Abstract
Progesterone participates in the regulation of several functions in mammals including brain differentiation and dopaminergic transmission but the role of progesterone in dopaminergic cell differentiation is unknown. We investigated the effects of progesterone on dopaminergic differentiation of embryonic stem cells using a 5-stage protocol. Cells were incubated with different progesterone concentrations during the proliferation (stage 4) or differentiation (stage 5) phases. Progesterone added at 1, 10 and 100 nM during stage 4 increased 72, 80 and 62% respectively the number of dopamine neurons at stage 5 as compared to control group. The administration of progesterone at stage 5 did not induce significant changes in the number of dopamine neurons. These actions were not mediated by the activation of intracellular progesterone receptors, because RU 486 did not block the positive effects of progesterone on differentiation to dopaminergic neurons. Our results suggest that progesterone should be useful to produce higher proportions of dopamine neurons from embryonic stem cells aimed for treating Parkinson's disease.
Keywords: Dopamine neurons, Embryonic stem cells, Progesterone, Tyrosine hydroxylase
Introduction
Embryonic stem cells (ESC) are pluripotent cells derived from the inner cell mass of pre-implantation embryos. Because of their ability to self-renew, proliferate undifferentiated in vitro, while maintaining the potential to give rise to any of the hundreds of cell types in the human body, ESC are useful to dissect critical steps of cell development. Furthermore, they also represent a potential source for biomedical research and regenerative medicine (1, 2). Many studies have demonstrated that mouse and human ESC can form dopamine neurons. In this context, it has been shown that midbrain dopamine neurons can be induced in vitro from mouse ESC with a 5-stage method and that these dopamine neurons have improved parkinsonism in rodents for over 32 weeks after grafting (3-5).
Sex steroid hormones, oestradiol and progesterone play major roles in the development, differentiation, function and protection of the Central Nervous System in mammals (6-8). There is strong evidence indicating that oestrogens can induce the development and protection of nigrostriatal dopaminergic neurons (9, 10), while the role of progesterone in the dopamine system has not been completely determined. It has been reported that progesterone protects dopaminergic neurons against degeneration induced by MPTP (11, 12) and methamphetamine (13) in rodents. Furthermore, progesterone at physiological doses and independently of oestrogens can rapidly increase dopamine release in the striatum of rats of both sexes (14, 15).
Dopamine neurons can be identified by expression of tyrosine hydroxylase (TH), the enzyme responsible for catalyzing the conversion of L-tyrosine to L-DOPA, which is the dopamine precursor. Progesterone receptor (PR) KO male mice have a diminished number of TH immune-reactive cells in the substantia nigra pars compact as compared with wild type animal (16). Progesterone also increases the content of TH in the olfactory bulb of gonadectomized male rats (17), but no information is available regarding the effects of progesterone on ESC-derived neural cells.
Many progesterone actions in brain are mediated by its intracellular receptors (18). We have previously reported the expression of sex steroid hormone receptors during a 5-stage protocol of dopamine cells differentiation from mouse ESC. The last two phases of this procedure produce nestin-positive cells (stage 4, neural precursors) that respond to the application of Fibroblast growth factor (FGF) 2, FGF 8 and Sonic hedgehog (SHH) to produce terminally differentiated dopaminergic neurons (stage 5). We found an increase in the content of both PR isoforms when cells were at the neural/neuronal stages compared with the content of these receptors in initial pluripotent conditions (3). Ninety-two percent of dopamine neurons co-expressed PR and TH (3). These results suggest PR activation by progesterone should participate in the dopamine cell differentiation process.
In the present study, we examined the effects of progesterone in the last stages of differentiation of ESC to dopamine neurons in vitro. We found a dose-dependent increase in the number of TH-positive neurons when ESC were incubated with progesterone during this neural precursor state.
