hydrogen atom donation by the different phenolic and flavonoid compounds, and their hydrogen donor capacity, most probably accounts in large part for the antioxidant activity and may provide a basis for the pharmacological activity and therapeutic applications of this extract.
A. vera gel extract did not show any DPPH radical scavenging activity.
Superoxide radical scavenging activity.
Many enzymatic and autoxidation reactions within the body produce the superoxide anion radical, by addition of an electron to molecular oxygen. Superoxide is poorly reactive but can take part in further reactions leading to the formation of more reactive oxygen species, contributing to tissue damage and various diseases.33 Therefore, the study of the scavenging effects of A. vera leaf extract on superoxide anion is one of the most important ways of illustrating the mechanism of antioxidant activity.
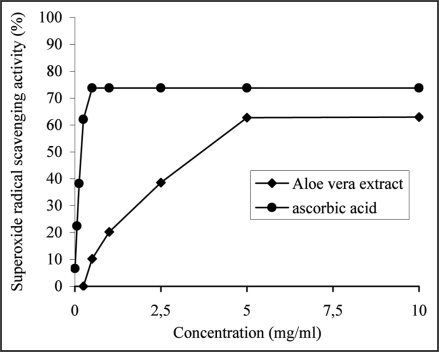
The extract was found to be an efficient scavenger of superoxide radical generated in xanthine/xanthine oxidase system in vitro and its activity of 62.72 ± 2.06% at 5 mg/ml was comparable (p > 0.05) to that of ascorbic acid (62.05 ± 1.44%) at 0.5 mg/ml. Figure 4 shows that both the extract and ascorbic acid exhibited dose-dependent inhibition on superoxide radical. α-Tocopherol did not show any detectable superoxide radical scavenging activity.
Figure 4.
The ability of the aqueous extract from Aloe vera leaves and ascorbic acid, used as a reference antioxidant to inhibit the generation of superoxide radical by xanthine/xanthine oxidase system was estimated as a function of increasing extract (from 0.5 to 5 mg/ml) and ascorbic acid (from 0.05 to 0.25 mg/ml) concentration and by recording the decrease in absorbance at 560 nm. The results were expresed as percent inhibiton of NBT reduction by the extract (from 10.2 ± 1.33 to 62.72 ± 2.06%) and ascorbic acid (from 6.63 ± 1.23 to 62.06 ± 1.44%), derived from three separate assays. No difference was observed between the superoxide radical scavenging activities of the extract at 5 mg/ml and ascorbic acid at 0.25 mg/ml, p > 0.05.
Based on the EC50 values, the superoxide radical scavenging activity of A. vera extract was significantly lower (p < 0.05) with an EC50 value of 5.76 ± 0.19 mg/ml in comparison with ascorbic acid (EC50 = 0.389 ± 0.010 mg/ml).
Ferric reducing antioxidant power (FRAP).
The FRAP value reflects the reducing power of the extract. In the FRAP assay, the extract revealed the ability to reduce Fe3+ to Fe2+. The FRAP value of the extract (1.02 ± 0.003 mM/L of Fe2+ at a concentration of 60 mg/ml or 1.14 ± 0.041 mM of Fe2+ per gram of FW) was found to be significantly (p < 0.05) below those of ascorbic acid (1.84 ± 0.011 mM/L Fe2+) at 0.5 mg/ml and α-tocopherol (1.81 ± 0.01 mM/L Fe2+) at 1 mg/ml.
The reducing power was highly correlated with phenolic (r2 = 0.9918) and flavonoid (r2 = 0.9717) contents. The antioxidant activity (r2 = 0.9955), DPPH (r2 = 0.9857), ABTS (r2 = 0.9916) radical scavenging activities were correlated with the ferric reducing antioxidant capacity of the extract. These results revealed that the antioxidant activity of the extract might also be due to its redox properties, which allowed the extract to act as reducing agent or hydrogen-atom donor and free radical scavenger.
The antioxidant activity of the aqueous extract of A. vera leaves, measured in a phosphatidylcholine liposome system was significantly correlated with its scavenging activity on ABTS•+ (r2 = 0.9835) and DPPH• (r2 = 0.9860) radicals, indicating that the antioxidant activities measured by the three methods complemented each other.
Discussion
Because antioxidant nutrients such as vitamin C, vitamin E, carotenoids and numerous polyphenolic compounds directly scavenge reactive oxidants, they are hypothesized to constitute a vital endogenous defense against oxidative cell and tissue injury. Therefore, the natural antioxidants contained in a plant may contribute to the antioxidant activity and thus towards the total or partial alleviation of some clinical disorders. A medicinal plant removing ROS is the most effective defense of a living body against disease.
