Abstract
A unifying mechanism for abused drugs has been proposed previously from the standpoint of electron transfer. Mescaline can be accommodated within the theoretical framework based on redox cycling by the catechol metabolite with its quinone counterpart. Electron transfer may play a role in electrical effects involving the nervous system in the brain. This approach is in accord with structure activity relationships involving mescaline, abused drugs, catecholamines and etoposide. Inefficient demethylation is in keeping with the various drug properties, such as requirement for high dosage and slow acting.
There is a discussion of receptor binding, electrical effects, cell signaling and other modes of action. Mescaline is a nonselective, seretonin receptor agonist. 5-HTP receptors are involved in the stimulus properties. Research addresses the aspect of stereochemical requirements. Receptor binding may involve the proposed quinone metabolite and/or the amino sidechain via protonation. Electroencephalographic studies were performed on the effects of mescaline on men. Spikes are elicited by stimulation of a cortical area. The potentials likely originate in nonsynaptic dendritic membranes. Receptor-mediated signaling pathways were examined which affect mescaline behavior. The hallucinogen belongs to the class of 2AR agonists which regulate pathways in cortical neurons. The research identifies neural and signaling mechanisms responsible for the biological effects. Recently, another hallucinogen, psilocybin, has been included within the unifying mechanistic framework. This mushroom constituent is hydrolyzed to the phenol psilocin, also active, which is subsequently oxidized to an ET o-quinone or iminoquinone.
Key words: mescaline, mechanism, metabolism, catechol, quinone, redox, electrochemistry, SAR, receptor, cell signaling, neuropharmacology
Introduction
The use of mescaline (peyote) (1) (Scheme 1), a psychedelic drug present in various types of cactus, was reported in religious ceremonies of North American natives by early Europeans. For centuries, North American Indians used mescaline as a medicine, amulet and hallucinogenic religious sacrament.1 Primitive peoples associated the plant action with the supernatural. Vestiges of the cults that survived, flourished and expanded to a large intertribal religion as late as 1973. No significant harm is evident from chronic consumption during these ceremonies, although adverse effects have been reported. It is claimed that the drug is neither habit-forming nor addicting. Abuse of the substance is less than might be expected. Among the reasons are bitter taste, nausea and low potency. Research on the substance started more than 100 years ago, and has continued at a vigorous pace. The drug was ruled illegal in the US in 1970.
Scheme 1.
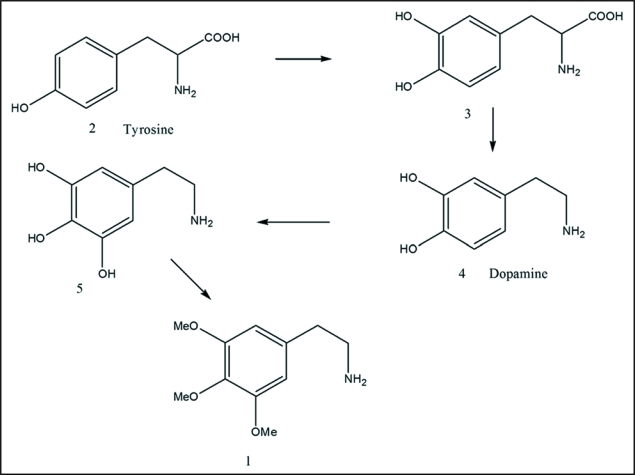
Biosynthesis of mescaline. Steps include oxidation of (2) to (3), decarboxylation to (4), oxidation to (5) and methylation to (1).
In vivo synthesis of mescaline in 1950, in the cactus was proposed as shown in Scheme 1.2 In 1969,3,4 intermediate steps were further elaborated. Starting with tyrosine (2), the main intermediates are dopa (3) dopamine (4) and 3,4,5-trihydroxy-βphenyethylamine (5), leading to mescaline (1).
Synthesis of Mescaline
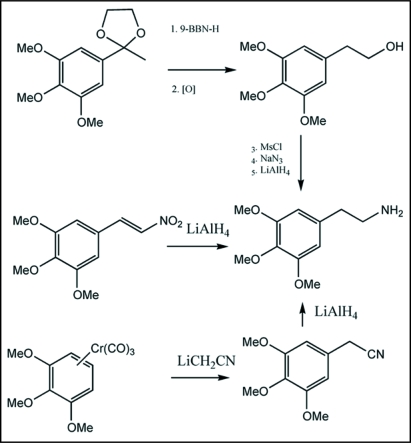
Over the years, several synthetic methods have appeared for making mescaline from readily available trimethoxybenzene compounds. Soderquist et al.5 converted trimethoxybenzene ketal to mescaline in good yields (Scheme 2). Another synthesis involving a chromium complex was reported by Rose-Munch et al.6 There are also several earlier references to the synthesis of mescaline.7–9
Scheme 2.
Synthetic routes to mescaline. Various routes have been reported for laboratory synthesis of mescaline. Key starting materials incorporate acetal, nitro alkene and chromium complex.
The preponderance of bioactive substances or their metabolites incorporate ET functionalities, which, we believe, play an important role in physiological responses. These main groups include quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). The quinone category is the main focus herein.
A brief summary of the fundamental biochemistry of the quinone ET functionality would be instructive as foundation for the ensuing sections. Quinones undergo redox cycling with the corresponding hydroquinone or catechol with intermediate involvement of semiquinones. In vivo redox cycling with oxygen can occur giving rise to oxidative stress (OS) through generation of reactive oxygen species (ROS). In some cases, ET results in interference with normal electrical effects, e.g., in respiration or neurochemistry. ET may be associated with bioelectrical events occurring in the brain in response to the action of psychic drugs. Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range, i.e., more positive than −0.5 V. o-Quinones possess particularly favorable reduction potentials. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g., anti-infective agents,10 anticancer drugs,11 carcinogens,12 reproductive toxins,13 nephrotoxins,14 hepatotoxins,15 cardiovascular toxins,16 nerve toxins,17 mitochondrial toxins,18 abused drugs,19 ototoxins,20 immunotoxins,21 eye toxins,22 pulmonary toxins23 and various other categories, including human illnesses.24 This review demonstrates that the ET-ROS-OS unifying theme, which has been successful for many other classes of toxins and abused drugs, can also be applied to mescaline.
