Abstract
Objective
Estrogens decrease atherosclerosis progression, mediated in part through changes in plasma lipids and lipoproteins. This study aimed to determine estrogen-induced changes in hepatic cholesterol metabolism, plasma lipoproteins, and the relationship of these changes to atherosclerosis extent.
Methods and Results
Ovariectomized monkeys (n=34) consumed atherogenic diets for 30 months which contained either no hormones (control, n=17) or conjugated equine estrogens (CEE, n=17) at a human dose equivalent of 0.625 mg/d. Hepatic cholesterol content, low-density lipoprotein (LDL) receptor expression, cholesterol 7α-hydroxylase and acyl-coenzyme A:cholesterol acyltransferase (ACAT) activity and expression levels were determined. CEE treatment resulted in lower plasma concentrations of very-low- and intermediate density lipoprotein cholesterol (V+IDLC; p=0.01), smaller LDL particles (p=0.002) and 50% lower hepatic cholesterol content (total, free and esterified; p<0.05 for all). Total ACAT activity was significantly lower (p=0.01), explained primarily by reductions in the activity of ACAT2. Estrogen regulation of enzymatic activity was at the protein level as both ACAT1 and 2 protein, but not mRNA levels, were lower (p=0.02 and <0.0001, respectively). ACAT2 activity was significantly associated with hepatic total cholesterol, plasma V+IDLC cholesterol, and atherosclerosis.
Conclusions
Atheroprotective effects of estrogen therapy may be related to reduced hepatic secretion of ACAT2-derived cholesteryl esters in plasma lipoproteins.
Condensed Abstract
Estrogen inhibits atherogenesis. We demonstrate in ovariectorized monkeys that estrogen therapy led to lower hepatic and circulating lipoprotein cholesterol, and lower ACAT2 protein and associated activity levels as compared to controls. Hepatic ACAT2 activity was highly correlated with, and was an independent predictor of coronary artery atherosclerosis extent.
Keywords: ACAT2, coronary artery atherosclerosis, estrogen, hepatic cholesterol, lipoproteins
Estrogens have beneficial effects on plasma lipid and lipoprotein concentrations and reduce atherosclerosis extent in a number of animal models including mice, rabbits and monkeys 1, 2. In postmenopausal women, hormone therapy improves lipoprotein concentrations and reduces atherosclerosis (as determined by carotid-intimal medial thickness) in primary prevention studies 3 but not when cardiovascular disease (CVD) is present 4. In the Women’s Health Initiative (WHI), an increased number of CVD events in the first year of therapy with combined conjugated equine estrogens (CEE) plus progestin (medroxyprogesterone acetate) was reported in 2003 5. In 2004, the results from WHI-estrogen-only trial showed no increase in overall CVD events and potential benefit for heart disease in women 50 to 59 years old 6. More recently, a subsequent report from the WHI showed that women 50 to 59 years of age had 42% reduced coronary calcification with estrogen therapy, which was even greater (61%) in those women adherent to treatment 7 indicative of reduced or less complicated atherosclerotic lesions. Hypothesized mechanisms underlying these discrepancies include pro-inflammatory effects which may be particularly important when advanced lesions are present 8, 9.
The mechanisms for atheroprotection with estrogens are complex and have been shown to be due, in part, to beneficial effects on plasma lipids and lipoproteins 2, 8 mediated primarily by changes in hepatic cholesterol metabolism 8, 10. The effects of exogenous estrogens on plasma lipoproteins vary with dose, route of administration, and formulation but generally are characterized by a decrease in total and low-density-lipoprotein cholesterol (TPC and LDLC, respectively) and an increase in high-density-lipoprotein cholesterol (HDLC) and triglycerides (TG). Oral estrogens dose-dependently lower plasma LDLC concentrations due to a first-pass effect that results in increased hepatic LDL catabolism 11, 12. Studies in animal models and cell culture systems suggest this is a result of estrogen-induced hepatic LDL receptor upregulation; however our previous results in monkeys treated with clinically relevant estrogen doses found no increase in LDL receptor mRNA abundance despite decreased hepatic cholesterol content 13. This suggests that changes in LDL receptor may be a secondary response to estrogen-related changes in hepatic cholesterol metabolism.
The purpose of this study was to expand upon our previous studies and assess other known steps in hepatic cholesterol metabolism that may be estrogen-sensitive, specifically hepatic cholesterol 7α-hydroxylase (C7H) and acyl-coenzyme A:cholesterol acyltransferase (ACAT) activities. C7H is the rate-limiting step in hepatocyte bile acid synthesis in the primary pathway for cholesterol catabolism and elimination from the body 14. Two ACAT isoforms (ACAT1 and ACAT2) are responsible for cholesteryl ester formation in tissues. ACAT2 is found in hepatocytes and facilitates cholesteryl ester incorporation into apoprotein (apo) B-containing lipoproteins and represents the majority of hepatic ACAT activity 15. ACAT2 is also the dominant cholesterol esterifying enzyme in the enterocyte 16. By contrast, ACAT1 is found in liver Kupffer cells and macrophages of many tissues 16 and, in these locations, is assumed to be responsible for cellular cholesterol homeostasis 17. Previous studies in monkeys have shown a high correlation between hepatic ACAT activity, VLDL secretion, LDL particle size, and coronary atherosclerosis 18, 19. In this study, we extend our previously published findings that estrogens decrease progression of coronary artery atherosclerosis in ovariectomized (OVX) monkeys 1 by documenting associated effects on hepatic cholesterol metabolism and reporting a high and significant correlation between estrogen-induced alterations in hepatic cholesterol metabolism and coronary artery atherosclerosis extent.
