Abstract
PURPOSE OF THE REVIEW:
Particularities of the fetal immune response to infection cause a heightened inflammatory state that acts synergistically with microbial insult to induce damage. Proteomics offers the opportunity for detecting fetuses at risk of sepsis and neurological injury.
RECENT FINDINGS:
Molecular tools (16S-rRNA) demonstrate the diversity of microbial agents of intra-amniotic infection exceeds what is suspected clinically or is documented by cultures. The resulting inflammatory process has the potential to damage the fetus in utero. Stepwise algorithms [mass restricted (MR) score] have been developed to extract proteomic profiles characteristic of amniotic fluid (AF) inflammation. The MR score includes 4 proteomic biomarkers: defensin-2, defensin-1, S100A12 and S100A8 proteins. Other AF biomarkers relevant for preterm birth are S100A9 and insulin-like-growth-factor-binding protein 1 (IGFBP-1). S100A12, ligand for the receptor of advanced glycation end-products (RAGE), has the strongest association with histological chorioamnionitis and funisitis. Presence of S100A12 and S100A8 in AF is predictive of early-onset neonatal sepsis and poor neuro-developmental outcome.
SUMMARY:
Presence of AF proteomic biomarkers of inflammation is associated with increased inflammatory status of the fetus at birth. Future challenges are finding biomarkers that provide insight into molecular mechanisms of chronic fetal and neonatal cellular damage and identify candidates for early neuro-protection strategies.
Keywords: amniotic fluid, biomarkers, inflammation, defensin, calgranulin, S100 proteins
INTRODUCTION
Despite significant research and a variety of interventions, preterm birth (PTB) has reached an unprecedented 12.8% of all deliveries in the USA [1]. Thus, studies to identify biomarkers that can predict adverse outcomes for infants born preterm are critical [2,3].
In the U.S., 500,000 premature infants are born annually, 60,000 of which have a birthweight less than 1,500 g [4]. The survival rate of premature infants has continued to improve as medical technology and care of the neonate in intensive care units has progressed [5,6**]. The margin of viability and the likelihood of a favorable outcome have steadily increased for infants born at 23 weeks' gestational age (GA) and survival to hospital discharge for infants ranging from 23 to 26 weeks' gestation is about 70% [6]. A significant step forward has been the development of web-based tools that allow for rapid and more thorough parental counseling regarding the likelihood of survival, benefit from intensive care therapy and likelihood of short and long-term complications and disabilities that relate to PTB. Physicians are encouraged to consult and use in their practice one such tool recently developed by the NICHD at www.nichd.nih.gov/neonatalestimates.
Preterm parturition is the leading cause of neonatal morbidity and mortality
PTB remains the leading cause of neonatal mortality, morbidity and long term neuro-developmental disability [7]. African-American infants, in particular, are at higher risk of death in both neonatal and post-neonatal periods, although the exact causes of this ethnic disparity are not well understood [8]. Prematurity accounts for approximately 50% of long-term neurological handicaps, including blindness, deafness and cerebral palsy [9,10,11]. Although the increased risk of cerebral palsy with decreasing GA is well established, [11] compelling evidence suggests that the poor outcome observed in many children delivered prematurely is not entirely dependent on their GA at birth or birth weight [6]. In addition to GA, factors such as fetal gender, exposure to antenatal corticosteroids, singleton versus high order gestation, histological chorioamnionitis, a robust fetal inflammatory response to infection and differences in genetic make-up should be taken into consideration [6,12**,13].
The societal burden of prematurity is underscored by the fact that medical and other supportive care expenses exceed the estimated lifetime earnings per survivor with a birth weight less than 900 grams (~27 wks) [7]. Unfortunately, although a significant effort has been made to identify women at risk of PTB, the appropriate therapy still remains unknown [14,15]. For years the attention has focused on cessation of myometrial contractions using tocolytics [16]. Regretfully, the efficacy of this approach has been disappointing and subject to powerful debate [17] as data from randomized clinical trials suggests tocolytics cannot prolong pregnancy more than 48 hours [18,19]. A small benefit, however, still remains plausible since prolongation of gestation might be beneficial (in select cases where intrauterine infection has been ruled out) to allow for administration of corticosteroids, which have been unanimously successful for prevention of several complications related to prematurity, such as respiratory distress syndrome, intra-ventricular hemorrhage and necrotizing enterocolitis [6,16].
Human brain development is uniquely complex and the developing brain is in particular more vulnerable to injury than the adult brain [20]. When brain growth is stopped or delayed by infection or exposure to noxious endogenous or environmental factors, virtually no potential for subsequent regeneration and repair to its pre-injured state exists. This inevitably leads to fetal death in utero, long-lasting or permanent consequences for the newborn [21,22,23].
The most recent randomized trial conducted by Rouse et al. and the Maternal Fetal Medicine Units Network proposed that fetal exposure to magnesium sulfate before early preterm delivery may reduce the rate of cerebral palsy among survivors [24**]. Though provocative, the results of this trial have generated more questions than answers given that overall magnesium sulfate did not impact the primary composite outcome of moderate or severe cerebral palsy or death. Uncovering the mechanism responsible for magnesium sulfate's selective protection of survivors of early preterm birth against cerebral palsy will be an important next step.
The prevalence of intra-amniotic infection and microbial diversity in PTB is higher than suspected clinically
Worldwide, infection is the primary known etiological factor of PTB [25]. Adverse pregnancy outcomes related to infection are due to a direct microbial attack on the fetus and/or activation of the myometrial contractile machinery and premature expulsion of the baby from the maternal womb [26,27**]. Over 30 years ago Larsen et al. provided the first evidence that intrauterine infection is an important determinant of PTB [28]. Yet, the true involvement of infection as a disease mechanism for PTB is still difficult to determine due to the large variety of clinical indications for amniotic fluid (AF) sampling, diverse clinical indications for histological and microbiological examination of the placenta, various GA at the time of evaluation and often absence of maternal symptoms of clinical chorioamnionitis [26,29,30,31].