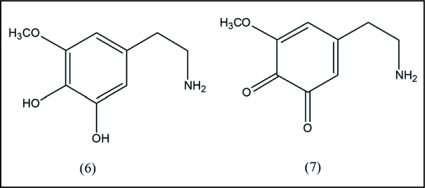
There is substantial precedent for ET involvement of catechol and o-quinone as a redox couple (see above). In the mescaline case, the suggested partners involved are catechol (6) and o-quinone (7), together with a semiquinone. A unifying mode of action based on ET has been applied to the principal abused drugs as well as abused therapeutic drugs19 (see below). The term bioelectronome refers to ET processes which are not only widespread in living systems, but are likely the most important chemical transformations.25 It is important to recognize that in vivo mode of action is usually multifaceted with various factors involved, some of which are interactive. The present proposed mechanism is the first one addressing events at the fundamental molecular level, which also fits into a unifying theme for abused drugs (Fig. 1).
Figure 1.
Catechol and o-quinone metabolites of mescaline. The catechol-type metabolite (6) is part of a class that readily undergoes redox cycling with generation of an o-quinone product (7). The process is an oxidative transformation. In addition, the catechol group readily complexes with heavy metals. Generally, such complexes are known to undergo electron transfer reactions which, in the brain, may be involved in some of the physiological effects elicited by mescaline.
Mescaline Metabolism
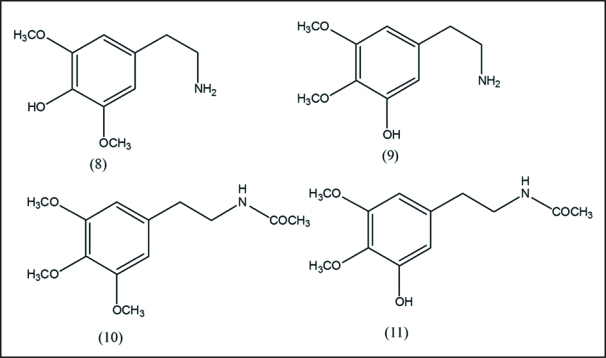
In vivo studies on the metabolism of mescaline have shown that a large part of the mescaline combines with liver protein26 and varying amounts are excreted unchanged or as the oxidatively deaminated 3,4,5-trimethoxyphenyacetic acid.27,28 Harley-Mason et al. isolated 3,4-dihydroxy-5-methoxyphenylacetic acid from human urine after the ingestion of mescaline,27 Ratcliffe and Smith29 have found 3,4-dimethoxy-5-hydroxyphenyethylamine as a minor metabolite of mescaline in humans. Friedhoff and Goldstein30 have reported 3,4,5-trimethoxyphenyethanol as a mescaline metabolite in rats. Charalampous et al.28 feeding 14C labeled mescaline orally isolated N-acylated mescaline and the 3,4-dimethoxy-5-hydroxy N-acetyl derivative from the urine. Interestingly, the N-acetyl derivative at high level of dosage 300–750 mg did show a mild degree of drowsiness (Fig. 2).
Figure 2.
In vivo metabolism products of mescaline. These include demethylation and side chain modification.
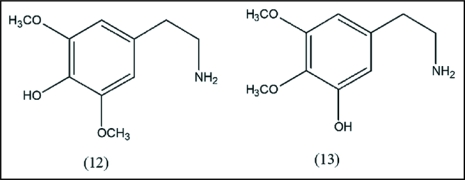
In an in vitro study using 14C-labelled mescaline and rabbit liver enzyme preparation, Daly et al.31 showed that mescaline is demethylated to the 2-hydroxy-1,3-dimethoxyderivative (12) and the 3-hydroxy-1,2-dimethoxyderivative (13) (Fig. 3).
Figure 3.
In vitro metabolic intermediates of mescaline. The process entails monodemethylation to phenolic diethers.
Metabolism (Demethylation and Oxidation)
In most cases, the products isolated result from sidechain oxidation, predominantly 3,4,5-trimethoxyphenylacetic acid,31–33 and to a lesser extent the corresponding aldehyde precursor.34 Other metabolites in this category include the hydroxyethyl derivative30 and 3,4,5-trimethoxybenzoic acid.35
Our mechanistic approach requires dealkylation of the ether groups to phenols. In an earlier study, compounds isolated comprise 3,4-dimethoxy-5-hydroxyphenethylamine, the 3,5-dimethoxy-4-hydroxy isomer and 3,4-dihydroxy-5-methoxyphenylacetic acid.31 Of most relevance is conversion to the catechol form which, according to the hypothetical approach, is precursor to an ET o-quinone. The rate of demethylation is slow,36 as also pointed out in another section. Data suggest that mescaline may be converted into some other substance which is directly responsible for the effects on the central nervous system.37
Relation of Demethylation Rate to Drug Properties
The rate of demethylation of anisole derivatives was determined by relative activity studies, ranging from 0 to 200%.38 The result was a quite low figure of 13% for mescaline which can be related to various drug properties. Large amounts of the drug are excreted unchanged,39 in accord with inefficient metabolism. Some have proposed that all of the drug is excreted suggesting that the parent is the active form. This statement is not valid since there are appreciable numbers of reports on metabolism. Also, the drug is 1,000–3,000 times less potent than LSD. The effective human dose is 300–500 mgs, which is much larger than for other related agents. The time needed for maximum effect may be up to 2 hours, which is longer than for other drugs in the psychic class.33 It is important to recognize that only small amounts are needed for noticeable effect since the quinone ET action is catalytic, as is the case for many physiologically-active materials.
Structure-Activity Relationship of Phenylethylamine Hallucinogens
Mescaline is orally active, but it is the least potent of all the classical hallucinogens. Despite its low potency, mescaline has served as a prototype hallucinogen because of the similarity of its psychopharmacolgy to other hallucinogens and also because it is extensively used up to the present day in the form of peyote during religious services of the Native American Church. It also has served as the lead molecule in structure-activity relationship studies of the phenethylamines.
Mescaline.
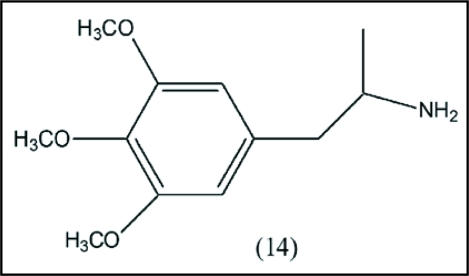
A quite thorough investigation was carried out more than 40 years ago on SAR.40 The monosubstituted methoxy counterparts are inactive. Also inactive are all disubstituted methoxy isomers. All trisubstituted compounds are inactive, except mescaline itself. The 2,3,4,5-tetramethoxy derivative is somewhat more active than mescaline. The pentamethoxy analog is the most active, indicating that sidechain cyclization to the indole derivative may not be important for mescaline. In relation to the o-quinone approach, it is evident that all active compounds incorporate the o-dimethoxy precursor of the subsequent catechol. Apparently, other factors are essential, such as metabolism and receptor binding. Other SAR studies involved sidechain homologs, such as 3,4,5-trimethoxyphenylisopropylamine (3,4,5-trimethoxyamphetamine) (14).41 It is about twice as potent as mescaline (Fig. 4).