In addition to its own stores of antioxidants, A. vera may also activate the body's endogenous antioxidant enzyme systems and play a vital role in the prevention and control of numerous diseases, especially those related to aging, such as cancer, coronary vascular disease and diabetes, as well as immune system enhancers and in the management of oxidative stress. Several papers have shown the beneficial effect of A. vera administration both in vitro and in vivo. It was reported that internal administration of Aloe leaf extract elevates liver levels of cellular antioxidant enzymes in mice36 and shows by this way chemopreventive effect on experimentally induced skin carcinogenesis37 or protection against arsenic toxicity.38
There is strong evidence to suggest that oxidative stress may play a role in the pathogenesis of both Type 1 and Type 2 DM. Although autoimmune disease appears to play a predominant role in the pathogenesis of Type 1 DM, it is widely known that chemical agents, such as alloxan and streptozotocin, which can induce insulin deficiency (Type 1 DM) by the destruction of pancreatic β-cells, act through a mechanism that involves free radical generation. It has also been shown that hyperglycaemia can cause oxidative stress in cells through different pathways that include enchanced production of toxic peroxides, glucose autoxidation and generation of increased levels of fructose and sorbitol.39 Okyar et al.16 reported that A. vera leaf pulp extract was effective in reducing blood sugar, suggesting that it might be useful in the scavenging of free radicals. It was reported that treatment with A. vera increased antioxidant enzymes and significantly reduced lipid peroxidation products in streptozotocin-induced diabetic rats, showing the relationship between antioxidant activity and the onset of diabetes.40–43 In the present study the strong antioxidative effect of the extract was manifested as an inhibition of peroxidation of liposomes. The findings showed that A. vera extract caused reduction of the MDA levels suggesting that prooxidative damage may decrease with the administration of the plant extract. A. vera extracts of different growing stages of the plant were reported to exhibit strong inhibition activity on lipid oxidation.30 Yagi et al.44 reported that isorabaichromone from A. vera showed antioxidative activity against microsomal and non-enzymatic mitochondrial lipid peroxidation.
The synthetic nitrogen-centered ABTS•+ and DPPH• radicals are not biologically relevant, but are often used as “indicator compounds” in testing hydrogen-donation capacity and thus antioxidant activity. Reactions are only likely to be significant with good H-atom donors, a necessary requirement for antioxidant function in biological systems. The extract was found to possess high antioxidant activity as determined by the ability to scavenge the nitrogen centered ABTS•+. Our results were of very good agreement with TEAC values of the extracts of leaf skin and leaf gel of Aloe vera.45
Aloe extract was found to be effective in scavenging the stable DPPH• radicals. It was interesting to note that the effective extract concentration at which the DPPH• radicals were scavenged by 50% was almost identical to the EC50 value of the extract found to inhibit Fe3+/ascorbic acid induced peroxidation in liposomes. The closeness of these two concentrations indicates that the extract is equally effective in inhibiting oxidative process and scavenging stable free radicals. These results confirm the radical scavenging activity found in the TEAC assay. A. arborescens leaf skin extract was found effective in inhibiting the destruction of pancreatic islets by streptozotocin and alloxan and this effect was attributed to its DPPH radical scavenging activity.46
In order to study the ability of the extract to scavenge O2•− in vitro, xanthine/xanthine oxidase system was used. Xanthine oxidase, implicated in ischaemia/reperfusion injury in vivo47 was used as a generator of O2•−. In the present study Aloe extract showed good potency as a superoxide scavenger, however, the effect of the extract on superoxide anion formation was not similar to the one obtained on lipid peroxidation. This difference may be due to the different mechanisms of production of oxidative stress in these two methods. EC50 value, in scavenging ability on superoxide radicals, was lower than the corresponding value generated in the assay using the lipid peroxidation of soybean phosphatidylcholine liposomes, suggesting that the extract was better at scavenging superoxide anion than at inhibiting oxidative degradation. As the extract was shown to efficiently scavenge O2•−, it may be effective in reducing the extent of ischemia-reperfusion damage.