Material and methods
ESC differentiation to dopamine neurons
We used male R1 mouse ESC from Dr. Andras Nagy's laboratory (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Canada) (19), which have been used to produce dopamine neurons (3-5). The differentiation procedure was performed as reported (3) and is summarized in Table 1. Briefly, undifferentiated ESC (stage 1) were grown on gelatin-coated tissue culture plates in the presence of 1000 U/ml of Leukemia inhibitory factor (LIF) (Chemicon, USA) in medium supplemented with ESC-tested fetal calf serum (Wisent, Canada). To induce formation of floating embryoid bodies (stage 2), cells were dissociated into cell suspension with trypsin and plated onto bacterial dishes at a density of 2 × 106 cells/57 cm2 in the presence of Leukemia inhibitory factor. Embryoid bodies were cultured for 4 days and then plated onto adhesive tissue culture surface. Selection of nestin-positive cells (stage 3) was initiated in serum-free insulin / transferrin / selenite / fibronectin (ITSFn) medium for 9-11 days. Cells in the last two stages of dopamine neuron differentiation are cultured in N2 medium prepared in our laboratory (20, 21), consisting of DMEM/F12 supplemented with 25 μg/ml insulin, 100 μg/ml apotransferrin, 100 μM putrescine, 30 nM selenite and 20 nM progesterone. For this study, we used progesterone-free N2 medium, and this hormone was added at the indicated concentrations. After stage 3, cells were dissociated with trypsin and plated at a density of 1.5 × 105 cell/cm2 in progesterone-free N2 medium with or without phenol red. These neural stem cells were plated on dishes pre-coated with 15 μg/ml poly-L-ornithine and 1 μg/ml mouse laminin (Becton Dickinson, USA), treated with 10 ng/ml of FGF 2, 100 ng/ml of FGF 8b and 100 ng/ml of human SHH (growth factors from R&D Systems and Peprotech, USA) for 4 days to expand/instruct dopamine precursors (stage 4). Differentiation (stage 5) was induced by growth factor withdrawal and feeding with the corresponding progesterone-free N2 medium with 200 μM ascorbic acid for 6 days. This differentiation protocol produces only dopamine neurons and not other catecholaminergic neurons (21), together with serotonin and GABA neurons (4, 21). With a similar protocol, astrocytes (12%) and oligodendrocytes (< 1%) are present in stage 5 (22).
Table 1. Generation of dopamine neurons from undifferentiated ESC by a 5-stage procedure.
| Stage | Culture condition and cell types present |
|---|---|
| 1 | Expansion of undifferentiated ESC in serum-containing medium in the presence of LIF |
| 2 | Embryoid bodies growing in suspension in serum-containing medium with LIF |
| 3 | Selection of nestin-positive neural precursors in serum-free medium |
| 4 | Proliferation of nestin-positive neural cells stimulated by FGF2, FGF8 and SHH |
| 5 | Dopamine neuron differentiation by growth factor withdrawal |
Progesterone and RU 486
To determine the effects of progesterone on differentiation of ESC to dopamine neurons, cells were treated with different steroid concentrations only at the period of proliferation (stage 4) or added only during differentiation (stage 5). Water soluble progesterone (cyclodextrin-encapsulated; Sigma, St. Louis, MO, USA) was used at several concentrations ranging from 1 nM to 10 μM, encompassing physiological concentrations, ranging from 3 to 64 nM (23, 24). During proliferation, progesterone was applied in the first and third days of this stage in N2 medium without progesterone. For the experiments in the phase of differentiation, progesterone was added every other day, starting at day one until day five. Controls received 0.02% water soluble cyclodextrin (Sigma).
In another series of experiments, we applied the PR antagonist RU 486 at 10 μM (Sigma) from an ethanol stock solution (0.1% final ethanol concentration; controls received ethanol only) to determine whether the effect of progesterone was mediated through its nuclear receptors. This concentration of RU 486 is effective in antagonizing PR-mediated progesterone actions in cultured cells (25). The following progesterone treatments and its antagonist were applied only in stage 4 of the protocol in progesterone-free N2 medium in the presence or absence of phenol red: 1) Control (cyclodextrin). 2) 10 μM RU 486. 3) 10 nM progesterone. 4) 10 nM progesterone + 10 μM RU 486.
Immunofluorescence
Immunofluorescence procedures were carried out using described standard protocols (3, 26, 27). After fixing cells with 4% paraformaldehyde, primary antibodies were applied as follows: rabbit anti-TH antibody, 1:1000 (Pel-Freeze, USA), mouse anti-β Tubulin III (a neuronal marker) monoclonal antibody, 1:1000 (Covance, USA). Appropriate fluorescently-labeled secondary antibodies (Molecular Probes, USA) were used at 1:1000 dilution. Nuclei were stained with 1 ng/ml of Hoechst 33258 (Sigma). Negative controls consisted of cells in which the primary antibody was omitted. These experiments did not produce any staining (data not shown). As previously reported, all TH-positive cells also expressed β Tubulin III (3).