Figure 4.
Sidechain homolog of mescaline. The data show that homologation of the sidechain to the isopropyl structure enhances the potency. This side chain is also present in Figure 5.
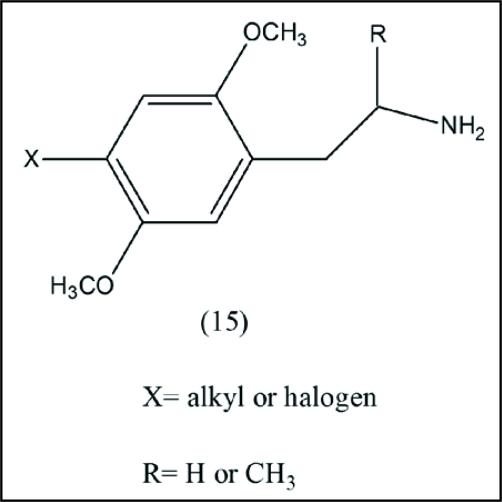
Extensive structure-activity relationship studies, carried out over several decades in various laboratories42–45 including that of Nichols, have led to a good general understanding of the structural and stereochemical features that lead to hallucinogenic activity in substituted phenyethylamine derivatives, leading ultimately to extremely potent substituted amphetamines such as 2,5-dimethoxy-4-bromoamphetamine (15) and 2,5-dimethoxy-4-iodoamphetamine (15).
Interesting results are reported for compounds of structure(14) in which nuclear substitution is quite different from that of mescaline (1) (Fig. 5).46
Figure 5.
2,5-Dimethoxyphenyl analog of mescaline. This analog on demethylation would yield a hydroquinone which could undergo redox cycling with its p-quinone partner.
Agents of general structure(14) are among some of the most potent hallucinogens known. These compounds have low nanomolar affinities for serotonin 5-HT2A and 5-HT2c receptors, the primary sites believed to be involved in mediation of the unique behavioral effects of hallucinogens. Although the 1,2-dimethoxy grouping, essential for catechol type formation, is missing, the present authors believe that substances of type (14) can be accommodated within the general mechanistic framework. The 1,4-dimethoxy arrangement in (14) can serve as precursor of a hydroquinone structure via demethylation, which might then undergo redox cycling with the corresponding p-benzoquinone, analogous to the mechanistic proposal for mescaline.
Studies were made on the activity of the aldehyde and carboxylic acid metabolites of mescaline (see Metabolism).34 Pharmacological experiments on mice indicate that the metabolites are much less active or inactive in cataleptogenic effect and phenobarbital-induced sleep prolongation as compared with the parent compound. The reason is most likely the absence of the aminoethyl sidechain which may be important for binding. Other SAR data are available in the sections on abused drugs and catecholamines.
Mescaline and abused drugs.
A recent review provides a unifying mechanism for toxicity and addiction by abused drugs based on ET-ROS.19 Evidence indicates that mescaline can also be incorporated within the broad framework. The drugs involved are nicotine, alcohol, phencyclidine, cocaine, methylenedioxymethamphetamine (MDMA, ecstasy), amphetamine, methamphetamine, morphine, heroin, delta-9-tetrahydrocannabinol, and lysergic acid diethylamide. Usually, metabolites are the active forms involved in ET and OS. Structural analogy can be drawn involving mescaline and several of these, based on catechol and o-quinone as the active entities, namely MDMA, amphetamine, methamphetamine, morphine and heroin. In the amphetamine series, the 3,4,5-compound was twice as active as mescaline; the 2,4,5-isomer was very active, and the 2,3,5-isomer was inactive.40
Various therapeutic drugs fall in the abused category, e.g., benzodiazepines, phenytoin, phenobarbital, aspirin and acetaminophen.19 Similarly, mescaline can be mechanistically associated with a number of these via catechol and o-quinone metabolites. The ones falling in this category are phenytoin, phenobarbital and aspirin.
Mescaline and catecholamines.
Catecholamines comprise an important class whose most prominent members are dopamine, epinephrine (adrenaline), norepinephrine (noradrenaline) and 3,4-dihydroxyphenylalanine (DOPA). The substances are involved in neurotransmission and neurotoxicity.47 Much of their biochemistry stems from the catechol moiety which undergoes oxidation to o-quinone and semiquinone. Thus, the catechol undergoes redox cycling with its quinone counterpart. Reported conversion of mescaline to catechols (see Metabolism section) lends credence to participation of ET as in the case of the catecholamine analogs. There is also similarity in the presence of an aminoethyl sidechain which is substituted in some cases.
Mescaline and etoposide.
This drug, a 2,6-dimethoxyphenol derivative, is a semisynthetic analog of the antitumor antibiotic podophyllotoxin.11 Etoposide is metabolically activated by oxidation and demethylation to an o-quinone derivative. The presence of molecular oxygen contributes to cytotoxicity, and DNA cleavage is inhibited by radical scavengers. Also, the model 3-methoxy1,2-quinone exhibits a comparatively positive reduction potential (-0.16 V), making for facile electron uptake. This compound can also serve as a close model for both the etoposide metabolite and the proposed metabolite of mescaline. Metals, such as Fe or Cu, may play a role in ET via complex formation with the intermediate catechol.
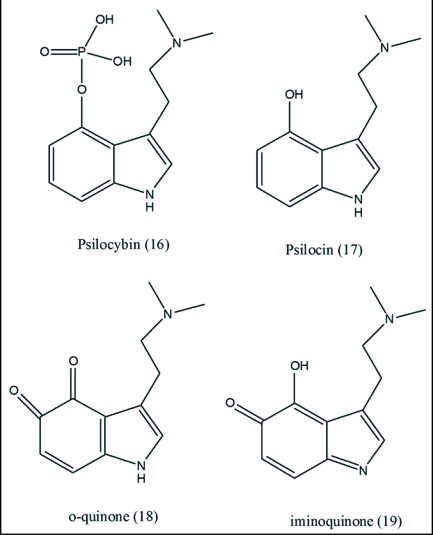
Recently, psilocybin (16) has been added to drugs of the abused class19 which operate by ET mode of action.48 The substance, present in certain mushrooms, is a hallucinogen belonging to the tryptamine family. It has been banned in most countries. In vivo, (16) is converted by dephosphorylation into a phenol (17) (psilocin) which also exhibits psychedelic (hallucinogenic) properties. Studies have been carried out entailing enzymatic oxidation which provides important evidence concerning the mechanistic mode. The product from psilocin, possessing a deep blue color, is believed to contain an o-quinone structure (18).49 A similar result was obtained in a subsequent investigation in which the product was assigned either an o-quinone (18) or iminoquinone structure (19).50 Psilocybin yielded a similar result in accord with facile hydrolysis to (17). o-Quinones display quite favorable ET properties. The data fit nicely into the prior unifying framework involving participation of ET entities in the physiological activity.19 There has been scant attention to action mode at the fundamental molecular level.