Methods
Study Design
Monkeys evaluated were OVX cynomolgus macaques (n=34) fed a moderately atherogenic diet (40% of calories supplied as fat supplemented with 0.28 mg cholesterol/kcal) for 30 months 1. Diet was fed with either no hormones (control, n=17) or with conjugated equine estrogens (CEE, n=17) at a human dose equivalent of 0.625 mg/day. At necropsy, the liver was weighed, and portions of liver were minced prior to immediate freezing in liquid nitrogen for storage at -70° C until analysis.
Plasma lipoprotein cholesterol and LDL molecular weight
Plasma collected at study end was measured for TPC, HDLC, TG and average LDL molecular weight as previously described 1. The cholesterol distribution among lipoproteins was determined after isolation by ultracentrifugation and separation of lipoprotein classes with high performance liquid chromatography (HPLC) 1.
Hepatic Cholesterol Concentrations
Lipids were extracted 20 with total and free cholesterol concentrations (TC and FC) determined enzymatically 21. Esterified cholesterol (EC) concentration was calculated as the difference between TC and FC. TG content was measured as previously described 22.
Hepatic C7H and ACAT Activity
Hepatic microsomes were prepared from frozen tissue after homogenization and centrifugation to remove membranes and cell debris. The supernatant was recentrifuged and the resulting microsomal pellet was resuspended in Tris buffer whereupon protein concentration was measured 23. C7H activity was determined as described by Rudel 24 and co-workers adapted from the method of Ogishima and Okuda 25. Total ACAT activity was determined as described previously 17, 18. Microsomes were first pre-incubated for 30 minutes with a cholesterol-saturated solution of β-hydroxypropyl cyclodextrin and the ACAT reaction was then initiated in the presence or absence of a specific ACAT2 inhibitor 26, pyripyropene A, at a concentration of 5 μmol/l to separately identify ACAT1 (uninhibited) and ACAT2 (total - ACAT1) activities 15. ACAT and C7H activities are expressed as nmol/min/mg and pmol/min/mg microsomal protein, respectively.
Immunoblotting for LDL receptor, C7H, ACAT1 and 2
LDL receptor was quantified in membrane proteins, while microsomal protein was used to quantify ACAT and C7H. Protein samples were electrophoresed on Novex pre-cast Tris-Glycine gels (Invitrogen, Carlsbad CA) prior to transfer to polyvinylidene fluoride membranes for LDL receptor, or nitrocellulose for ACAT and C7H. Nonspecific binding was blocked by incubation in 5% non-fat dried milk in Tris-buffered saline with Tween-20. Monospecific fusion protein antibodies to the N-terminal 100-110 amino acids of ACAT1 and ACAT2 were prepared in rabbits and purified as described by Lee et al. 17. C7H antibodies are fusion protein antibodies prepared in chickens against the sequence from AA353-436 of African green monkey C7H. The primary ACAT and C7H antibodies were applied to the nitrocellulose membrane in blocking solution at 3 μg/ml for ACAT1 and 2, and 1 μg/ml for C7H. A donkey anti-rabbit secondary antibody conjugated to horseradish peroxidase in a 1:20000 dilution was applied to membranes for detection of ACAT1 and 2, whereas a goat anti-chicken secondary antibody conjugated to horseradish peroxidase was used for C7H at a 1:15 000 dilution (Amersham Pharmacia Biotech, Buckinghamshire, UK). The peroxidase signal was obtained using chemiluminescence reagents (ECL+ Western Blotting Detection, Amersham Biosciences UK, Buckinghamshire, UK) and detected using phosphorimaging (Storm 860, Molecular Dynamics, Sunnyvale, CA). The membranes were stripped of antibody and then re-probed for actin (Oncogene Research Products, San Diego, CA) according to manufacturer instructions. Quantification was determined using ImageQuant 5.2 software (Molecular Dynamics, Sunnyvale, CA) and results expressed as the ratio to actin densities.
RNA preparation and C7H and ACAT mRNA determination
Total RNA was extracted with Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Quantification of mRNAs was performed by the SYBR Green real-time polymerase chain reaction (PCR) in an ABI PRISM 7000 thermocycler (Applied Biosystems, Foster City, CA) using primer sequences for ACAT1, ACAT2, ABCG5 and C7H (CYP 7A) as previously published 15. Primer sequences used for HMG-CoA reductase were forward 5′-GACGCAACCTTTATATCCGTTT-3′ and reverse 5′-TTGAAAGTGCTTTCTCTGTACCC-3′; HMG-CoA synthase (cytoplasmic) forward 5′-TTCGTGGCTCACTCCCTTT-3′ and reverse 5′-CTGTCACTGTTTCCTCCTTGG-3′; HMG-CoA synthase (mitochondrial) forward 5′-CTCGCTTCTGTCCCACCA-3′ and reverse 5′-AAGAGAAGGCACCAATCCTG-3′. Data are expressed in arbitrary units normalized by the qRT-PCR signal obtained in the same cDNA preparation for glyceraldehyde 3-phosphate dehydrogenase mRNA.