Activated phagocytic cells such as neutrophils, monocytes, macrophages and eosinophils, generate superoxide as part of their response to foreign organisms or particles. In chronic inflammation, this normal protective mechanism may become damaging.33 The role of ROS in inflammation where oxygen metabolites are thought to mediate tissue injury, is clearly demonstrated by the antiinflammatory effects of the antioxidants. The wound healing effect of A. vera is thought to be related to its antioxidant properties.11
Inflammatory cells may also increase DNA damage by activating pro-carcinogens to DNα-damaging species by ROS-dependent mechanisms. Most cancers can be considered as degenerative diseases of old age, related to the effects of continuous damage over a life span by toxic oxygen. Thus, tumor promotion can be inhibited in animal models by the use of agents that inhibit the phagocyte respiratory burst.48
As results from the literature, the antioxidant properties of the dried flower49 or gel extracts50 of Aloe species were mainly connected with the presence of phenolic substances, like aloesin derivatives44,51 anthraquinones,52 and polysaccharides53,54 and many of these phenolic compounds have been shown to be tumor protective by reducing oxidative stress thereby giving a solid basis to the proposal that the antioxidant content of A. vera could account for its antitumor properties.27 Indeed in recent studies, one of the main phenolic substances present in A. vera: aloin55 and aloe-emodin56 were suggested to show their antitumor effects via apoptotic cell death mediated by their antioxidative effect.
The antioxidant activity of A. vera leaf extract may be also due to its redox properties, which can play an important role in neutralizing free radicals or decomposing peroxides. Transition metals accelerate lipid oxidation reactions by hydrogen abstraction and peroxide decomposition, resulting in the formation of free radicals.
According to the results presented in this study, termination of free radical reaction, reducing power, quenching of DPPH•, ABTS•+ and superoxide radicals were suggested to be, in part, responsible for the antioxidant activity of the aqueous extract from A. vera leaves devoid of the inner gel. This is of pharmacological relevance because these events are implicated in several pathological processes related to oxidative stress, mentioned above. It is suggested that the antioxidant activity of the A. vera extract, could be the cause of the health promoting potential of the plant and that activity could be due to the synergistic effect of antioxidant vitamins and phenolic substances. The fact that the gel portion of the leaves frequently used for its wound healing effect, due to the polysaccharides and glycoproteins, did not exhibit antioxidant activity, tends to propose that the effect of polysaccharides in antioxidant activity could be negligible.
Materials and Methods
Plant material.
Specimens of Aloe vera (L.) Burm. fil. (in Turkish Sarisabir) were collected from Kale (Demre) in Antalya, identified by Prof. Dr. Nurhayat Sütlüpinar (May 1993; a voucher specimen was deposited in the Herbarium of the Faculty of Pharmacy, ISTE No. 65118). Since that time the plant was cultivated in the greenhouse of the Istanbul University, Faculty of Sciences, Department of Botany. The fresh leaves of this cultivated plant were used in the study.
Preparation of A. vera leaf extract.
Freshly chopped A. vera leaves, were washed and cut from the middle, the gel was separated by scraping with a spoon, homogenized in a Waring blender, lyophylized (5.05 g) and conserved at −20°C. The remaining leaves (50 g) were cut in small pieces and the aqueous leaf extract was prepared by heating the leaves in a flask with 250 ml distilled water for 1 h. After cooling the mixture was filtered through cloth and the filtrate was concentrated by means of a rotary evaporator. The dark-brown solid residue weighing 1.32 g (2.64% w/w) was preserved at −20°C till further utilization.
Determination of antioxidant components.
Phenolic compounds in the aqueous leaf extract of A. vera were estimated by a colorimetric assay, based on a procedure described by Slinkard and Singleton57 with some modifications. Aliquots (0.1 ml) of the extract (10–60 mg/ml) were transferred into the test tubes and their volumes made up to 4.6 ml with distilled water. After addition of 0.1 ml Folin-Ciocalteu reagent (previously diluted 3-fold with distilled water) and 0.3 ml 2% aqueous sodium carbonate solution, tubes were vortexed and the absorbance of the blue colored mixture recorded after 2 h at 760 nm, against a blank containing 0.1 ml of the extraction solvent. Gallic acid (0.05–0.4 mg/ml) was used for calibration of a standard curve. The results were expressed as GAE/g of FW. Data were presented as the average of triplicate analyses.
Total flavonoid content was determined by using a colorimetric method described by Sakanaka et al.58 Briefly, 0.25 ml of the extract (10–60 mg/ml) or (+)-catechin standard solution (15–250 µg/ml) was mixed with 1.25 ml of distilled water in a test tube, followed by addition of 75 µl of a 5% sodium nitrite solution. After 6 min, 150 µl of a 10% aluminium chloride solution was added and the mixture was allowed to stand for a further 5 min before 0.5 ml of 1 M sodium hydroxide was added. The mixture was brought to 2.5 ml with distilled water and mixed well. The absorbance was measured immediately at 510 nm. The results were expressed as means (± SD) mg of CA per g of FW.