Pharmacological studies were reported on receptor participation.51 Psilocin behaves as a partial agonist at the 5-HT2A serotonin receptor in the brain. It is not surprising that the effects are somewhat related to those of serotonin which possesses a closely related structure (Fig. 6).
Figure 6.
Metabolism of psilocybin to o- and imino-quinones. The process entails dephosphorylation to the phenol followed by oxidation to quinone-type products.
Physiological Effects
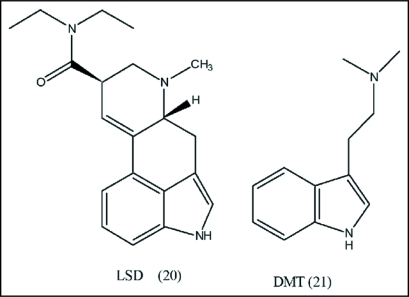
Mescaline is about 1,000–3,000 times less potent than LSD (20) and about 30 times less potent than psilocybin (16).43,52,53 Despite its low potency, mescaline has served as prototype hallucinogen because of the similarity of its psychopharmacology to other hallucinogens. Death in animals results from convulsions and respiratory arrest. An effective dose of the sulfate, 200–400 mg, causes hallucination lasting for about 10–12 h,54 compared with DMT (21) (<1 h) or psilocybin (4–6 h). Brain imaging showed mescaline selectively increases neuronal activity, especially in the striatolimbic systems in the right hemisphere. Pàlenicek et al.55 showed mescaline had a delayed onset of the main behavioral changes in rats compared to other hallucinogens. Subcutaneous injection of mescaline leads to rapid increase in blood serum levels in 30 min and subsequently quickly decreased. Mescaline entered the brain slowly, and showed peak levels at 60 min and remained high for an additional 60 min. Reaction to mescaline mimics the symptoms produced by norepinephrine and epinephrine, namely increase in heart rate, increase in temperature, nausea, dizziness, heavy perspiration, dilation of pupils, dry mouth and anxiety. In addition, coordination and reflux disruption, muscle weakness, increased blood pressure (hypertension), contraction of the intestines and the uterus are experienced by individuals after exposure to mescaline.56 Hermie et al.57 showed subjects given 500 mg of mescaline sulfate produced “hyperfrontal” pattern of increased blood flow, which was correlated with mescaline-induced psychological effects (Fig. 7).
Figure 7.
Hallucinogens. These psychic drugs are used in comparison studies involving physiological activity.
Pharmocokinetics
Animals show slow tolerance to repeated administration. Intoxication can be alleviated or stopped with chlorpromazine or valium.
Psychic Effects
Individuals experience various distortions, involving sensory perception, space, time, color, sounds and shapes, as well as dreamlike feeling.46,56,57 There have been very few clinical studies employing mescaline, although it has a long history of use among the Native American population. Mescaline was studied to determine whether its effects were similar to the symptoms of acute psychosis. Hermie et al.57 showed, in normal male volunteers, that mescaline produced an acute “psychotic state” 3.5–4 h after drug administration as measured by the Brief Psychiatric Rating Scale (BPRS) and Paranoid Depression Scale (PDS).
Receptor Binding
Serotonin receptors are made up of G protein-coupled types and ligand-gated ion channels present in the CNS, which mediate neurotransmission via the release of neurotransmitters, in addition to hormones. These receptors play an important role in a variety of processes, such as anxiety, cognition, aggression, learning, memory, nausea, sleep and mood. As a result, they are the target of therapeutic agents, as well as illicit drugs, including hallucinogens. There is a detailed discussion of hallucinogens and receptors in a 2004 review.46
Mescaline is a nonselective, serotonin receptor agonist.55 Data suggest that 5-HT2 receptors are involved in the stimulus properties of the drug.58,59 Another article deals with receptor interaction in the rat striatum.60 Some research addresses the aspect of stereochemical requirements. One study involved cis-and trans-2-(2,4,5-trimethoxyphenyl)-cyclopropylamine.61 The trans isomer is qualitatively indistinguishable from mescaline in relation to behavioral effects. Potency is somewhat greater, and duration of action is briefer. In contrast, the cis isomer is almost inactive. Calculations on the docked conformation indicate that this form is higher in energy than that in which the side chain is in the anti conformation. Insight was gained from investigations on C-(4,5,6trimethoxyindan-1-yl)methanamine, in which the side chain is considerably constrained by covalent linkage to the benzene ring.62 The compound possessed threefold higher affinity and potency than mescaline, as well as equal efficacy at the 5-HT2A receptor. In drug discrimination studies, the indan derivative was fivefold more potent. Resolution into the enantiomers showed the R-(+) isomer to be the active form. These studies of the effects of sidechain alteration are also relevant to the SAR section.
The quinone metabolite may be difficult to isolate since this class is known to bind readily to protein via conjugate addition by thiol or amino nucleophiles. Subsequent oxidation can regenerate the quinone which would then redox cycle at the active site. This view is in keeping with the observation that large amounts of the drug are bound to protein.31 The side chain amino group of mescaline may also be involved in receptor binding via protonation by protein carboxyl.
Receptor chemistry in relation to ET-ROS and electrostatics is provided elsewhere (see sections on Electrical Effects and Cell Signaling).