Statistical Methods
Data are presented as mean ± standard error (SEM) for each group. Data were analyzed for normality and logarithmically transformed where necessary prior to comparisons by unpaired Student’s t-tests. Pair-wise associations between variables were evaluated by Pearson’s correlation coefficient. To assess associations between atherosclerosis and other variables, multiple regression analysis was performed with backward stepwise evaluation of independent variables. Significant predictors of atherosclerosis extent determined by the multiple regression model were used as covariates in ANCOVA to assess the contribution of these variables to group differences in atherosclerosis extent. Statistical analysis was performed using Statistica 6 (StatSoft Inc., Tulsa, OK) with significance set at α < 0.05.
Results
Table 1 details body and liver weights. Liver weight was not different despite the 60% lower TG content in the CEE group. Plasma lipid and lipoprotein cholesterol concentrations are also reported in Table 1. CEE-treatment resulted in a statistically significant lowering of cholesterol concentration in very-low and intermediate-density lipoproteins (V+IDLC; p=0.01). Total, LDLC and HDLC were not changed. Plasma TG concentrations were increased (p<0.001) and LDL particle size, as estimated by molecular weight, was decreased (p=0.002) in the CEE-treated animals compared to controls.
Table 1.
Bodyweight (BW), liver actual and relative weight, total liver triglyceride (TG) (Mean ± SEM, n=13 -17/group), plasma cholesterol (TPC), very-low + intermediate-density lipoprotein cholesterol (V+IDLC), low- and high-density lipoprotein cholesterol (LDLC and HDLC) and triglycerides (TG) concentrations and LDL molecular weight (LDL mol.wt.) in control and CEE-treated OVX monkeys (Mean ± SEM, n=17/group). Conversion to mmol/L cholesterol and TG by multiplying by 0.0259 and 0.0113, respectively
| CTL | CEE | p-value | ||
|---|---|---|---|---|
| BW | (kg) | 3.13 (0.27) | 2.80 (0.098) | 0.3 |
| Liver Wt | (g) | 72.74 (5.80) | 68.22 (2.74) | 0.6 |
| Relative Liver Wt | (%) | 2.34 (0.12) | 2.46 (0.090) | 0.41 |
| Liver TG | (mg/g) | 74.96 (13.95) | 41.86 (7.15) | 0.04 |
| TPC | (mg/dL) | 419.69 (23.55) | 384.56 (26.25) | 0.32 |
| V+IDLC | (mg/dL) | 49.03 (6.18) | 29.31 (4.31) | 0.01 |
| LDLC | (mg/dL) | 322.21 (21.81) | 314.32 (25.70) | 0.81 |
| HDLC | (mg/dL) | 49.76 (5.19) | 41.24 (2.88) | 0.13 |
| TG | (mg/dL) | 34.41 (6.88) | 65.76 (19.22) | <0.001 |
| LDL mol.wt. | (g/mmol) | 4.0 (0.12) | 3.5 (0.10) | 0.002 |
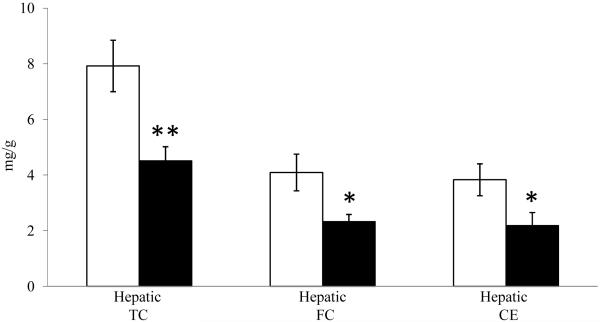
Hepatic TC was reduced approximately 50% by CEE-treatment (p<0.001, Figure 1) compared to control. This was due to equivalent reductions in both FC and CE concentrations (p<0.05 for both). There were no differences in total hepatic protein concentrations (mg/g wet wt) between groups (data not shown; p=0.33).
Figure 1.
Mean (± SEM) hepatic cholesterol content (total, free and esterified: TC, FC, and CE respectively) in control (□) and CEE-treated (■) monkeys (n=17/group). ** p<0.01; * p<0.05.