During the last two decades it has become clear that intra-amniotic infection has a strong association with PTB [29,32**,33,34,35]. Data from our studies support a causal role of infection in at least 10% of patients with preterm labor and intact membranes and 38% of patients with preterm premature rupture of membranes (PPROM) [12,32].
A variety of microbial pathogens have been implicated as etiologic agents of intra-amniotic infection [36,37,38]. The infectious etiology attendant PTB was extensively reviewed [29,38,39,40,41] and there is consensus that the spectrum is most often polymicrobial [29,38]. The most frequently involved pathogens are thought to originate primarily from the genital flora (Gardnerella vaginalis, Mycoplasma hominis, Ureaplasma, Peptostreptococcus and Bacteroides species) [38,39]. Unfortunately, at the time of this writing, confirmation of an infectious etiology in both mother and the neonate is based on a limited number of laboratory techniques for cultivation and growth of the pathogens [42]. The disadvantage of using a culture-based approach for microbial identification is that only a limited number of pathogens are targeted for identification. Thus, “uncultivated” or “difficult-to-cultivate” bacteria cannot be found when relying on culture conditions alone [43**,44].
Culture-independent methods, such as PCR, can detect AF bacterial DNA in 35% to 60% of pregnancies complicated by PTB [45,46]. Using mass spectrometry technology we showed that presence in AF of proteomic biomarkers characteristic of inflammation is not always accompanied by a positive microbial culture result [32]. We questioned why the difference? A recent study conducted by Han et al. provided a comprehensive and unbiased screening of the AF bacterial diversity in pregnancies complicated by intra-amniotic infection [47,48**] and showed that that approximately 60% of the species detected by culture-independent methods could not be identified by culture alone [48]. The key finding of this study is that both uncultivated and difficult-to-cultivate species, such as Fusobacterium nucleatum, Leptotrichia/Sneathia, Bergeyella, Peptostreptococcus, Ureaplasma parvum, Bacteroides and Clostridiales species, can be isolated from AF of women with proteomic biomarkers characteristic of intra-amniotic inflammation even in the absence of a positive microbial culture [48]. This suggests that in pregnancies complicated by PTB both the prevalence of AF infection and microbial diversity is underestimated.
Neonatal sepsis is a major cause of morbidity and mortality in the newborn
Intrauterine infection and subsequent inflammation contributes disproportionately to neonatal mortality and morbidity when adjusted for GA at delivery [23,49,50]. Both early- and late-onset sepsis occur with increased frequency in premature neonates [50,51,52,53,54]. Importantly, intrauterine infection is a risk factor for early onset neonatal sepsis (EONS), which is defined as confirmed or suspected sepsis in the first 72 hours post-delivery [32]. In statistics published by Yale-New Haven Hospital, which has the longest running, single-center database of neonatal sepsis started in 1928, mortality attributable to sepsis remains at 11% despite advances in neonatal care [36].
Antibiotics are routinely used ante- and post-partum to decrease the risk of neonatal group B streptococcal (GBS) infections and reduce the risk of neonatal illness following delivery [55,56,57,58]. There is a widely held view that the widespread use of antibiotics to either prolong pregnancy or prevent GBS sepsis has led to a shift in pathogens with the majority of EONS now caused by Gram-negative bacteria, with Escherichia coli as the most frequent pathogen rather than by Gram-positive species (i.e. GBS) [50,51]. Not surprisingly, there is concern that increased use of antibiotics may further change the diversity of microbes in EONS, increasing the diagnostic challenges [59]. A positive blood or other body fluid culture is regarded as the gold standard for diagnosing sepsis. Unfortunately, a positive culture ranges from 8% to 73% in the diagnosis of suspected neonatal sepsis [12,32,60]. Furthermore, the clinical symptoms of EONS are nonspecific and often manifest in the absence of a positive culture. There are several explanations for why it is difficult to diagnose EONS. One obstacle is related to the narrow spectrum of pathogens looked for in the microbiology laboratory. For example, searching for Ureaplasma and Mycoplasma species is not a routine practice in neonates who have a microbiological work-up for sepsis, and yet a recent study by Goldenberg et al. to evaluate the frequency of umbilical cord blood infections with these species in PTBs at 23-32 weeks found that 23% of the blood cultures proved positive for one of these pathogens [61*]. These results may thus explain why neonates can manifest clinical symptoms or have hematological indices suggestive of EONS in the absence of positive microbial culture results [12,62,63]. It is also plausible that analogous to intra-amniotic inflammation, “uncultivated” and “difficult-to-cultivate” species are responsible for fetal and neonatal sepsis as well. Data supporting this premise has shown that 16S rDNA PCR technology can improve the accuracy of culture-based methods for diagnosis of neonatal sepsis [64].
The recent analysis of childhood outcomes of the ORACLE I & II clinical trials [57,58] showed that antibiotics given to women in threatened preterm labor increased the risk of cerebral palsy and thus generated major clinical concern [65**]. The reason antibiotics do not decrease cerebral palsy as would be expected if pathogens are etiologic agents of PTB is unknown. Our proposed explanation for this finding is that antibiotics can kill the microbes but do not curtail the inflammatory process underway and specifically antibiotics do not destroy the bacterial wall pathogen-associated molecular pattern molecules (PAMPs). We propose that these bacterial PAMPs continue to engage Toll-like receptors (TLRs), participate in the release of cellular endogenous danger signal molecules and perpetuate the inflammatory cascade leading to brain cell damage even after antibiotic treatment. Therefore, there is much interest in identifying markers and biomarkers that can reliably predict sepsis in the neonate [59].