Electrical Effects
A review was recently published on the importance of electrical effects in living systems.63 Electroencephalographic (EEG) studies were performed on the effects of mescaline on man.64 All-or-nothing spikes are elicited by antidromic, afferent or electrical stimulation of a cortical area treated with the drug.65 The potentials likely originate in nonsynaptic dendritic membranes. A depressant cortical reaction induced by afferent stimulation is influenced by the dendritic action potential from application of mescaline. An EEG arousal is brought about in the rabbit by administration of the drug.66 Evidence indicates that some biochemical and neural patterns are common to both mescaline and morphine.67,68 Another section of this review addresses similar ET mechanisms for both cases, in line with the experimental observation. Results indicate an association between mescaline-induced pathological aggression and abnormal electrical activity in the amygdale.69 A possible mechanism is discussed in rat studies, in which the drug inhibited the spontaneous firing of noradrenergic neurons of the locus ceruleus.70 There was increased reactivity of these neurons to the excitory influences of peripheral stimuli. Results suggest that nanosecond-pulsed electric fields modulate cell signaling from the plasma membrane to intracellular structures and function.71 This technology could provide a powerful, unique tool to recruit signaling mechanisms that can eliminate aberrant cells by apoptosis. A 1993 symposium,72 focused on bioelectricity of cell signaling. Living creatures can be regarded as complex electrochemical systems that evolved over billions of years. Organisms interacted with and adapted to an environment of such fields whose long-term effects are unknown.
Cell Signaling
A study was made of receptor-mediated signaling pathways which affect mescaline behavior.59 Mescaline belongs to the class of 5-HT<sub> receptor (2AR) agonists. The 2AR-regulated pathways on cortical neurons are sufficient to mediate the response. The research identifies the long-elusive neural and signaling mechanism responsible for the unique hallucinogenic effects. A review addresses redox aspects of cell signaling by catecholamines and their metabolites.73
Considerable research has been done on the basic mechanism of cell signaling based on radicals and electrons. More than 10 years ago, ROS attracted attention in relation to cell signaling. Since then, several books have addressed this aspect,74,75 including RNS, e.g., NO. Evidence has accumulated that ROS, such as hydrogen peroxide, superoxide and the hydroxyl radical, are important chemical mediators that regulate the transduction of signals by modulating protein activity via redox chemistry.76 Authors have proposed that ROS have been conserved throughout evolution as universal second messengers.77 Nearly every step in signal transfer is sensitive to ROS, which can function as primary signals and as second messengers in the activation of transcription factors.74 The approach is elaborated in a recent review.78 A 2004 review79 summarized the present status of electrophysiological effects and deserves special attention. After a burst of research dealing with electrical coupling, gap junctions became less popular among neurobiologists versus the ionic approach. Recent reports have brought gap junctions back into the spotlight, suggesting that this type of cell-cell signaling may be interrelated with, rather than an alternative to, chemical transmission. We believe the thesis is credible, since electromagnetic effects of electrons and radicals in motion should have an influence on positive and negative charges associated with the central nervous systems (CNS).
Substantial evidence supports the contention that ET and ROS play important roles in cell signaling.78,80 The proposed quinone metabolite could then be involved in ET. Also, electrostatic fields are believed to participate.63,80 In the case of mescaline, molecular electrostatic potential would be associated with the alkyl ammonium ion that may play a part in receptor binding, in addition to the dipolar carbonyl groups.
Other Modes of Action
Various mechanisms have been advanced for mescaline action, but with little focus at the molecular level. Some reports suggest conversion to an active form,81 with which our view is in accord. Other suggestions are listed in Table 1.
Table 1.
Other mescaline mechanisms
| References | ||
| 1 | Alteration of normal metabolism in such a way as to cause hallucinations. | 81 |
| 2 | A mescaline-protein complex is responsible for the characteristic central effects.a | 81 |
| 3 | Competition of mescaline for adrenergic receptors. | 81 |
| 4 | Relationships between catecholamine metabolites and mescaline.b | 81 |
| 5 | Cyclization to an indole. | 81 |
| 6 | In the periphery, mescaline blocks the release of acetylcholine in muscle | 82 |
This proposal is similar to the one in an earlier section dealing with receptor binding by the quinone metabolite.
Which is related to a prior section.
CNS effects.
The behaviorial effects of mescaline on retention of spatial orientation were explored.83 The intensity and time courses of the neurotoxic effect depended on the brain area administered. Lengthening of the latencies in reaching the goal was observed. The neuropsychological and neurometabolic effects of the drug were investigated with human subjects.84 Specific effects in the visual systems resulted. A hyperfrontal pattern in the face was induced with emphasis on the right hemisphere; which was correlated with induced psychotomimetic psychopathology. Mescaline operates selectively in the brain, stimulating regions to increased neuronal sctivity.85 Face-test studies were performed under mescaline-induced psychosis. Apparently, the potentiating effect of the drug is triggered by preferential actions on the serotogenic muscles.86,87 The behavioral effects were investigated for mescaline via intracerebroventricular administration.88 Rats exposed to mescaline exhibited enhanced spontaneous motility with no effect on exploratory behavior.89 The influence on behavior may be related to change in the brain cholinergic system.
Hypotheses Testing
Experiments can be designed to test validity of the proposed action mechanism. The hypothesized catechol metabolite should be synthesized and evaluated for activity. Oxidation yields the o-quinone whose activity and reduction potential could be determined. In the enzyme system used for demethylation, addition of o-phenylenediamine can serve to trap the proposed o-quinone derivative, with formation of a readily identified product. Similarly, heavy metal ions can act to form complexes with the proposed catechol derivative.
Acknowledgements
Editorial assistance by Angelica Ruiz, Michael McGovern, Ashley Berry and Thelma Chavez is acknowledged. Helpful discussion with Phillip White is appreciated.