Ascorbic asid was determined according to the method of Omaye et al.59 25 mg A. vera leaf extract was extracted with 5 ml 1% metaphosphoric acid for 45 min at room temperature and filtered through Whatman No. 4 filter paper. The filtrate (2 ml) was mixed with 1 ml of 2,6-dichlorophenol-indophenol sodium and the absorbance was measured within 15 seconds at 520 nm against a blank. Content of ascorbic acid was calculated on the basis of the calibration curve of L-ascorbic acid (0–80 µg/ml). The assays were carried out in triplicate. The results were mean values ± SD and expressed as mg of ascorbic acid/g FW.
β-Carotene was determined according to the method of Nagata and Yamashita.60 The extract (25 mg) was vigorously shaken with 10 ml af acetone-hexane mixture (4:6) and filtered through Whatman No. 4 filter paper. The absorbance of the filtrate was measured at 453, 505 and 663 nm. The assays were carried out in triplicate. The results were mean values ± SD and expressed as µg of carotenoid/g of FW.
The content of β-carotene was calculated according to the following equation:
β-carotene (mg/100 ml) = 0.216 A663 − 0.304 A505 + 0.452 A453
The content of α-tocopherol was estimated by the method of Desai.61 The extract (25 mg) was suspended in 3 ml of 2% pyrogallol in 95% ethanol and 1 ml of 10 N aqueous potassium hydroxide solution, and the resulting mixture was saponified at 70°C for 30 min. The tubes were immediately cooled in an ice bath, 1 ml of distilled water was added and the mixture was extracted with 3 ml of n-hexan. The tubes were shaken vigorously for 2 min and centrifuged at 3,000 rpm for 10 min to separate the phases. The organic layer was evaporated to dryness under a gentle stream of nitrogen. The residue was redissolved in 1 ml of absolute alcohol. To this 0.2 ml of 0.2% bathophenanthroline was added. The assay mixture was protected from light and 0.2 ml of 0.001 M ferric chloride was added followed by 0.2 ml of 0.0001 M o-phosphoric acid. The color developped was read at 530 nm. The content of α-tocopherol was calculated on the basis of the calibration curve of α-tocopherol (1.25–20 µg/ml). The assays were carried out in triplicate. The results were mean values ± SD and expressed as µg of α-tocopherol/g of FW.
Antioxidant activity on liposome peroxidation.
Phospholipids are regarded as valuable substrates for the appraisal of potential food antioxidants and are considered to be ideal models for the study of dietary components and drugs on membrane lipid peroxidation.62
Lipid peroxidation assay was based on the method described by Duh et al.63 Lecithin (300 mg) was suspended in 30 ml phosphate buffer (10 mmol/L, pH 7.4). This suspension was then sonicated with a rod using an ultrasonic homogenizer (Bandelin, Berlin, Germany) at 30 second intervals for 10 min until an opalescent suspension was obtained.
The sonicated solution (10 mg/ml), FeCl3, ascorbic acid and the plant extract (5–60 mg/ml) or α-tocopherol (0.31–0.625 mg/ml) used as a reference antioxidant were mixed to produce a final concentration of 3.08 mg liposome/ml, 123.2 µmol FeCl3 and 123.2 µmol ascorbic acid. After 1 h incubation at 37°C, the formation of lipid peroxidation products was assayed by the measurement of MDA levels on the basis that MDA reacted with TBA at 532 nm according to Buege and Aust.64 Briefly, 500 µl of this reaction mixture was mixed with 1,000 µl TCα-TBA reagent (consisting of 15% w/v TCA and 0.375% TBA in 0.25 N HCl) and 14 µl BHT (2% in absolute ethanol). The mixture was vortexed and heated for 20 min in a boiling water bath. After cooling, an equal volume of n-butanol was added and the mixture was shaken vigorously. The n-butanol layer was separated by centrifugation at 3,000 rpm for 10 min. The absorbance of the sample was read at 532 nm against a blank which contained all reagents except lecithin. The values of TBARS were calculated from a standard curve prepared with TEP (1–32 µmol) and expressed as nmol/g of lecithin. The percentage inhibition of lipid peroxidation was calculated by comparing the results of the samples with those of controls not treated with the extract using the following equation:
Inhibition effect (%) = [1 − (Absorbance of sample at 532 nm/Absorbance of control at 532 nm)] × 100.