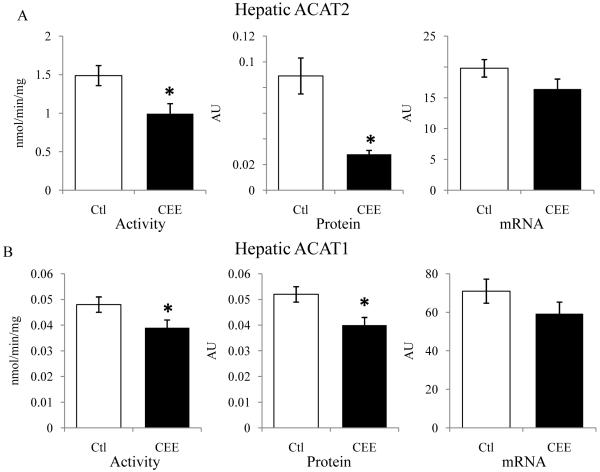
Hepatic total ACAT activity was significantly less in CEE-treated monkeys (1.54±0.13 nmol/min/mg vs. 1.03±0.13 nmol/min/mg, p=0.01). ACAT2 represents the majority (>95%) of hepatic ACAT enzymatic activity and ACAT2 activity was about a third lower with CEE treatment (1.49±0.13 vs. 0.99±13 nmol/min/mg, p=0.01), depicted in Figure 2A. CEE treatment resulted in lower ACAT2 protein concentrations by more than two thirds (0.089±0.014 vs. 0.028±0.003 AU, p<0.001), while ACAT2 mRNA abundance was not statistically different (19.7±1.4 vs. 16.4±1.6 AU, p=0.13) (Figure 2A). For ACAT1, CEE treatment resulted in about 20% lower activity (0.048±0.003 vs. 0.039±0.003 nmol/min/mg, p<0.03), and similarly decreased ACAT1 protein (0.052±0.005 vs. 0.040±0.009 AU, p<0.02) while not affecting ACAT1 mRNA (70.9±6.3 vs. 59.3±6.0±0.04 AU, p=0.20) (Figure 2B). Since ACAT2 is expressed in hepatocytes while ACAT1 is expressed primarily in Kupffer cells, these results suggest that estrogen effects on protein and activity are present in both cell types through mechanisms that are not mediated principally through gene expression. The majority of estrogen-mediated reduction in ACAT activity was on the ACAT2 isoform present in hepatocytes, however.
Figure 2.
Mean (± SEM) activity (ACT), protein (Pro), and mRNA comparisons for ACAT2 and ACAT1 in livers of control (□) and CEE-treated (■) monkeys (n=17/group). * p<0.05.
Only ACAT2 protein was associated with total ACAT activity and hepatic TC content (R=0.64, p<0.001; R=0.48, p<0.01 respectively). Additionally, ACAT2 activity correlated highly with ACAT2 protein (R=0.64, p<0.001), ACAT2 mRNA (R=0.56, p=0.002), TPC (R=0.38, p=0.03), V+IDLC (R=0.45, p=0.01), and LDL size (R=0.55, p=0.001). ACAT1 protein also was associated with V+IDLC (R=0.38, p=0.03) and LDL size (R=0.48, p=0.006).
CEE-treatment resulted in a significant 85% higher C7H protein mass estimated by Western blotting (p=0.005, Table 2) which paralleled C7H activity and mRNA expression though the latter were not statistically different from control (p=0.23, and 0.22, respectively; Table 2). There was no significant effect of CEE on expression of LDL receptor protein (p=0.15) or mRNA levels of several other proteins commonly associated with cholesterol efflux and synthesis (Table 2).
Table 2.
Hepatic C7H activity, protein, and mRNA and LDLR protein and selected mRNA of genes that modulate hepatic cholesterol levels (cholesterol efflux: ABCA1, ABCG8 and synthesis: HMG-CoA reductase and synthases) in control and CEE-treated monkeys (Mean ± SEM, n=17/group)
| CTL | CEE | p-value | ||
|---|---|---|---|---|
| LDLR protein | (AU) | 6.57 (0.5) | 7.64 (0.52) | 0.15 |
| C7H activity | (pmol/min/mg) | 1.17 (0.20) | 2.48 (0.58) | 0.23 |
| C7H protein | (AU) | 3.48 (0.50) | 6.50 (0.88) | 0.005 |
| C7H mRNA | (AU) | 35.86 (9.61) | 61.24 (16.88) | 0.22 |
| ABCA1 mRNA | (AU) | 9.33 (0.62) | 9.41 (0.75) | 0.60 |
| ABCG8 mRNA | (AU) | 142.38 (8.70) | 151.23 (10.29) | 0.52 |
|
HMG-CoA Reductase mRNA |
(AU) | 6.19 (0.69) | 5.98 (0.47) | 0.80 |
|
HMG-CoA Synthase (cytoplasmic) mRNA |
(AU) | 0.30 (0.06) | 0.55 (0.11) | 0.061 |
|
HMG-CoA Synthase (mitochondrial) mRNA |
(AU) | 1612.30 (176.32) | 1709.16 (200.89) | 0.72 |
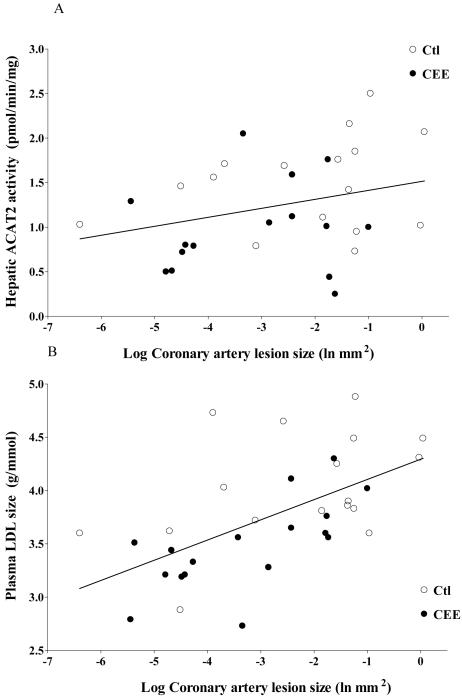
As previously reported, coronary artery atherosclerosis extent, determined as intimal area (mm2) [defined as the cross-sectional area occupied by intimal lesion (plaque size)] was significantly lower with CEE-treatment (0.255±0.08 vs. 0.09 ±0.02 mm2) 3. Correlation and multiple regression analyses were done to evaluate CEE-treatment effects on cholesterol metabolism and atherosclerosis. Coronary artery intimal area (mm2) was positively associated with ACAT2 activity (R=0.44, p=0.01, Figure 3A) with LDL size (R=0.58, p<0.001, Figure 3B). In regression analysis using all study outcome variables as independent variables, ACAT2 protein and LDLR were significant positive predictors while HDLC and C7H protein were significant negative predictors of atherosclerosis cumulatively accounting for 67% of variability in lesion size (Table 3). ACAT2 activity was then used as a covariate in ANCOVA which eliminated group differences in hepatic FC and CE content, providing evidence that CEE mediates effects on hepatic cholesterol metabolism though reduction in ACAT2 activity.