Proteomics, general principles
Recent advances in basic sciences are transforming medical research and offer the promise of answering many of the questions related to human diseases, including accurate diagnosis of EONS. Proteomics is the study of expressed proteins and, as such, is much more clinically relevant and easier to translate into diagnostic tools and therapeutical strategies than genomics, which studies DNA [66,67*]. For a comprehensive review of the proteomic technology, principles and data analysis the reader is referred to several review articles on this subject [67,68*,69,70,71].
Proteomic profiling of AF reveals a set of biomarkers characteristic of intra-amniotic inflammation, histological chorioamnionitis and funisitis
A comprehensive mapping of the AF proteome was provided by Tsangaris et al. [72], and a comparative proteomic analysis of human maternal plasma and AF to identify protein biomarkers predicting PPROM was reported by Michel et al. [73]. In recent years, proteomics also has been extensively used to search for biologically-relevant biomarkers to generate protein profiles characteristic of intra-amniotic inflammation [32,74,75,76,77,78,79,80**]. Almost 99% of the neutrophils identified in the AF are of fetal origin [81]. Thus, the AF proteome reflects closely the fetal inflammatory response to intra-amniotic infection. These studies were sparked by the clinical observation that although inflammatory intrauterine processes adversely affect the fetus prior to birth [54,82,83,84,85], neither the biophysical profile nor evaluation of the fetal heart rate reliably provide early identification of the sick fetuses and need for immediate delivery [86,87,88].
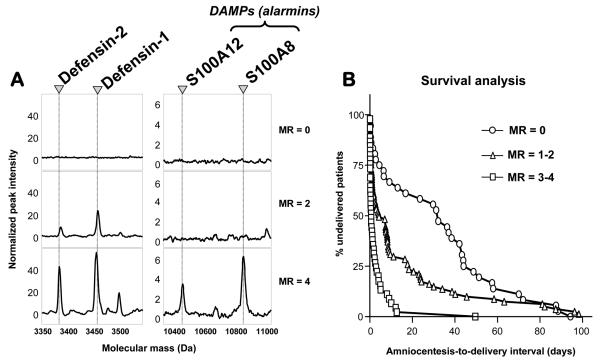
A vast amount of information is generated in a typical clinical proteomics study but only a fraction of this is ultimately biologically relevant. Surface enhanced laser desorbtion ionization-time of flight (SELDI-TOF) outputs are Cartesian sequences of numbers with molecular weight (m/z in fact) on the x-axis and normalized peak intensity on the y-axis and for instance a reading of one spot on a Protein Chip array generates about 20,000 data points. To analyze proteomics data from SELDI-TOF outputs, our group devised a novel, stepwise strategy to extract relevant proteomic biomarkers characteristic for intra-amniotic inflammation based on filter preferences applied sequentially [74]. This strategy was named mass restricted (MR) scoring [74]. The goal of MR scoring was to reduce the proteomics information into a numeric variable that characterizes each sample, could be prospectively tested and compared to other diagnostic modalities using standard statistical methods and could be directly related to outcomes. We used 4 peaks (and thus the proteins they represent) to devise our MR score, which ranges from 0 to 4 depending on the presence or absence of each of these 4 biomarkers. In the original study, an MR score of 3-4 indicated the presence of inflammation and a score of 0-2 was considered to exclude it [74] (Figure 1A). While the presence or absence of these 4 biomarkers was adequate and sufficient for a diagnosis of intra-amniotic inflammation, a better comprehension of the pathophysiology of the inflammatory process required identification of the corresponding proteins. On-chip immunoassays and peptide mass fingerprinting, confirmed by Western blotting, established that the 4 biomarkers were neutrophil defensin-2, neutrophil defensin-1, S100A12 (calgranulin C) and A100A8 (calgranulin A), all members of the innate immunity arm of antimicrobial defense [89].
Figure 1. Proteomic profiling of amniotic fluid and risk for preterm birth.
Panel A: Representative SELDI-TOF mass spectrometry profiles of the amniotic fluid based on the “severity” of inflammation (MR=0 “no” inflammation; MR=2 “minimal” inflammation; MR=3-4 “severe” inflammation). CHCA and SPA denote the energy absorbing molecules α-cyano-4-hydroxycinnamic acid or sinnapinic acid, respectively. The CHCA chip allows for the identification of defensins 2 and 1. The SPA chip allows for identification of the peaks corresponding to S100A12 (calgranulin C) and S100A8 (calgranulin A). The x axis of the tracings represents the molecular mass in Daltons; the y axis represents the relative peak intensity. Panel B: Cumulative probability of pregnancy maintenance for the 169 patients illustrating the duration from amniocentesis-to-delivery in women with MR (Mass Restricted) scores of 0 (zero), MR scores of 1-2, and MR scores 3-4. Originally published in Buhimschi et al. PLoS Med. 2007 Jan 16; 4(1):e18 (Reference 32). Republication by authors permitted under Creative Commons Attribution License.
Two other independent reports applied SELDI-TOF and a variety of proteomics validation tools (gel electrophoresis, tandem mass spectrometry, and Western blotting) to identify biomarkers characteristic of intra-amniotic inflammation [78,79]. Gravett et al. identified S100A9 (calgranulin-B), a unique fragment of insulin like growth factor binding protein 1 (IGFBP-1), and several immuno-regulator proteins as biomarkers characteristic of intra-amniotic infection [78]. Similarly, Ruetschi et al. reported that human neutrophil defensins 1-3 and S100A12 and S100A9 proteins are part of the AF fingerprint characteristic of intra-amniotic inflammation and/or infection [79].
We next validated prospectively the clinical utility of the MR score in predicting PTB and neonatal sepsis in a cohort of 169 consecutive women with singleton pregnancies [32]. An MR score 3-4 had the highest accuracy (92.6%) in diagnosing intra-amniotic inflammation and was significantly better than white blood cell (WBC) count or IL-6 [32]. This is not surprising given that AF WBCs are frequently destroyed by invading bacteria.