Total radical antioxidant potential (TRAP).
The total radical antioxidant potential of the extract was measured using TEAC assay as described by Re et al.65 with minor modifications. The TEAC value is based on the ability of the antioxidant molecules to quench the long-lived ABTS•+ cation radical, a blue-green chromophore with characteristic absorption at 734 nm, compared to that of Trolox, a water-soluble vitamin E analogue and reflects the electron-donating ability of the antioxidants.
After addition of 990 µl of ABTS•+ solution to 10 µl of A. vera extract (5–60 mg/ml) or ascorbic acid (0.0625–1 mg/ml), α-tocopherol (0.125–1 mg/ml) or Trolox standards (final concentration 0–20 µM in ethanol), the decrease in absorbance at 734 nm was monitored exactly 6 min after the initial mixing. Appropriate ethanol blanks were run in each assay. The ability to scavenge ABTS•+ radical was calculated by the following equation:
ABTS radical scavenging activity (%) = [1 − (Absorbance of sample at 734 nm/Absorbance of control at 734 nm)] × 100.
DPPH radical scavenging activity.
To determine the hydrogen-donating ability of the extract a method based on the reduction of a methanolic solution of the colored free radical DPPH to the non-radical form was used.66 A. vera leaf extract or gel extract solution (0.1 ml) in methanol at different concentrations (5–40 mg/ml), ascorbic acid (0.008–0.25 mg/ml) or α-tocopherol (0.031–0.5 mg/ml) were added to 3.9 ml of 6 × 10−5 M methanolic solution of DPPH•. The mixture was shaken vigorously and allowed to stand in the dark at room temperature for 30 min. The decrease in absorbance of the resulting solution was then measured spectrophotometrically at 517 nm against methanol. Two controls were used for this test, a negative control (containing all reagents except the test sample) and positive controls (using the reference antioxidants: ascorbic acid or α-tocopherol) for comparison. The ability to scavenge DPPH• radical was calculated by the following equation:
DPPH radical scavenging activity (%) = [1 − (Absorbance of sample at 517 nm/Absorbance of control at 517 nm)] × 100.
Superoxide radical scavenging activity.
Superoxide radical scavenging activity was assayed by using a colorimetric assay in which xanthine oxidase serves as a free radical generator and causes the reduction of NBT.67 Antioxidant inhibits the reduction of NBT by scavenging the free radicals generated by xanthine oxidase.
Reaction mixtures contained, in a final volume of 3 ml, 100 µl A. vera extract (0.625–5 mg/ml) or ascorbic acid (0.04–0.625 mg/ml), 100 µl 30 mM EDTA, 10 µl 30 mM xanthine in 50 mM KOH, 100 µl of 3 mM NBT and 50 mM (final concentration) KH2PO4-KOH buffer, pH 7.4. The reaction was started by adding 100 µl of xanthine oxidase (freshly diluted in the above phosphate buffer to give one unit of enzyme activity per ml) and the rate of NBT reduction was measured at 560 nm. The positive and negative controls were subjected to the same procedures as the sample, except that for the negative control, only the solvent was added, and for the positive control, sample was replaced with ascorbic acid or α-tocopherol. The abilities to scavenge the superoxide radical were calculated using the following equation:
Superoxide radical scavenging activity (%) = [1 − (Absorbance of sample at 560 nm/Absorbance of control at 560 nm)] × 100.
Ferric reducing antioxidant power (FRAP).
Antioxidant potential of the extract was also estimated from its ability to reduce Fe(III)-TPTZ complex to Fe(II)-TPTZ, the resulting intense blue color being linearly related to the amount of reductant (antioxidant) present.
The FRAP assay was carried out according to the procedure of Benzie and Strain.68 The FRAP reagent contained 2.5 ml of 10 mM TPTZ solution in 40 mM HCl plus 2.5 ml of 20 mM FeCl3.6H2O and 25 ml of 0.3 M acetate buffer, pH 3.6. FRAP reagent (900 µl), prepared freshly and incubated at 37°C, was mixed with 90 µl of distilled water and 30 µl of A. vera extract (2.5–80 mg/ml), ascorbic acid (0.031–0.5 mg/ml), α-tocopherol (0.0625–1 mg/ml) or water for the reagent blank. The increase in absorbance at 593 nm was measured at 4 min. The standard curve was constructed using iron sulfate heptahydrate solution (125–2,000 µM), and the results were expressed as mM Fe2+ equivalents/g FW.