Figure 3.
Scatterplot (n=34) demonstrating the relationship between coronary artery atherosclerosis extent (depicted on the x-axis as the logarithmic transformation of intimal area (mm2) and A) Total hepatic ACAT2 activity (R=0.44; p=0.01) and B) plasma LDL molecular weight (R=0.58; p<0.001) in control (○) and CEE (●) treated monkeys.
Table 3.
Multiple regression analysis (overall model R2 = 0.67; p < 0.0001) with parameter estimates of β (slope), the standard error (SE) of β, squared semi-partial correlation coefficients (SrII2; percent of variance uniquely accounted for by the model parameter) and statistical results for significant predictors of coronary artery atherosclerosis
| β-coefficient | SE of β | SrII2 | p-value | |
|---|---|---|---|---|
| HDLC | -0.407 | 0.127 | 11.94% | 0.004 |
| ACAT2 protein | 0.397 | 0.133 | 10.23% | 0.007 |
| LDLR | 0.385 | 0.124 | 11.16% | 0.005 |
| C7H protein | -0.302 | 0.118 | 7.61% | 0.02 |
Discussion
The major finding from this study is that estrogens (CEE) decrease total hepatic ACAT activity in ovariectomized monkeys, due primarily to reductions in ACAT2, the isoform of the enzyme found in hepatocytes. ACAT2 activity was assayed utilizing the selective inhibition by pyripyropene A and found to be responsible for over 95% of hepatic microsomal activity, a finding consistent with our previous report 26. CEE treatment resulted in a greater reduction of ACAT2 protein levels compared to that of ACAT1. Further, ACAT2 activity was significantly and independently associated with atherosclerosis extent. This suggests that a primary mechanism for the atheroprotective effects of estrogens could be through a reduction in the ACAT2 enzyme activity in hepatocytes and the associated reduction in the product cholesteryl esters that get secreted into apoB-containing lipoproteins 18, 19.
Elevation in plasma concentrations of apoB-containing lipoproteins is a known risk factor for atherosclerosis development. Both the concentration of these lipoproteins and the size of the particles are considered important in atherogenesis 27. The incorporation of cholesteryl esters into apoB-containing lipoprotein particles is regulated by ACAT2. Higher ACAT2 activity results in increased hepatic secretion of VLDL particles that are enriched in cholesteryl oleate (as oleoyl-CoA is a primary substrate for ACAT). Resulting LDL particles are larger and more cholesteryl oleate-enriched 19. ACAT activity has been associated with increased atherosclerosis extent in African green monkeys 18 and deletion of the ACAT2 gene has been shown to protect against atherosclerosis in mice 22, 28. The present studies suggest that estrogen can act to limit ACAT2-derived cholesteryl oleate enrichment of apoB-containing lipoproteins, thus providing an additional mechanism underlying the atheroprotective effects of estrogen.
The effect of CEE treatment on reducing hepatic total ACAT activity was determined to be primarily the result of decreased activity and protein levels of the ACAT2 isoform. This enzyme is expressed in the hepatocytes of liver and in the enterocytes of the intestine of human and nonhuman primates, the two cell types in the body where apoB-containing lipoprotein production, assembly and secretion occurs 15, 17. CEE treatment did not result in significantly lower ACAT2 mRNA although the trend was in the same direction as the protein, suggesting that ACAT2 protein and activity is primarily modified post-transcriptionally and may be related to hepatic free cholesterol content as shown previously 29. This is consistent with the significant correlation that was found between hepatic cholesterol content and ACAT2 protein and ACAT activity.
Findings related to the effects of estrogen on ACAT have been inconsistent, with studies of rats reporting no effect on total ACAT activity 30 and baboons showing a 40% reduction that did not reach statistical significance 31. However recent studies have shown that tamoxifen (a selective estrogen receptor modulator) and other estrogen receptor ligands (including estradiol) display three-dimensional structural homology with SAH 58-035, shown in vitro to be a prototypical competitive inhibitor of the ACAT enzyme 32. Estradiol directly inhibits ACAT only at supraphysiologic concentrations (IC50≈20μM) 32 whereas estrogen concentrations measured in premenopausal women and CEE-treated primates are in the nanomolar range 1, suggesting significant direct inhibition of ACAT2 in vivo is unlikely. Estrogens appear more likely to exert effects on ACAT through estrogen’s pleiotropic nuclear receptor action and regulation of ACAT synthesis, or through reductions in hepatic cholesterol content.