The value of these studies extends beyond the diagnostic impact of the MR score. We observed a sequential appearance of the biomarkers as the process of intra-amniotic inflammation developed from acute to chronic, with S100A12 and S100A8 appearing last. This finding enabled us to stratify the study population based on the severity of inflammation (MR 0 absent; MR 1-2 mild; MR 3-4 severe) (Figure 1B). Presence in the AF of S100A12 [EN-RAGE, ligand for the advanced glycation end products (RAGE) receptor] [90**] had the strongest correlation with stage III chorioamnionitis [91] and funisitis [92]. Detection of monomeric S100A8 (calgranulin A) in the AF was associated with EONS [92]. This classification would not be possible based on receiver operating curves for glucose, WBC count, IL-6 or MMP-8 levels which do not consistently concord in discriminating “diseased” versus “non-diseased” cases [32]. Second, these findings are important because the biomarkers comprising the MR score have a unique ability to predict in utero clinically-relevant histological chorioamnionitis [91] and funisitis, which are known risk factors for sepsis and poor neonatal outcome [93,94]. Presence of the “alarmins” S100A12 and S100A8 in the AF of pregnancies complicated by intra-amniotic infection and/or inflammation pointed our attention toward their potential involvement as mediators of fetal inflammatory damage and to the opportunity to employ targeted therapeutic interventions to reverse the process [12,95].
Proteomic profiling of AF predicts early-onset neonatal sepsis
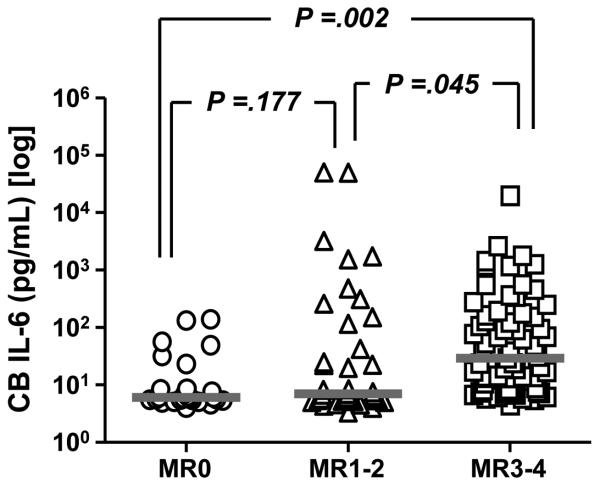
We further sought to provide direct insight into the relationship between the presence of AF biomarkers characteristic of inflammation and fetal inflammatory status at birth [12]. In a recent study we showed that premature infants delivered by mothers with MR score 3-4 had higher umbilical cord IL-6 as compared to neonates delivered by mothers with absent or mild inflammation (Figure 2) [12]. The combination of GA and presence of S100A12 and S100A8 in AF significantly predicted neonatal neuro-developmental impairment in our preliminary analysis [12]. Presence of S100A12 and S100A8 in AF resulted in a 5.3 increase in odds ratio for neuro-developmental injury at 18-22 months corrected age (Buhimschi et al. unpublished data). Interestingly, there were no significant differences in birthweight, AF or cord blood IL-6 levels between the infants with and without neuro-developmental damage. The lack of differences in cord blood IL-6 levels suggest that evaluation of acute phase response reactants such as IL-6 is not sufficient to predict which neonates will suffer from irreversible injury. Rather, evaluating oxidative insult [96] or other markers of fetal injury (S100B proteins) may be more informative [97]. The model of fetal inflammatory response to infection needs a fundamental revision since evidence is mounting that chronic rather than acute endogenous inducers of inflammation are more likely to be responsible for tissue injury and poor clinical outcome of the neonate [89]. Our proposed working model of the pathogenic mechanisms leading to fetal damage in presented in Figure 3.
Figure 2. Relationship between presence in the amniotic fluid of biomarkers characteristic of intra-amniotic inflammation and umbilical cord blood (CB) interleukin-6 (IL-6) levels in a prospective cohort of 132 consecutive premature newborns admitted to Newborn Special Care Unit at Yale New Haven Hospital.
The gestational age at delivery for the cohort was median [range] 29 [23-34] weeks and birthweight 1373 [536-2,980] grams. The MR score ranges from 0 to 4. MR score 0 (zero) indicates “no” inflammation; MR score 1-2 indicates “minimal” inflammation; MR score 3-4 indicates “severe” inflammation. Data is presented in logarithmic format. (pg=picograms; mL=milliliters). Originally published in Buhimschi et al. BJOG 2008; DOI: 10.1111/j.1471-0528.2008.01925.x. Republication with permission from Blackwell Publishing.
Figure 3.
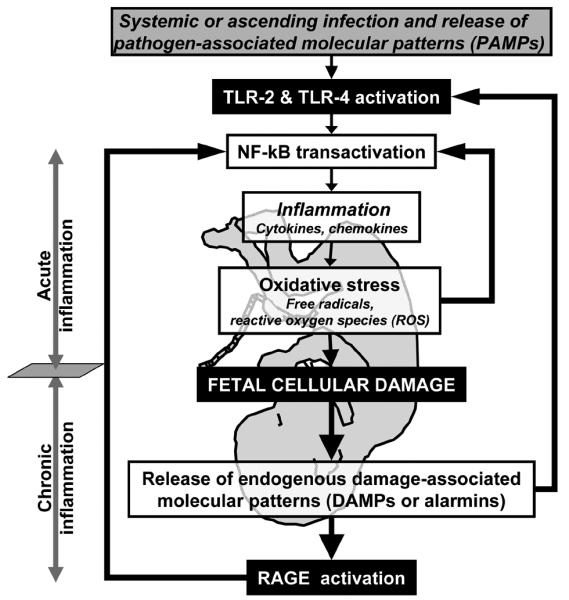
Working model for the potential roles of DAMPs (alarmins) in promoting cellular damage to the fetus exposed to intrauterine infection, inflammation and oxidative stress.