Abbreviations
- OS
oxidative stress
- ROS
reactive oxygen species
- ET
electron transfer
- SAR
structure activity relationship
- MDMA
methylenedioxymethamphetamine
- EEG
electroencephalographic
- LSD
lysergic acid diethylamide
- DMT
N, N-dimethyltryptamine
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: http://www.landesbioscience.com/journals/oximed/article/9380
References
- 1.Mc Laughlin JL. Peyote: an introduction. Lloydia. 1973;36:1–8. [PubMed] [Google Scholar]
- 2.Paul AG. Biosynthesis of the peyote alkaloids. Lloydia. 1973;36:36–45. [PubMed] [Google Scholar]
- 3.Lundstöm J, Agurell S. A complete biosynthetic sequence from tyrosine to mescaline. Tetrahedron Lett. 1969:3371–3374. doi: 10.1016/s0040-4039(00)99766-1. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia GJ, Vaishnav YN, Fayez MBE. Peyote alkaloids IX: Identification and synthesis of demethylmescaline, a plausible intermediate in the biosynthesis of the cactus alkaloids. J Pharmaceut Sci. 1969;58:1157–1159. doi: 10.1002/jps.2600580932. [DOI] [PubMed] [Google Scholar]
- 5.Soderquist JA, Kock I, Estrella ME. Reductive cleavage of acetals and ketals with 9-borabicyclo[3.3.1]nonane. Org Proc Res Develop. 2006;10:1076–1079. [Google Scholar]
- 6.Rose-Munch F, Chavignon R, Tranchier J-P, Gagliardini V, Rose E. Mescaline synthesis via tricarbonyl (η6-1,2,3-trimethoxybenzene)chromium complex. Inorg Chim Acta. 2000;302:693–697. [Google Scholar]
- 7.Tsao M. A new synthesis of mescaline. J Am Chem Soc. 1951;73:5495–5496. [Google Scholar]
- 8.Max E, Ramirez F. The reduction of β-nitrostyrene with lithium aluminum hydride. Helv Chim Acta. 1950;33:912–916. [Google Scholar]
- 9.Banholzer K, Campbell TW, Schimid H. Synthesis of mescaline, N-methyl- and N,N-dimethylmescaline. Helv Chim Acta. 1952;35:1577–1588. [Google Scholar]
- 10.Kovacic P, Becvar LE. Mode of action of anti-infective agents: emphasis on oxidative stress and electron transfer. Curr Pharmaceut Des. 2000;6:143–167. doi: 10.2174/1381612810006020143. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic P, Osuna JA. Mechanisms of anticancer agents: emphasis on oxidative stress and electron transfer. Curr Pharmaceut Des. 2000;6:277–309. doi: 10.2174/1381612003401046. [DOI] [PubMed] [Google Scholar]
- 12.Kovacic P, Jacintho JD. Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–796. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 13.Kovacic P, Jacintho JD. Reproductive toxins: pervasive theme of oxidative stress and electron transfer. Curr Med Chem. 2001;8:863–892. doi: 10.2174/0929867013372878. [DOI] [PubMed] [Google Scholar]
- 14.Kovacic P, Sacman A, Wu-Weis M. Nephrotoxins: widespread role of oxidative stress and electron transfer. Curr Med Chem. 2002;9:823–847. doi: 10.2174/0929867024606803. [DOI] [PubMed] [Google Scholar]
- 15.Poli G, Cheeseman KH, Dianzani MV, Slater TF, editors. Free Radicals in the Pathogenesis of Liver Injury. Pergamon: New York; 1989. [Google Scholar]
- 16.Kovacic P, Thurn LA. Cardiovascular toxins from the perspective of oxidative stress and electron transfer. Curr Vasc Pharmacol. 2005;3:107–117. doi: 10.2174/1570161053586912. [DOI] [PubMed] [Google Scholar]
- 17.Kovacic P, Somanathan R. Neurotoxicity: The broad framework of electron transfer, oxidative stress and protection by antioxidants. Curr Med Chem-CNS Agents. 2005;5:249–258. [Google Scholar]
- 18.Kovacic P, Pozos RS, Somanathan R, Shangari R, O'Brien PJ. Mechanism of mitochondrial uncouplers, inhibitors and toxins: focus on electron transfer, free radicals and structure-activity relationships. Curr Med Chem. 2005;5:2601–2623. doi: 10.2174/092986705774370646. [DOI] [PubMed] [Google Scholar]
- 19.Kovacic P, Cooksy AL. Unifying mechanism for toxicity and addiction by abused drugs: electron transfer and reactive oxygen species. Med Hypotheses. 2005;64:366–367. doi: 10.1016/j.mehy.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Kovacic P, Somanathan R. Ototoxicity and noise trauma: reactive oxygen species, cell signaling, electrical effects and protection by antioxidants. Practical medical aspects Med Hypotheses. 2008;70:914–923. doi: 10.1016/j.mehy.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Kovacic P, Somanathan R. Integrated approach to immunotoxicity: electron transfer, reactive oxygen species, antioxidants, cell siganling and receptors. J Recept Signal Transduct. 2008;28:323–346. doi: 10.1080/10799890802305217. [DOI] [PubMed] [Google Scholar]
- 22.Kovacic P, Somanathan R. Unifying mechanism for eye toxicity: electrón transfer, reactive oxygen species, antioxidant benefits, cell signaling and cell membranes. Cell Memb Free Radic Res. 2008;1:56–69. [Google Scholar]
- 23.Kovacic P, Somanathan R. Pulmonary toxicity and environmental conatamination; radicals, electron transfer and protection by antioxidants. In: Whitcare DM, editor. In Reviews of Environmantal Contamination and Toxicology. Vol. 201. Springer NY; 2009. pp. 41–69. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford UP: New York; 1999. [Google Scholar]
- 25.Kovacic P, Pozos RS. Review: Bioelectronome: Integrated approach to receptor chemistry, radicals electrochemistry, cell signaling and physiological effects based on electron transfer. J Recept Signal Transduct. 2007;27:261–294. doi: 10.1080/10799890701509133. [DOI] [PubMed] [Google Scholar]
- 26.Block W, Block K, Patzig B. Physiology of the 14C-radioactive mescaline in animal experiment II. Distribution of radioactivity in organs in relation to time. Z Physiol Chem. 1952;290:230–236. [PubMed] [Google Scholar]
- 27.Harley-Mason J, Laird AH, Smythies JR. The metabolism of mescaline in the human; delayed clinical reactions to mescaline. Conf Neurolog. 18:152–155. doi: 10.1159/000105047. [DOI] [PubMed] [Google Scholar]
- 28.Charlampous KD, Walker KE, Kinross-Wright J. Metabolic fate of mescaline in man. Psychopharmacol (Berlin) 1966;9:48–63. doi: 10.1007/BF00427703. [DOI] [PubMed] [Google Scholar]
- 29.Ratcliffe J, Smith P. Metabolism of mescaline. Chem Ind (London) 1958 [Google Scholar]
- 30.Friedhoff AJ, Goldstein M. New developments in metabolism of mescaline and related amines. Ann NY Acad Sci. 1961;95:5–13. doi: 10.1111/j.1749-6632.1962.tb50097.x. [DOI] [PubMed] [Google Scholar]
- 31.Daly J, Axelrod J, Witkop B. Methylation and demethylation in relation to the in vitro metabolism of mescaline. Ann NY Acad Sci. 1962;96:37–43. doi: 10.1111/j.1749-6632.1962.tb50099.x. [DOI] [PubMed] [Google Scholar]
- 32.Charalampous KD, Orengo A, Walker KE, Wright JK. Metabolic fate of 3,4,5-trimethoxyphenyl-ethylamine (mescaline) in humans: isolation and identification of 3,4,5-trimethoxyphenylacetic acid. J Pharmacol Exper Therapeut. 1964;145:242–246. [PubMed] [Google Scholar]
- 33.Neff N, Rossi GV, Chase GD, Rabinowitz JL. Distribution and metabolism of mescaline in the cat brain. J Pharmacol Exper Therapeut. 1964;144:1–7. [PubMed] [Google Scholar]
- 34.Wantanabe K, Kayano Y, Matsunaga T. Trimethoxyphenylacetaldehyde, an intermediate metabolite of mescaline, is a substrate for microsomal aldehyde oxgenase in the mouse liver. Biol Pharm Bull. 1995;18:696–699. doi: 10.1248/bpb.18.696. [DOI] [PubMed] [Google Scholar]
- 35.Demisch L, Kaczmarczyk P, Seiler N. 3,4,5-Trimethoxybenzoic acid, a new mescaline metabolite in humans. Drug Metab Dispos. 1978;6:507–509. [PubMed] [Google Scholar]
- 36.Datta RK, Ghosh JJ. Mescaline-induced changes of brain cortex ribosomes. Mescaline demethylase of brain cortex soluble supernatant. Naunyn Schmiedebergs Arch Pharmacol. 1977;296:297–300. doi: 10.1007/BF00498697. [DOI] [PubMed] [Google Scholar]
- 37.Mokrasch LC, Stevenson I. The metabolism of mescaline with a note on correlations between metabolism and psychological effects. J New Ment Dis. 1959;129:177–183. doi: 10.1097/00005053-195908000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Axelrod J. The enzymic cleavage of aromatic ethers. Biochem J. 1956;63:634–639. doi: 10.1042/bj0630634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mescaline, Wikipedia encyclopedia. 2008. Anonymous. http://en.wikipedia.org/wiki/Mescaline.