Statistical analysis.
Results were expressed as mean ± SD. Statistical comparisons were performed with Student's t-test. Differences were considered significant at p < 0.05. The correlation coefficient (r2) between the parameters tested was established by regression analysis.
Acknowledgements
This work is a part of Eda Candoken's Master Thesis (Istanbul 2008) and was supported by the Research Fund of Istanbul University: Project Number: T-1456. The authors wish to thank Prof. Dr. Nurhayat Sütlüpinar (Istanbul University, Faculty of Pharmacy, Department of Pharmacognosy) for the identification and Prof. Dr. Erdal Üzen (Istanbul University, Faculty of Science, Deparment of Botany) for the cultivation of the plant.
Abbreviations
- ABTS•+
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- BHT
butylated hydroxytoluene
- CA
catechin equivalents
- DM
diabetes mellitus
- DPPH•
2,2-diphenyl-1-picrylhydrazyl
- EC
extractable compounds
- EC50 values
effective concentration at which the antioxidant activity was 50%
- FRAP
ferric ion reducing antioxidant power
- FW
fresh weight
- MDA
malondialdehyde
- GAE
gallic acid equivalents
- NBT
nitroblue tetrazolium
- O2•−
superoxide anion radical
- ROS
reactive oxygen species
- SD
standard deviation
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive substances
- TCA
trichloroacetic acid
- TEAC
trolox equivalence antioxidant capacity
- TEP
1,1,3,3-tetraetoxypropane
- TPTZ
2,4,6-tripyridyl-s-triazine
- Trolox
6-hydroxy-2,5,7,8,-tetramethylchroman-2-carboxylic acid
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: http://www.landesbioscience.com/journals/oximed/article/8493
References
- 1.Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease menagement and the role of ‘Rasayana’ herbs of Ayurveda. J Ethnopharmacol. 2005;99:165–178. doi: 10.1016/j.jep.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 2.Kaliora AC, Dedoussis GVZ, Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Shahidi F, Wanasundara PKJPD. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 4.Saito H. Purification of active substances of Aloe arborescens Miller and their biological and pharmacological activity. Phytother Res. 1993;7:S14–S19. [Google Scholar]
- 5.Vázquez B, Avila G, Segura D, Escalante B. Antiinflammatory activity of extracts from Aloe vera gel. J Ethnopharmacol. 1996;55:69–75. doi: 10.1016/s0378-8741(96)01476-6. [DOI] [PubMed] [Google Scholar]
- 6.Shimpo K, Ida C, Chihara T, Beppu H, Kaneko T, Kuzuya H. Aloe arborescens extract inhibits TPα-induced ear oedema, putrescine increase and tumour promotion in mouse skin. Phytother Res. 2002;16:491–493. doi: 10.1002/ptr.938. [DOI] [PubMed] [Google Scholar]
- 7.Imanishi K. Aloctin A, an active substance of Aloe arborescens Miller as an immunomodulator. Phytother Res. 1993;7:S20–S22. [Google Scholar]
- 8.Qiu Z, Jones K, Wylie M, Jia Q, Orndorff S. Modified Aloe barbadensis polysaccharide with immunoregulatory activity. Planta Med. 2000;66:152–156. doi: 10.1055/s-2000-11125. [DOI] [PubMed] [Google Scholar]
- 9.Chitra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59:195–201. doi: 10.1016/s0378-8741(97)00124-4. [DOI] [PubMed] [Google Scholar]
- 10.Heggers JP, Kucukcelebi A, Stabenau CJ, Ko F, Broemeling LD, Robson MC, Winters WD. Wound healing effects of Aloe gel and other topical antibacterial agents on rat skin. Phytother Res. 1995;9:455–457. [Google Scholar]
- 11.Rajendran A, Narayanan V, Gnananvel I. Evaluation of therapeutic efficacy of Aloe vera sap in diabetes and treating wounds and inflammation in animals. J Appl Sci Res. 2007;3:1434–1436. [Google Scholar]
- 12.Koo MWL. Aloe vera: Antiulcer and antidiabetic effects. Phytotother Res. 1994;8:461–464. [Google Scholar]
- 13.Ghannam N, Kingston M, Al-Meshaal IA, Tariq M, Parman NS, Woodhouse N. The antidiabetic activity of Aloes: Preliminary clinical and experimental observations. Horm Res. 1986;24:288–294. doi: 10.1159/000180569. [DOI] [PubMed] [Google Scholar]
- 14.Ajabnoor MA. Effect of Aloes on blood glucose levels in normal and alloxan diabetic mice. J Ethnopharmacol. 1990;28:215–220. doi: 10.1016/0378-8741(90)90031-n. [DOI] [PubMed] [Google Scholar]
- 15.Beppu H, Nagamura Y, Fujita K. Hypoglycaemic and antidiabetic effects in mice of Aloe arborescens Miller var. natalensis Berger. Phytother Res. 1993;7:S37–S42. [Google Scholar]