By using a variety of SELDI Protein Chip arrays, Dammann et al. showed that dried neonatal blood reveals profiles similar those derived from serum at a mass molecular range of 3-10 kDa [98]. This study is important because it demonstrates that SELDI-TOF technology carries the attributes to allow for a direct, rapid and reliable identification of proteomic biomarkers directly from small amounts of neonatal blood.
Fetal systemic and central nervous system inflammation are critical to the genesis of brain injury in the neonate [99]. In humans, extremely low-birthweight infants with sepsis are more likely to have adverse neuro-developmental outcomes, and a link among sepsis, brain injury and adverse outcomes has long been proposed [100]. The mechanisms mediating periventricular leukomalacia in preterm infants with sepsis are postulated to include injury to pre-oligodendrocytes induced by free radicals (FR), reactive oxygen species (ROS) and inflammatory cytokines [100]. Injuries to endothelial cells induced by similar mechanisms add to the damage, leading to multisystemic organ dysfunction [101]. Given the large spectrum of signaling pathways and physiological responses to sepsis, the fetal brain proteomic database assembled by Fountoulakis et al. represents a valuable addition to basic and clinical research [102]. Over 1,700 proteins, products of 437 different genes were identified. Most of the identified proteins are enzyme subunits and are mainly localized in cytosol and mitochondria. The most frequently identified proteins were heat shock proteins, house-keeping enzymes, and structural proteins, such as tubulin chains. Seven gene products were identified for the first time in the fetal brain. The next step is to identify the biomarker sets that can provide better insight into the molecular mechanisms responsible for the defense immune function of the fetus and carry a useful diagnostic predictive value for brain injury. Functional analyses and logical interpretation of results using regulatory proteomic networks will be necessary to allow development of a novel fetal inflammatory response classification system based on unbiased discoveries [27].
CONCLUSION
The process leading to fetal sepsis and damage in utero, remains incompletely understood. Neither surrogate indicators of fetal exposure to acute inflammation (clinical chorioamnionitis), nor direct proof of exposure (umbilical cord IL-6, histological chorioamnionitis) can predict long term adverse neuro-developmental outcomes. The most recent advancements in proteomics lead to the discovery that the S100 (calgranulins) proteins may play a critical role in protecting the fetus. This finding advises that the model of fetal origin and physiological role of inflammation in response to infection needs to be revised. Mounting evidence suggests that the course of an inflammatory response is not entirely dependent on acute phase reactants (IL-6) or pathogen-associated molecular patterns (PAMPs), but also on intra-cellular “alarmins” known as damage associated molecular patterns (DAMPs), which includes high mobility group box-1 protein (HMGB1), heat shock proteins, altered matrix proteins and S100 proteins. They represent important danger signals that mediate inflammatory responses through TLRs and importantly through RAGE. It is recognized that DAMPs mediate the late response to infection. Based on our proteomic discoveries we propose that the chronic and not the acute phase of the fetal inflammatory response is maladaptive for the fetus, as exemplified by release of DAMPs. Expanding the list of chronic “stress” cellular protein biomarkers to change fetal outcome is difficult. Yet, there is hope that proteomics can successfully respond to this challenge by the ability to provide insight into molecular targets which could not have been envisioned through hypothesis-driven approaches.
Acknowledgments
FUNDING SOURCE
C.S.B. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), grant R03 HD50249 and the Yale WRHR Career Development Center (K12 HD1027766). This work was supported in part from National Institutes of Health grants RO1 HD47321 (IAB), RO1 DE14924(YWH) and R37 HL28373 (JAM). The funding source had no involvement in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
REFERENCES
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary Data for 2006. NVSR. 2008;56(7):1120. 18. PHS. [Google Scholar]
- 2.Green NS, Damus K, Simpson JL, et al. March Of Dimes Scientific Advisory Committee On Prematurity. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193:626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 3.Behrman RE, Butler AS, editors. Causes, Consequences, and Prevention. Institute of Medicine of the National Academies. The National Academies Press; Washington D.C: 2007. Preterm Birth. [PubMed] [Google Scholar]
- 4.Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. 2008;299:1429–1436. doi: 10.1001/jama.299.12.1429. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MM. The medically complex premature infant in primary care. J Pediatr Health Care. 2006;20:367–373. doi: 10.1016/j.pedhc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6**.Tyson JE, Parikh NA, Langer J, et al. National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med. 2008;358:1672–1681. doi: 10.1056/NEJMoa073059. Relevant, prospective study which showed that sex, antenatal corticosteroids, multi-fetal pregnancy and birth weight are important determinants of the neonatal outcome in intensive care units. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall MH, Danielian P, Lamont RF. The importance of preterm birth. In: Elger MG, Romero R, Lamont RF, editors. Preterm labor. Churchill Livingstone; New York: 1993. pp. 1–28. [Google Scholar]
- 8.Hessol NA, Fuentes-Afflick E. Ethnic differences in neonatal and postneonatal mortality. Pediatrics. 2005;115:e44–51. doi: 10.1542/peds.2004-0478. [DOI] [PubMed] [Google Scholar]
- 9.Berkovitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–413. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 10.Creasy RK. Preterm birth prevention: Where we are? Am J Obstet Gynecol. 1993;168:1223–1230. doi: 10.1016/0002-9378(93)90373-q. [DOI] [PubMed] [Google Scholar]