- 40.Smythies GR, Bradley RG, Johnston VS, Benington F, Morin RD, Clark LC., Jr Structure activity relationship studies on mescaline 3. The influence of the methoxy groups. Psychopharmacologia. 1967;10:379–387. doi: 10.1007/BF00403978. [DOI] [PubMed] [Google Scholar]
- 41.Shulgin T. Mescaline: The chemistry and pharmacology of its analogs. Lloydia. 1973;36:46–58. [PubMed] [Google Scholar]
- 42.Nichols DE. Structure-activity relationships of phenyethylamine hallucinogens. J Pharmaceut Sci. 1981;70:839–847. doi: 10.1002/jps.2600700802. [DOI] [PubMed] [Google Scholar]
- 43.Monte MP, Waldman ST, Marona-Lewicka D, Wainscott DB, Nelson DL, Sanders-Bush E, Nichols DE. Dihydrobenzofuran analogues of hallucinogens 4. Mescaline derivatives. J Med Chem. 1997;40:2997–3008. doi: 10.1021/jm970219x. [DOI] [PubMed] [Google Scholar]
- 44.Glennon RA. Arylalkylamine drugs of abuse: an overview of drug discrimination studies. Pharmacol Biochem Behav. 1999;64:43–67. doi: 10.1016/s0091-3057(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 45.Shulgin A, Shulgin A. PIHKAL a chemical love story. Berkeley, CA: Transform Press; 1991. [Google Scholar]
- 46.Nichols DE. Hallucinogens. Pharmacol Therapeut. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Jacintho J, Kovacic P. Neurotransmission and neurotoxicity by nitric oxide, catecholamines and glutamate. Unifying themes of reactive oxygen species and electron transfer. Curr Med Chem. 2003;10:2693–2703. doi: 10.2174/0929867033456404. [DOI] [PubMed] [Google Scholar]
- 48.Kovacic P. Unifying electron transfer mechanism for psilocybin and psilocin. Med Hypotheses. 2009 doi: 10.1016/j.mehy.2009.06.022. In press. [DOI] [PubMed] [Google Scholar]
- 49.Blaschko H, Levine WG. Enzymatic oxidation of psilocine and other hydroxyindoles. Biochem Pharmacol. 1960;3:168–169. [Google Scholar]
- 50.Horita A, Weber LG. The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem Pharmacol. 1961;7:47–54. doi: 10.1016/0006-2952(61)90124-1. [DOI] [PubMed] [Google Scholar]
- 51.Passie T, Seifert G, Schneider U, Enrich HM. The pharmacology of psilocybin. Addict Biol. 2002;7:357–364. doi: 10.1080/1355621021000005937. [DOI] [PubMed] [Google Scholar]
- 52.Nelson DL, Lucaites VL, Wainscot DB, Glennon RA. Comparison of hallucinogenic phenylisopropylamine binding affinities at cloned 5-HT2A, 5-HT2B receptors and 5-HT2C receptors. Naunyn Schmiedeberg's Arch Pharmacol. 1999;359:1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- 53.Davis WM, Bedford JA, Buelke JI, Guinn MM, Hatoum HT, Waters IW, et al. Acute toxicity and gross behavioral effects of amphetamine, four methoxyamphetamines and mescaline in rodents, dogs and monkeys. Toxicol Appl Pharmacol. 1978;45:49–62. doi: 10.1016/0041-008x(78)90027-3. [DOI] [PubMed] [Google Scholar]
- 54.Beyerstein BL, Dal Cason TA, Franzosa ES, Hugel J, Kalchik M, Laing R, et al. A Forensic Handbook. New York: Academic Press; 2003. Hallucinogens. [Google Scholar]
- 55.Pàlenicek T, Balíkovà M, Bubeníkovà-Valešovà, Horàcek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacol. 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- 56.Hollister LE. Effects of hallucinogens in humans. In: Jacobs BL, editor. Hallucinogens: neurochemical, behavioral and clinical perspective. New York: Raven Press; 1984. p. 19033. [Google Scholar]
- 57.Hermie L, Funfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, et al. Mescaline-induced psychopathological, neurophsiological and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 58.Appel JB, Callahan PM. Involvement of 5-HT receptor subtypes in the discriminative stimulus properties of mescaline. Eur J Pharmacol. 1989;159:41–46. doi: 10.1016/0014-2999(89)90041-1. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez-Maeso J, Weisstaub N, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Dill RE. Mescaline: receptor interaction in the rat striatum. Arch Int Pharmacodyn Ther. 1974;195:320–329. [PubMed] [Google Scholar]
- 61.Walters GC. Stereochemical requirements of the mescaline receptor. Nature. 1972;238:96–98. doi: 10.1038/238096a0. [DOI] [PubMed] [Google Scholar]
- 62.McLean TH, Chambers JJ, Parrish HC, Braden MR, Marona-Lewicka D, Kurrasch-Orbaugh D, Nichols DE. C-(4,5,6-trimethoxyindan-1-yl)methanamine: a mescaline analogue designed using a homology model of the 5-HT2A receptor. J Med Chem. 2006;49:4269–4274. doi: 10.1021/jm060272y. [DOI] [PubMed] [Google Scholar]
- 63.Kovacic P. Bioelectrostatics: review of widespread importance in biochemistry. J Electrostat. 2008;66:124–129. [Google Scholar]
- 64.Wikler A. Clinical and electroencephalographic studies on the effects of mescaline, N-allylnormorphine and morphine in man; a pharmacologic analysis of the functions of the spontaneous electrical activity of the cerebral cortex. J Nerv Ment Dis. 1954;120:157–175. [PubMed] [Google Scholar]