Lower hepatic cholesterol content may result from estrogen enhancement of biliary secretion of both free cholesterol and bile acids. The increase in the cholesterol to bile acid ratio can result in gallstone formation, which is a relatively common occurrence with hormone therapy 33. One mechanism for increased biliary sterol secretion involves C7H, the rate limiting enzyme in bile acid production. The increases in C7H protein expression and trend toward increased activity and mRNA with estrogen treatment is consistent with our prior report 13 and those of others 34, 35 on C7H message levels in monkeys. Hepatic sterol 27 hydroxylase is involved in another pathway for increased biliary sterol secretion. It catalyzes the first step in the “alternative” bile acid biosynthetic pathway and has also been found to be increased with estrogens 31. While a large portion (50-60%) of the secreted biliary cholesterol is reabsorbed in the GI tract, the loss of fecal cholesterol still represents the primary mechanism for cholesterol removal from the body. An increased conversion of cholesterol into bile acids via increased C7H would appear to be consistent with the decrease in hepatic cholesterol accumulation.
Our finding of reduced hepatic free cholesterol with CEE treatment, and reduced ACAT2 activity, is additionally supported by recent elucidation of the ‘alternative’ pathway for cholesterol excretion in ACAT2-deficient mice 36. In the alternative pathway, cholesterol is transported to proximal segments of the small intestine and is excreted directly and independently of bile. Evidence for this non-dietary sterol loss through the feces was also seen in dogs and people 37, 38 with a net reduction in enterohepatic recirculation of biliary cholesterol. Free cholesterol also was apparently not shunted to HDL biogenesis as HDL-associated cholesterol and ABCA1 levels were comparable between groups. Increased fecal cholesterol excretion is seen with ACAT2 gene disruption 36 and the use of bile acid sequestrants. Both interventions also result in higher plasma TG and greater fecal sterol loss, however ACAT2 gene disruption did not increase biliary sterol secretion. The similarity in clinical profile suggests that CEE-related reductions in ACAT2 activity may be coupled with free cholesterol loss through the ‘alternative’ pathway 36.
Reduction of cholesterol absorption from the intestine is another mechanism for reducing hepatic cholesterol concentration. While we did not determine estrogen effects on intestinal ACAT, it is possible that estrogen reduces intestinal ACAT2 (the predominant isoform in enterocytes) in a manner similar to hepatic ACAT2. CEE was not shown to have an effect on cholesterol absorption in a previous report using this same model 39, but decreases of 18% and 9% following oral and transdermal estrogen therapy, respectively, have been noted in women 40. Lower intestinal ACAT2 activity does lead to reduced cholesterol absorption from the intestine 41, 42 but regulation of intestinal and hepatic ACAT2 activity can be independent, as has been demonstrated in diabetes 43, allowing the possibility that ACAT2 activity reductions in liver may not be seen concurrently in the intestine. This is supported by studies in primates where similar plasma lipid improvements were documented following CEE treatment, although cholesterol absorption was not significantly altered39.
In this study there were no significant changes in hepatic LDL receptor protein with CEE treatment, as has been reported previously in the same model 13 and in rats 35. However we have seen increases in hepatic LDL fractional degradation rates with the same estrogen dose 10 although this would include both LDL receptor- and nonreceptor-mediated uptake. Increased catabolism of VLDL and LDL cholesterol, via receptor dependent and independent mechanisms, may factor into the observed predominance of smaller plasma LDL particles. Studies in women found that estrogen increases both the production and clearance of large LDL and small LDL, however the greater effect was on clearance of large LDL, resulting in an overall increase in concentration of small LDL. The notion that smaller particles are inherently more atherogenic has been questioned 44. A decreased CE secretion associated with the estrogen-related reduction in hepatic ACAT2 activity could result in plasma very low density LDL, intermediate density LDL and LDL particles with few CE molecules; such a compositional shift may indicate a situation where these lipoproteins are unable to result in the deposition of as much cholesterol in the arterial intima during atherogenesis.
Elevated plasma TG is seen commonly with oral estrogen therapy. Similarly, TG elevation is a consistent feature of ACAT2 disruption in mouse models 28, 41, 45, 46. The mechanisms are unknown, however unpublished data from our group demonstrates higher hepatic TG secretion rates in ACAT deficiency. One hypothetical mechanism undlerlying this effect is that hepatic TG is more efficiently mobilized when hepatic CE depletion is present. Hepatic lipase is generally thought to be decreased by oral estrogen treatment 47 and thus the contribution of liver TG and hepatic lipase activity on plasma lipids and atherosclerosis is controversial 48. Hepatic lipase activity was 40% lower with CEE treatment in the primate model, however the lipid measures in plasma and liver were of the same magnitude as reported here 10.
In conclusion, CEE-treatment of ovariectomized monkeys decreased hepatic ACAT activity with reductions of both ACAT1 and ACAT2 protein and activity, with the major reduction seen in hepatocyte-associated ACAT2. Increased bile acid secretion, following C7H upregulation, may contribute to the decrease in hepatic cholesterol content which is important in preventing down-regulation of hepatic LDL receptors. If ACAT2 inhibition by estrogen is not liver specific, reductions in dietary and endogenous cholesterol absorption from the intestinal tract could further contribute to the reductions in hepatic cholesterol. Of great interest, regression analysis showed that ACAT2 was significantly and independently associated with coronary atherosclerosis extent. This suggests that suppression of ACAT2 activity and the associated decrease in V+ILDL CE secretion could play an important role in estrogen-induced inhibition of the progression of atherosclerosis.