- 11.Nelson KB, Grether JK. Causes of cerebral palsy. Curr Opin Pediatr. 1999;11:487–491. doi: 10.1097/00008480-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 12**.Buhimschi CS, Dulay AT, Abdel-Razeq S, et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. 2009 doi: 10.1111/j.1471-0528.2008.01925.x. DOI: 10.1111/j.1471-0528.2008.01925.x. This study provides evidence that presence in the amniotic fluid of proteomic biomarkers characteristic of inflammation is associated with an increased inflammatory status of the fetus at birth, sepsis and increased risk of poor long-term neuro-developmental outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer GB. Genetics considerations in cerebral palsy. Semin Pediatr Neurol. 2008;15:21–26. doi: 10.1016/j.spen.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood CJ, Senyei AE, Dische MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669–674. doi: 10.1056/NEJM199109053251001. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein S, Heimler R, Sasidharan P. Approaching the management of the neonatal intensive care unit graduate through history and physical assessment. Ped Clin North Am. 1998;45:79–105. doi: 10.1016/s0031-3955(05)70584-8. [DOI] [PubMed] [Google Scholar]
- 16.Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357:477–487. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- 17.Macones G, Hauth JC, Lockwood CJ. Magnesium sulfate tocolysis: time to quit. Obstet Gynecol. 2007;109:450–451. doi: 10.1097/01.AOG.0000244696.81795.3f. [DOI] [PubMed] [Google Scholar]
- 18.Grimes DA, Nanda K. Magnesium sulfate tocolysis: time to quit. Obstet Gynecol. 2006;108:986–989. doi: 10.1097/01.AOG.0000236445.18265.93. [DOI] [PubMed] [Google Scholar]
- 19.Thornton JG. Maintenance tocolysis. BJOG. 2005;112:118–121. doi: 10.1111/j.1471-0528.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalia M. Brain development: anatomy, connectivity, adaptive plasticity, and toxicity. Metabolism. 2008;57(Suppl 2):S2–5. doi: 10.1016/j.metabol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196:433–444. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson KB, Chang T. Is cerebral palsy preventable? Curr Opin Neurol. 2008;21:129–35. doi: 10.1097/WCO.0b013e3282f4958b. [DOI] [PubMed] [Google Scholar]
- 23.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 24**.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. Large randomized study which showed that fetal exposure to magnesium sulfate did not reduce the combined risk of moderate or severe cerebral palsy or death, although the rate of cerebral palsy was reduced among survivors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steer PJ. The epidemiology of preterm labour--why have advances not equated to reduced incidence? BJOG. 2006;113(Suppl 3):1–3. doi: 10.1111/j.1471-0528.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32:523–559. doi: 10.1016/j.clp.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Buhimschi IA, Zhao G, Rosenberg VA, et al. Multidimensional proteomics analysis of amniotic fluid to provide insight into the mechanisms of idiopathic preterm birth. PLoS ONE. 2008;3:e2049. doi: 10.1371/journal.pone.0002049. The first description of an amniotic fluid proteomic profile characteristic of idiopathic preterm birth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen JW, Goldkrand JW, Hanson TM, et al. Intrauterine infection on an obstetric service. Obstet Gynecol. 1974;43:838–843. [PubMed] [Google Scholar]
- 29.Klein LL, Gibbs RS. Infection and preterm birth. Obstet Gynecol Clin North Am. 2005;32:397–410. doi: 10.1016/j.ogc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Simhan HN, Canavan TP. Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG. 2005;112(Suppl 1):32–37. doi: 10.1111/j.1471-0528.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier DW, Meyer WJ, Bieniarz A. Expectant management of premature rupture of membranes with amniotic fluid cultures positive for Ureaplasma urealyticum alone. Am J Obstet Gynecol. 1994;170:5875–90. doi: 10.1016/s0002-9378(94)70233-0. [DOI] [PubMed] [Google Scholar]
- 32**.Buhimschi CS, Bhandari V, Hamar BD, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. This study represents the first prospective testing of a previously described proteomic profile characteristic of intra-amniotic inflammation in a large population of women at risk for preterm birth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenstrom KD, Andrews WW, Hauth JC, et al. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 34.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 36.Bizzarro MJ, Dembry LM, Baltimore RS, et al. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 37.Larsen JW, Sever JL. Group B Streptococcus and pregnancy: a review. Am J Obstet Gynecol. 2008;198:440–448. doi: 10.1016/j.ajog.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Pettker CM, Buhimschi IA, Magloire LK, et al. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109:739–749. doi: 10.1097/01.AOG.0000255663.47512.23. [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg RL, Culhane JF. Infection as a cause of preterm birth. Clin Perinatol. 2003;30:677–700. doi: 10.1016/s0095-5108(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 40.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–478. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barron EJ, Jorgensen JH, Landry ML, et al. Bacteriology. In: Murray PR, editor. Manual of clinical Bacteriology. 9th ed ASM Press; Washington, DC: 2007. p. 974. [Google Scholar]
- 43**.Dong J, Olano JP, McBride JW, et al. Emerging pathogens: challenges and successes of molecular diagnostics. J Mol Diagn. 2008;10:185–197. doi: 10.2353/jmoldx.2008.070063. This study provides an overview of the ongoing challenge to the field of molecular diagnostics to further discoveries of novel pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuypers MM. Microbiology. Sizing up the uncultivated majority. Science. 2007;317:1510–1511. doi: 10.1126/science.1148538. [DOI] [PubMed] [Google Scholar]