- 16.Okyar A, Can A, Akev N, Baktir G, Sütlüpinar N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother Res. 2001;15:157–161. doi: 10.1002/ptr.719. [DOI] [PubMed] [Google Scholar]
- 17.Rajesekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004;7:61–66. doi: 10.1089/109662004322984725. [DOI] [PubMed] [Google Scholar]
- 18.Corsi MM, Bertelli AA, Gaja G, Fulgenzi A, Ferrero ME. The therapeutic potential of Aloe vera in tumor-bearing rats. Int J Tissue React. 1998;20:115–118. [PubMed] [Google Scholar]
- 19.Pecere T, Gazzola MV, Mucignat C, Parolin C, Della Vecchia F, Cavaggioni A, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumours. Cancer Res. 2000;60:2800–2804. [PubMed] [Google Scholar]
- 20.Capasso F, Borrelli F, Capasso R, Di Carlo G, Izzo AA, Pinto L, et al. Aloe and its therapeutic use. Phytother Res. 1998;12:S124–S127. [Google Scholar]
- 21.Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis Miller, Aloe vera. J Environ Sci Health C. 2006;24:103–154. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds T. Aloes, The Genus Aloe. USA: CRC Press LLC; 2004. [Google Scholar]
- 23.Park YI, Lee SK. New Perspectives on Aloe. USA: Springer Science + Business Media LLC; 2006. [Google Scholar]
- 24.Choi S, Chung M-H. A review on the relationship between Aloe vera components and their biologic effects. Semin Integr Med. 2003;1:53–62. [Google Scholar]
- 25.Eshun K, He Q. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries-a review. Crit Rev Food Sci Nutr. 2004;44:91–96. doi: 10.1080/10408690490424694. [DOI] [PubMed] [Google Scholar]
- 26.Saada HN, Ussama ZS, Mahdy AM. Effectiveness of Aloe vera on the antioxidant status of different tissues in irradiated rats. Pharmazie. 2003;58:929–931. [PubMed] [Google Scholar]
- 27.Kametani S, Kojimα-Yuasa A, Kikuzaki H, Kennedy DO, Honzawa M, Matsui-Yuasa I. Chemical constituents of cape Aloe and their synergistic growth-inhibiting effect on Ehrlich ascites tumor cells. Biosci Biotechnol Biochem. 2007;71:1220–1229. doi: 10.1271/bbb.60659. [DOI] [PubMed] [Google Scholar]
- 28.Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 29.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Therapeut. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Xu J, Hu Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis Miller) extracts. J Agric Food Chem. 2003;51:7788–7791. doi: 10.1021/jf034255i. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. Vitamin C: Antioxidant or pro-oxidant in vivo? Free Radic Res. 1996;25:439–454. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B. Tell me about free radicals, doctor: a review. J Roy Soc Med. 1989;82:747–752. doi: 10.1177/014107688908201216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson D, Phillips BJ. Comparative in vitro and in vivo effects of antioxidants. Food Chem Toxicol. 1999;37:1015–1025. doi: 10.1016/s0278-6915(99)00089-7. [DOI] [PubMed] [Google Scholar]
- 34.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740:101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement and significance. Am J Clin Nutr. 1993;57:715S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 36.Singh RP, Dhanalakshmi S, Rao AR. Chemomodulatory action of Aloe vera on the profiles of enzymes associated with carcinogen metabolism and antioxidant status regulation in mice. Phytomedicine. 2000;7:209–219. doi: 10.1016/S0944-7113(00)80006-9. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhary G, Saini MR, Goyal PK. Chemopreventive potential of Aloe vera against 7,12-dimethylbenz(a)anthracene-induced skin papillomagenesis in mice. ICT. 2007;6:405–412. doi: 10.1177/1534735407309079. [DOI] [PubMed] [Google Scholar]
- 38.Gupta R, Flora SJS. Protective value of Aloe vera against some toxic effects of arsenic in rats. Phytother Res. 2005;19:23–28. doi: 10.1002/ptr.1560. [DOI] [PubMed] [Google Scholar]
- 39.Opara EC. Oxidative stress micronutrients, diabetes mellitus and its complications. J Roy Soc Promot Health. 2002;122:28–34. doi: 10.1177/146642400212200112. [DOI] [PubMed] [Google Scholar]
- 40.Rajasekaran S, Sivagnanam K, Subramanian S. Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. JPP. 2005;57:241–246. doi: 10.1211/0022357055416. [DOI] [PubMed] [Google Scholar]