Acknowledgements
This work was supported in part by grant HL-P01-45666 and HL-49373, National Heart Lung and Blood Institute, Bethesda, MD and Training Grant 5 T32 HL07115 (KK), National Institutes of Health Cardiovascular Pathology Training Grant.
Footnotes
No financial conflicts of interest exist for any author contributing to this manuscript.
Kavanagh; Estrogen decreases ACAT2 and atherosclerosis
References
- 1.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JD. Effects of sex steroid treatment on the cardiovascular system. Infertility and Reproductive Medicine Clinics of North America. 2001;12:511–533. [Google Scholar]
- 3.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu Cr, Liu Ch, Azen SP. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. CR. CH. [DOI] [PubMed] [Google Scholar]
- 4.Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, Faxon DP, Cashin-Hemphill L, Sanmarco ME, French WJ, Shook TL, Gaarder TD, Mehra AO, Rabbani R, Sevanian A, Shil AB, Torres M, Vogelbach KH, Selzer RH. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–545. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 8.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149–156. doi: 10.1055/s-2005-869482. [DOI] [PubMed] [Google Scholar]
- 10.Wagner JD, Schwenke DC, Zhang L, Applebaum-Bowden D, Bagdade JD, Adams MR. Effects of short-term hormone replacement therapies on low-density lipoprotein metabolism in cynomologus monkeys. Arterioscler Thromb Vasc Biol. 1997;17:1128–1134. doi: 10.1161/01.atv.17.6.1128. [DOI] [PubMed] [Google Scholar]
- 11.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe BM, Barrett PH, Laurier L, Huff MW. Effects of continuous conjugated estrogen and micronized progesterone therapy upon lipoprotein metabolism in postmenopausal women. J Lipid Res. 2000;41:368–375. [PubMed] [Google Scholar]
- 13.Colvin PL, Jr., Wagner JD, Adams MR, Sorci-Thomas MG. Sex steroids increase cholesterol 7alpha-hydroxylase mRNA in nonhuman primates. Metabolism. 1998;47:391–395. doi: 10.1016/s0026-0495(98)90048-8. [DOI] [PubMed] [Google Scholar]
- 14.Shefer S, Hauser S, Lapar V, Mosbach EH. Regulatory effects of sterols and bile acids on hepatic 3-hydroxy-3-methylglutaryl CoA reductase and cholesterol 7alpha-hydroxylase in the rat. J Lipid Res. 1973;14:573–580. [PubMed] [Google Scholar]
- 15.Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, Tomoda H, Omura S, Willingham MC, Rudel LL. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 2004;110:2017–2023. doi: 10.1161/01.CIR.0000143163.76212.0B. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 17.Lee RG, Willingham MC, Davis MA, Skinner KA, Rudel LL. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res. 2000;41:1991–2001. [PubMed] [Google Scholar]
- 18.Carr TP, Parks JS, Rudel LL. Hepatic ACAT activity in African green monkeys is highly correlated to plasma LDL cholesteryl ester enrichment and coronary artery atherosclerosis. Arterioscler Thromb. 1992;12:1274–1283. doi: 10.1161/01.atv.12.11.1274. [DOI] [PubMed] [Google Scholar]
- 19.Rudel LL, Haines J, Sawyer JK, Shah R, Wilson MS, Carr TP. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clin Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee RG, Kelley KL, Sawyer JK, Farese RV, Jr., Parks JS, Rudel LL. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ Res. 2004;95:998–1004. doi: 10.1161/01.RES.0000147558.15554.67. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Rudel L, Deckelman C, Wilson M, Scobey M, Anderson R. Dietary cholesterol and downregulation of cholesterol 7 alpha-hydroxylase and cholesterol absorption in African green monkeys. J Clin Invest. 1994;93:2463–2472. doi: 10.1172/JCI117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogishima T, Okuda K. An improved method for assay of cholesterol 7 alpha-hydroxylase activity. Anal Biochem. 1986;158:228–232. doi: 10.1016/0003-2697(86)90613-5. [DOI] [PubMed] [Google Scholar]
- 26.Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res. 2004;45:378–386. doi: 10.1194/jlr.D300037-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Lada AT, Rudel LL. Associations of low density lipoprotein particle composition with atherogenicity. Curr Opin Lipidol. 2004;15:19–24. doi: 10.1097/00041433-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV., Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003;100:1262–1267. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudel LL, Davis M, Sawyer J, Shah R, Wallace J. Primates highly responsive to dietary cholesterol up-regulate hepatic ACAT2, and less responsive primates do not. J Biol Chem. 2002;277:31401–31406. doi: 10.1074/jbc.M204106200. [DOI] [PubMed] [Google Scholar]