- 45.Hitti J, Riley DE, Krohn MA, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 46.Markenson G, Martin R, Foley K, et al. The use of polymerase chain reaction to detect bacteria in amniotic fluid in pregnancies complicated with preterm labor. Am J Obstet Gynecol. 1997;177:1471–1477. doi: 10.1016/s0002-9378(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 47.Han YW, Zhang L, Chung P, et al. Evidence of uncultivated bacteria as etiologic agents of intra-amniotic infection and inflammation leading to preterm birth; Presented at: 28th Annual Meeting of the Society for Maternal-Fetal Medicine; Dallas, TX, USA. January 28-February 2, 2008. [Google Scholar]
- 48**.Han YW, Shen T, Chung P, et al. Uncultivable bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009 doi: 10.1128/JCM.01206-08. in press. Study demonstrating that previously unrecognized, uncultivated or difficult-to-cultivate species may play a key role in initiating human preterm birth. This study provides an explanation why women with amniotic fluid proteomic profiles characteristic of inflammation do not always have a positive microbial test result. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grigg J, Arnon S, Chase A, et al. Inflammatory cells in the lungs of premature infants on the first day of life. Perinatal risk factors and origin of cells. Arch Dis Child. 1993;69:40–43. doi: 10.1136/adc.69.1_spec_no.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005;24:635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 51.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 52.Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr. 2006;18:125–131. doi: 10.1097/01.mop.0000193293.87022.4c. [DOI] [PubMed] [Google Scholar]
- 53.Smulian JC, Bhandari V, Campbell WA, et al. Value of umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med. 1997;6:254–259. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<254::AID-MFM2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez BE, Mercado CK, Johnson L, et al. Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med. 2003;31:60–68. doi: 10.1515/JPM.2003.009. [DOI] [PubMed] [Google Scholar]
- 55.ACOG Committee on Practice Bulletins-Obstetrics ACOG Practice Bulletin No. 80: premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 56.American College of Obstetricians and Gynecologists ACOG Committee Opinion: number 279, December 2002. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2002;100:1405–1412. doi: 10.1016/s0029-7844(02)02629-7. [DOI] [PubMed] [Google Scholar]
- 57.Kenyon SL, Taylor DJ, Tarnow-Mordi W, et al. ORACLE Collaborative Group. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979–988. doi: 10.1016/s0140-6736(00)04233-1. [DOI] [PubMed] [Google Scholar]
- 58.Kenyon SL, Taylor DJ, Tarnow-Mordi W, et al. ORACLE Collaborative Group. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:989–994. doi: 10.1016/s0140-6736(00)04234-3. [DOI] [PubMed] [Google Scholar]
- 59.Mishra UK, Jacobs SE, Doyle LW, et al. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2006;91:F208–212. doi: 10.1136/adc.2004.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buttery JP. Blood cultures in newborns and children: optimising an everyday test. Arch Dis Child Fetal Neonatal Ed. 2002;87:F25–28. doi: 10.1136/fn.87.1.F25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:e1–5. doi: 10.1016/j.ajog.2007.07.033. Important study which demonstrates that Ureaplasma and Mycoplasma spp. are involved in fetal sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodwell RL, Taylor KM, Tudehope DI, et al. Hematologic scoring system in early diagnosis of sepsis in neutropenic newborns. Pediatr Infect Dis J. 1993;12:372–376. doi: 10.1097/00006454-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Bhandari V, Wang C, Rinder C, et al. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–134. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 64.Jordan JA, Durso MB, Butchko AR, et al. Evaluating the near-term infant for early onset sepsis: progress and challenges to consider with 16S rDNA polymerase chain reaction testing. J Mol Diagn. 2006;8:357–363. doi: 10.2353/jmoldx.2006.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Kenyon SL, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008 doi: 10.1016/S0140-6736(08)61203-9. DOI: 10.1016/s0140-6736(08)61203-9. Important study showing that prescription of erythromycin for women in spontaneous preterm labor with intact membranes was associated with an increase in functional impairment among their children at 7 years of age. [DOI] [PubMed] [Google Scholar]
- 66.Wilkins MR, Sanchez JC, Gooley AA, et al. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 67*.Buhimschi CS, Rosenberg VA, Dulay AT, et al. Multidimensional system biology: genetic markers and proteomic biomarkers of adverse pregnancy outcome in preterm birth. Am J Perinatol. 2008;25:175–187. doi: 10.1055/s-2008-1061497. Important review on the role of genomics and proteomics in improving clinical care of patients. [DOI] [PubMed] [Google Scholar]
- 68*.Buhimschi IA, Buhimschi CS. Proteomics of the amniotic fluid in assessment of the placenta. Relevance for preterm birth. Placenta. 2008;29(Suppl A):S95–101. doi: 10.1016/j.placenta.2007.12.001. Important review which details the general principles of proteomics research during pregnancy and relevance of proteomic biomarkers in evaluating the normal and pathologic states of the placenta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buhimschi CS, Weiner CP, Buhimschi IA. Clinical proteomics: a novel diagnostic tool for the new biology of preterm labor, part I: proteomics tools. Obstet Gynecol Surv. 2006;61:481–486. doi: 10.1097/01.ogx.0000224617.11789.ab. [DOI] [PubMed] [Google Scholar]
- 70.Hampton T. Comprehensive "proteomic profile" of amniotic fluid may aid prenatal diagnosis. JAMA. 2007;298:1751. doi: 10.1001/jama.298.15.1751. [DOI] [PubMed] [Google Scholar]
- 71.Shankar R, Cullinane F, Brennecke SP, et al. Applications of proteomic methodologies to human pregnancy research: a growing gestation approaching delivery? Proteomics. 2004;4:1909–1917. doi: 10.1002/pmic.200300790. [DOI] [PubMed] [Google Scholar]
- 72.Tsangaris G, Weitzdörfer R, Pollak D, et al. The amniotic fluid cell proteome. Electrophoresis. 2005;26:1168–1173. doi: 10.1002/elps.200406183. [DOI] [PubMed] [Google Scholar]