- 41.Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005;57:90–96. [PubMed] [Google Scholar]
- 42.Nwanjo HU. Antioxidant activity of the exudate from Aloe barbadensis leaves in diabetic rats. Biokemistri. 2006;18:77–81. [Google Scholar]
- 43.Ozsoy N, Yanardag R, Can A, Akev N, Okyar A. Effectiveness of Aloe vera versus glibenclamide on serum lipid parameters, heart and skin lipid peroxidation in type II diabetic rats. Asian J Chem. 2008;20:2679–2689. [Google Scholar]
- 44.Yagi A, Kabash A, Okamura N, Haraguchi H, Moustafa SM, Khalifa TI. Antioxidant, free radical scavenging and anti-inflammatory effects of aloesin derivatives in Aloe vera. Planta Med. 2002;68:957–960. doi: 10.1055/s-2002-35666. [DOI] [PubMed] [Google Scholar]
- 45.Hu Q, Hu Y, Xu J. Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chem. 2005;91:85–90. [Google Scholar]
- 46.Beppu H, Koike T, Shimpo K, Chihara T, Hoshino M, Ida C, Kuzuya H. Radical-scavenging effects of Aloe arborescens Miller on prevention of pancreatic islet B-cell destruction in rats. J Ethnopharmacol. 2003;89:37–45. doi: 10.1016/s0378-8741(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 47.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 48.Matés JM, Pérez-Gómez C, Núnez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 49.Keyhanian S, Stahl-Biskup E. Phenolic constituents in dried flowers of Aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. 2007;73:599–602. doi: 10.1055/s-2007-967202. [DOI] [PubMed] [Google Scholar]
- 50.Botes L, van der Westhuizen FH, Loots DT. Phytochemical contents and antioxidant capacities of two Aloe greatheadii var. davyana extracts. Molecules. 2008;13:2169–2180. doi: 10.3390/molecules13092169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KY, Weintraub ST, Yu BP. Isolation and identification of a phenolic antioxidant from Aloe barbadensis. Free Radic Biol Med. 2000;28:261–265. doi: 10.1016/s0891-5849(99)00235-x. [DOI] [PubMed] [Google Scholar]
- 52.Malterud KE, Farbrot TL, Huse AE, Sund RB. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology. 1993;47:77–85. doi: 10.1159/000139846. [DOI] [PubMed] [Google Scholar]
- 53.Chun-hui L, Chang-hai W, Zhi-liang X, Yi W. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007;42:961–970. [Google Scholar]
- 54.Kardošová A, Machová E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006;77:367–373. doi: 10.1016/j.fitote.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Niciforovic A, Adzic M, Spassic SD, Radojcic MB. Antitumour effects of natural anthracycline analog (Aloin) involve altered activity of antioxidant enzymes in HeLaS3 cells. Cancer Biol Ther. 2007;6:1200–1205. doi: 10.4161/cbt.6.8.4383. [DOI] [PubMed] [Google Scholar]
- 56.Lu GD, Shen H-M, Chung MCM, Ong CN. Critical role of oxidative stress and sustained JNK activation in aloe-emodin-mediated apoptotic cell death in human hepatoma cells. Carcinogenesis. 2007;28:1937–1945. doi: 10.1093/carcin/bgm143. [DOI] [PubMed] [Google Scholar]
- 57.Slinkard K, Singleton VL. Total phenol analyses: Automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- 58.Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:569–575. [Google Scholar]
- 59.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1971;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 60.Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi. 1992;39:925–928. [Google Scholar]
- 61.Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–147. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 62.Chatterjee SN, Agarwal S. Liposomes as a membrane model for study of lipid peroxidation. Free Radic Biol Med. 1988;4:51–72. doi: 10.1016/0891-5849(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 63.Duh P-D, Tu Y-Y, Yen G-C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) LWT. 1999;32:269–277. [Google Scholar]
- 64.Buege JA, Aust SD. Lipid peroxidation. Methods Enzymol. 1978;51:324–437. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 65.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 66.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT. 1995;28:25–30. [Google Scholar]
- 67.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 68.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]