- 30.Letterie GS. Impact of subdermal norgestrel on hepatic acyl-coenzyme A:cholesterol-acyltransferase (ACAT) activity: possible antiatherogenic effect. Contraception. 2000;61:391–394. doi: 10.1016/s0010-7824(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 31.Kushwaha RS, Guntupalli B, Jackson EM, McGill HC., Jr. Effect of estrogen and progesterone on the expression of hepatic and extrahepatic sterol 27-hydroxylase in baboons (Papio sp) Arterioscler Thromb Vasc Biol. 1996;16:1088–1094. doi: 10.1161/01.atv.16.8.1088. [DOI] [PubMed] [Google Scholar]
- 32.de Medina P, Payre BL, Bernad J, Bosser I, Pipy B, Silvente-Poirot S, Favre G, Faye JC, Poirot M. Tamoxifen is a potent inhibitor of cholesterol esterification and prevents the formation of foam cells. J Pharmacol Exp Ther. 2004;308:1165–1173. doi: 10.1124/jpet.103.060426. [DOI] [PubMed] [Google Scholar]
- 33.Everson GT, McKinley C, Kern F., Jr. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J Clin Invest. 1991;87:237–246. doi: 10.1172/JCI114977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kushwaha RS, Born KM. Effect of estrogen and progesterone on the hepatic cholesterol 7-alpha-hydroxylase activity in ovariectomized baboons. Biochim Biophys Acta. 1991;1084:300–302. doi: 10.1016/0005-2760(91)90072-p. [DOI] [PubMed] [Google Scholar]
- 35.Parini P, Angelin B, Stavreus-Evers A, Freyschuss B, Eriksson H, Rudling M. Biphasic effects of the natural estrogen 17beta-estradiol on hepatic cholesterol metabolism in intact female rats. Arterioscler Thromb Vasc Biol. 2000;20:1817–1823. doi: 10.1161/01.atv.20.7.1817. [DOI] [PubMed] [Google Scholar]
- 36.Brown JM, Bell TA, 3rd, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG, Graham MJ, Crooke RM, Rudel LL. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pertsemlidis D, Kirchman EH, Ahrens EH., Jr. Regulation of cholesterol metabolism in the dog. I. Effects of complete bile diversion and of cholesterol feeding on absorption, synthesis, accumulation, and excretion rates measured during life. J Clin Invest. 1973;52:2353–2367. doi: 10.1172/JCI107424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng SH, Stanley MM. Secretion of cholesterol by intestinal mucosa in patients with complete common bile duct obstruction. Proc Soc Exp Biol Med. 1959;101:223–225. doi: 10.3181/00379727-101-24890. [DOI] [PubMed] [Google Scholar]
- 39.Greaves KA, Wilson MD, Rudel LL, Williams JK, Wagner JD. Consumption of soy protein reduces cholesterol absorption compared to casein protein alone or supplemented with an isoflavone extract or conjugated equine estrogen in ovariectomized cynomolgus monkeys. J Nutr. 2000;130:820–826. doi: 10.1093/jn/130.4.820. [DOI] [PubMed] [Google Scholar]
- 40.Karjalainen A, Heikkinen J, Savolainen MJ, Backstrom AC, Kesaniemi YA. Mechanisms regulating LDL metabolism in subjects on peroral and transdermal estrogen replacement therapy. Arterioscler Thromb Vasc Biol. 2000;20:1101–1106. doi: 10.1161/01.atv.20.4.1101. [DOI] [PubMed] [Google Scholar]
- 41.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 42.Krause BR, Anderson M, Bisgaier CL, Bocan T, Bousley R, DeHart P, Essenburg A, Hamelehle K, Homan R, Kieft K, et al. In vivo evidence that the lipid-regulating activity of the ACAT inhibitor CI-976 in rats is due to inhibition of both intestinal and liver ACAT. J Lipid Res. 1993;34:279–294. [PubMed] [Google Scholar]
- 43.Hori M, Satoh M, Furukawa K, Sakamoto Y, Hakamata H, Komohara Y, Takeya M, Sasaki Y, Miyazaki A, Horiuchi S. Acyl-coenzyme A:cholesterol acyltransferase-2 (ACAT-2) is responsible for elevated intestinal ACAT activity in diabetic rats. Arterioscler Thromb Vasc Biol. 2004;24:1689–1695. doi: 10.1161/01.ATV.0000137976.88533.13. [DOI] [PubMed] [Google Scholar]
- 44.Sacks FM, Campos H. Clinical review 163: Cardiovascular endocrinology: Low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 45.Bell TA, 3rd, Brown JM, Graham MJ, Lemonidis KM, Crooke RM, Rudel LL. Liver-specific inhibition of acyl-coenzyme a:cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein B100-only low-density lipoprotein receptor-/- mice. Arterioscler Thromb Vasc Biol. 2006;26:1814–1820. doi: 10.1161/01.ATV.0000225289.30767.06. [DOI] [PubMed] [Google Scholar]
- 46.Bell TA, 3rd, Kelley K, Wilson MD, Sawyer JK, Rudel LL. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiency in ApoB100 only, LDLr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:1396–1402. doi: 10.1161/ATVBAHA.107.142802. [DOI] [PubMed] [Google Scholar]
- 47.Colvin PL, Jr., Auerbach BJ, Case LD, Hazzard WR, Applebaum-Bowden D. A dose-response relationship between sex hormone-induced change in hepatic triglyceride lipase and high-density lipoprotein cholesterol in postmenopausal women. Metabolism. 1991;40:1052–1056. doi: 10.1016/0026-0495(91)90129-k. [DOI] [PubMed] [Google Scholar]
- 48.Santamarina-Fojo S, Gonzalez-Navarro H, Freeman L, Wagner E, Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1750–1754. doi: 10.1161/01.ATV.0000140818.00570.2d. [DOI] [PubMed] [Google Scholar]