- 73.Michel PE, Crettaz D, Morier P, et al. Proteome analysis of human plasma and amniotic fluid by Off-Gel isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis. 2006;27:1169–1181. doi: 10.1002/elps.200500680. [DOI] [PubMed] [Google Scholar]
- 74.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 75.Buhimschi IA, Buhimschi CS, Weiner CP, et al. Proteomic but not enzyme-linked immunosorbent assay technology detects amniotic fluid monomeric calgranulins from their complexed calprotectin form. Clin Diagn Lab Immunol. 2005;12:837–844. doi: 10.1128/CDLI.12.7.837-844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buhimschi IA, Buhimschi CS, Christner R, et al. Proteomics technology for the accurate diagnosis of inflammation in twin pregnancies. BJOG. 2005;112:250–255. doi: 10.1111/j.1471-0528.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- 77.Buhimschi CS, Pettker CM, Magloire LK, et al. Proteomic technology and delayed interval delivery in multiple pregnancies. Int J Gynaecol Obstet. 2005;90:48–50. doi: 10.1016/j.ijgo.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 78.Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–469. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 79.Ruetschi U, Rosen A, Karlsson G, et al. Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation. J Proteome Res. 2005;4:2236–2242. doi: 10.1021/pr050139e. [DOI] [PubMed] [Google Scholar]
- 80**.Weiner CP, Lee KY, Buhimschi CS, et al. Proteomic biomarkers that predict the clinical success of rescue cerclage. Am J Obstet Gynecol. 2005;192:710–718. doi: 10.1016/j.ajog.2004.10.588. This is the first study to demonstrate that presence in the amniotic fluid of biomarkers characteristic of inflammation and bleeding (hemoglobin chains) is associated with a high pregnancy failure in women with cervical incompetence. Importantly, the sensitivity of proteomics based technologies is much higher than ELISA in identifying presence of hemoglobin biomarkers in the amniotic fluid. [DOI] [PubMed] [Google Scholar]
- 81.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 82.Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Dev Med Child Neurol. 1993;35:553–558. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- 83.Costantine MM, How HY, Coppage K, et al. Does peripartum infection increase the incidence of cerebral palsy in extremely low birthweight infants? Am J Obstet Gynecol. 2007;196:e6–8. doi: 10.1016/j.ajog.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Keogh JM, Badawi N. The origins of cerebral palsy. Curr Opin Neurol. 2006;19:129–134. doi: 10.1097/01.wco.0000218227.35560.0d. [DOI] [PubMed] [Google Scholar]
- 85.Babnik J, Stucin-Gantar I, Kornhauser-Cerar L, et al. Intrauterine inflammation and the onset of peri-intraventricular hemorrhage in premature infants. Biol Neonate. 2006;90:113–121. doi: 10.1159/000092070. [DOI] [PubMed] [Google Scholar]
- 86.Buhimschi CS, Abdel-Razeq S, Cackovic M, et al. Fetal heart rate monitoring patterns in women with amniotic fluid proteomic profiles indicative of inflammation. Am J Perinatol. 2008;25:359–372. doi: 10.1055/s-2008-1078761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aina-Mumuney AJ, Althaus JE, Henderson JL, et al. Intrapartum electronic fetal monitoring and the identification of systemic fetal inflammation. J Reprod Med. 2007;52:762–768. [PubMed] [Google Scholar]
- 88.Ghidini A, Salafia CM, Kirn V, et al. Biophysical profile in predicting acute ascending infection in preterm rupture of membranes before 32 weeks. Obstet Gynecol. 2000;96:201–206. doi: 10.1016/s0029-7844(00)00908-x. [DOI] [PubMed] [Google Scholar]
- 89.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 90**.Buhimschi IA, Zhao G, Pettker CM, et al. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am J Obstet Gynecol. 2007;196:181.e1–13. doi: 10.1016/j.ajog.2006.09.001. This study describes for the first time the presence and role of the receptor for advanced glycation end products (RAGE) receptor in the human amniotic fluid and fetal membranes. [DOI] [PubMed] [Google Scholar]
- 91.Buhimschi IA, Zambrano E, Pettker CM, et al. Using proteomic analysis of the human amniotic fluid to identify histologic chorioamnionitis. Obstet Gynecol. 2008;111:403–412. doi: 10.1097/AOG.0b013e31816102aa. [DOI] [PubMed] [Google Scholar]
- 92.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007;61:318–324. doi: 10.1203/01.pdr.0000252439.48564.37. [DOI] [PubMed] [Google Scholar]
- 93.Murphy DJ, Sellers S, MacKenzie IZ, et al. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995;346:449–1454. doi: 10.1016/s0140-6736(95)92471-x. [DOI] [PubMed] [Google Scholar]
- 94.Martius JA, Roos T, Gora B, et al. Risk factors associated with early-onset sepsis in premature infants. Eur J Obstet Gynecol Reprod Biol. 1999;85:151–158. doi: 10.1016/s0301-2115(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 95.Dulay AT, Buhimschi IA, Zhao G, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426.e1–9. doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buhimschi IA, Zhao G, Funai E, et al. Intra-amniotic inflammation induces damage to DNA Present in amniotic cavity. Reprod Sci. 2007;14(Suppl):63A. abstract. [Google Scholar]
- 97.Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- 98.Dammann CE, Meyer M, Dammann O, et al. Protein detection in dried blood by surface-enhanced laser desorption/ionization-time of flight mass spectrometry (SELDI-TOF MS) Biol Neonate. 2006;89:126–132. doi: 10.1159/000088716. [DOI] [PubMed] [Google Scholar]
- 99.Ferriero DM. Neonatal brain. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 100.Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005;58:821–828. doi: 10.1002/ana.20662. [DOI] [PubMed] [Google Scholar]
- 101.Hack CE, Zeerleder S. The endothelium in sepsis: source of and a target for inflammation. Crit Care Med. 200;29:S21–27. doi: 10.1097/00003246-200107001-00011. [DOI] [PubMed] [Google Scholar]
- 102.Fountoulakis M, Juranville JF, Dierssen M, et al. Proteomic analysis of the fetal brain. Proteomics. 2002;2:1547–1576. doi: 10.1002/1615-9861(200211)2:11<1547::AID-PROT1547>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]