- 65.Ramos JG. The mescaline or strychnine spikes as a tool for physiological and pharmacological studies. Acta Physiol Lat Am. 1977;27:16–30. [PubMed] [Google Scholar]
- 66.Ramos JG. A depressant cortical reaction induced by afferent stimulation. Acta Physiol Lat Am. 1976;26:38–50. [PubMed] [Google Scholar]
- 67.Ferri S, Santagostino A, Braga PC. Development of tolerance to the antinociceptive effect of mescaline intraventricularly administered to rabbits. Psychopharmacology. 1976;47:261–265. doi: 10.1007/BF00427610. [DOI] [PubMed] [Google Scholar]
- 68.Ferri S, Santagostino A, Braga PC. Cross tolerance to antinociception elicited by intracerebroventricular administration of mescaline and morphine to rabbits, and EEG correlates. Psychopharmacology. 1976;47:267–269. doi: 10.1007/BF00427611. [DOI] [PubMed] [Google Scholar]
- 69.Shull RN, Sbordone RJ, Gorelick DA. EEG correlates of mescaline-induced pathological aggression in rats. Physiolog Psycholog. 1981;9:208–212. [Google Scholar]
- 70.Aghjanian GK. Mescaline and LSD facilitate the activation of locus ceruleus neurons by peripheral stimuli. Brain Res. 1980;186:492–498. doi: 10.1016/0006-8993(80)90997-x. [DOI] [PubMed] [Google Scholar]
- 71.Beebe SJ, Fox PM, Rec LJ. Nanosecond, high-intensity pulsed electric fields induce apoptosis in living cells. FASEB. 2003;17:1493–1495. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 72.Frey AH. Electromagnetic field interaction with biological systems. FASEB. 1993;17:272–281. doi: 10.1096/fasebj.7.2.8440406. [DOI] [PubMed] [Google Scholar]
- 73.Smythies J. Redox aspect of signaling by catecholamines and their metabolites. Antioxid Redox Sig. 2000;2:573–583. doi: 10.1089/15230860050192332. [DOI] [PubMed] [Google Scholar]
- 74.Forman HG, Cadenas E, editors. Oxidative Stress and Signal Tranduction. New York: Chapman and Hall; 1997. p. 475. [Google Scholar]
- 75.Hancock JT, editor. Cell Signalling. New York: Oxford University Press; 2005. p. 296. [Google Scholar]
- 76.Veriweij CL, Gringhuis SJ. Oxidants and tyrosine phosphorylation; role of acute and chronic oxidative stress in T- and β-lymphocyte signaling. Antioxid Redox Signal. 2002;5:543–551. doi: 10.1089/15230860260196344. [DOI] [PubMed] [Google Scholar]
- 77.Schulze-Osthhoff K, Bauer MK, Vogt M, Wesselborg S. Oxidative stress and signal transduction. Int J Vitam Nutr Res. 1997;67:336–342. [PubMed] [Google Scholar]
- 78.Kovacic P, Pozos RS. Cell signaling (mechanism and reproductive toxicity): redox chains, radicals, electrons, relays, conduit, electrochemistry and other medical implications. Birth Defects Res Part C. 2006;78:333–344. doi: 10.1002/bdrc.20083. [DOI] [PubMed] [Google Scholar]
- 79.Hormuzdi SG, Filippov MA, Mitropoulou G, Monyer H, Buzzone R. Electrical synopsis: a dynamic signaling system that shapes the activity of neuronal networks. Biochim Biophys Acta. 2004;1662:113–137. doi: 10.1016/j.bbamem.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 80.Kovacic P, Pozos RS, Draskovich CD. Unifying electrostatic mechanism for receptorligand activity. J Recept Signal Transduct. 2007;27:411–432. doi: 10.1080/10799890701699686. [DOI] [PubMed] [Google Scholar]
- 81.Neff N, Rossi GB. Mescaline. Am J Pharm Sci Support Public Health. 1963;135:319–327. [PubMed] [Google Scholar]
- 82.Diaz J. How Drugs Influence Behavior. Englewood Cliffs: Prentice Hall; 1996. [Google Scholar]
- 83.Koupilova M, Herink J, Krs O. Influencing of spatial memory in rats by DSP-4 and mescaline. Acta Medica (Hrade Kralove, Czech Republic) 1999;42:69–72. [PubMed] [Google Scholar]
- 84.Hermie L, Gouzoulis-Mayfrank E, Spitzer M. Blood flow and cerebral laterality in the mescaline model of psychosis. Pharmacopsychiatry. 1998;31:85–91. doi: 10.1055/s-2007-979352. [DOI] [PubMed] [Google Scholar]
- 85.Oepen G, Harrington A, Hermie L, Botsch H, Fuenfgeld M, Spitzed M. Increased right-hemisphereic (RH) striatolimbic uptake of technetium-99m-HMPAO in mescaline-induced schizophrenic-like psychosis. Nuklearmedizin (Suppl) (Stuttgart) 1989;25:300–303. [Google Scholar]
- 86.Fink H, Oelssner W. LSD, mescaline and serotonin injected into medial nucleus potentiate apomorphine hypermotility. Eur J Pharmacol. 1981;75:289–296. doi: 10.1016/0014-2999(81)90556-2. [DOI] [PubMed] [Google Scholar]
- 87.Aghajanian GK, Foote WE, Sheard MH. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Expt Therap. 1970;171:178–187. [PubMed] [Google Scholar]
- 88.Mokler DJ, Rech RH. Behavioral effects of intracerebroventricular administration of LSD DOM, mescaline or lisuride. Pharmacol Biochem Behav. 1984;21:281–287. doi: 10.1016/0091-3057(84)90227-2. [DOI] [PubMed] [Google Scholar]
- 89.Torre E, Carbonatto P, Torre M. Effect of mescaline on cerebral cholinesterases and the exploring behavior of rats. Bollet Soc Ital Biol Speriment. 1979;55:1509–1515. [PubMed] [Google Scholar]