2.4. RNA extraction and RT-PCR
Assays were performed as described (26). Total RNA was isolated from differentiated (stage 5) cultures after progesterone treatment only during stage 4, using TRIZOL (Invitrogen). RNA (1 μg) was reverse transcribed with random hexamers and 2 μl from the reaction were used in PCR containing 2 U of Taq polymerase (Invitrogen), 20 pmol of specific primers (Sigma), 500 μM deoxynucleoside triphosphates and 1.5 mM MgCl2. For cDNA amplification, the following reported (4, 28) forward and reverse primer sequences were used: dopamine transporter (DAT), aromatic L-amino acid decarboxylase (AADC), Engrailed-1 (En-1), Lmx1b, Nurr 1, Pitx3, c-RET, β Tubulin III and Glyceraldehyde phosphate dehydrogenase (GADPH). Primer sequences are listed in Table 2. Cycling parameters were as follows: denaturalization at 94°C for 1 min, annealing at 57-62°C for 1 min depending on the primers, and elongation at 72°C for 1 min. The number of cycles varied between 25 and 35, depending on the gene of interest. Final extension at 74°C for 10 min was terminated by rapid cooling at 4°C. PCR products were analyzed on 2% agarose gel electrophoresis, and the size of products was determined by comparison with molecular weight standards after eithidium bromide staining. As a negative control for PCR amplification, reactions with RNA in the absence of retrotranscription (-RT) were included.
Table 2. Primers used for amplification of neuronal and dopaminergic markers.
| Target | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| DAT | GGACCAATGTCTTCAGTGGTGGC | GGATCCATGGGAGGTCCATGG | 520 |
| AADC | CCTACTGGCTGCTCGGACTAA | GCGTACCAGTGACTCAAACTC | 368 |
| Engrailed-1 | TCAAGACTGACTCACAGCAACCCC | CTTTGTCCTGAACCGTGGTGGTAG | 381 |
| Lmx1b | CCTCAGCGTGCGTGTGGTC | AGCAGTCGCTGAGGCTGGTG | 323 |
| Nurr1 | TGAAGAGAGCGGAGAAGGAGATC | TCTGGAGTTAAGAAATCGGAGCTG | 255 |
| Pitx 3 | AGGACGGCTCTCTGAAGAA | TTGACCGAGTTGAAGGCGAA | 373 |
| c-RET | GCGCCCCGAGTGTGAGGAATGTGG | GCTGATGCAATGGGCGGCTTGTGC | 440 |
| β Tubulin III | TCAGCGATGAGCACGGCATA | CACTCTTTCCGCACGACATC | 301 |
| GADPH | ATCACCATCTTCCAGGAGCG | CCTGCTTCACCACCTTCTTG | 573 |
Cell counting
At the final stage of differentiation, cell counts from immunofluorescence experiments were performed from pictures taken with a Nikon (Tokyo, Japan) digital camera DMX1200F and the Nikon ACT-1 imaging software. Analysis of cultures was performed by counting in duplicate the number of TH+ and β Tubulin III+ cells in 8 random fields taken at 400×, from 3-6 independent experiments. Some images were acquired in a FV1000 confocal microscope (Olympus, Tokyo, Japan) using a super apochromat objective (N.A. 0.40), to detect Alexa 488 and Alexa 568 fluorescence in a sequential fashion, by exciting with different lasers. The examiner was unaware of the treatment condition of cells.
Statistical analysis
Data are expressed as mean ± SEM of at least 3 independent experiments performed by duplicate, unless otherwise noted. Statistical analysis was made by ANOVA followed by Fisher's test. GB-STAT Version 7.0 program (Dynamic Microsystem, USA) was used for calculating probability values. P < 0.05 was considered statistically significant.
Results
To establish the effects of progesterone on the number of dopamine neurons during the proliferation stage, we performed a set of experiments with several hormone concentrations in N2 medium without progesterone together with FGF 2, FGF 8 and SHH. All cultures started at the same initial cell density, were incubated for 4 days in the presence of cytokines and then allowed to differentiate for 4-6 days in progesterone-free N2 medium with ascorbic acid. Representative pictures of differentiated (day 6 of stage 5) cultures treated with 10 nM progesterone in stage 4 are shown to document that this hormone increased the number of dopaminergic cells without changing notably the total (Hoechst-labeled) cell number (Fig. 1). We quantified the number of neurons (β Tubulin III-positive cells) and dopamine (TH-positive) neurons by performing double immunodetection of these markers in control and hormone-treated cultures. When we treated cells in stage 4 (proliferation) with progesterone, we did not find significant effects in the number of β Tubulin III-positive cells. However, progesterone significantly increased the number of TH-positive cells at 1 nM (72%), 10 nM (80%) and 100 nM (62%) relative to control treatment (Fig. 2A). When progesterone was added in the differentiation stage, we did not find any significant effect in the number of neurons or dopamine cells (Fig. 2B).
Figure 1. Effects of progesterone, added in the phase of proliferation (stage 4) of neural precursors derived from ESC, on dopamine neuron differentiation.
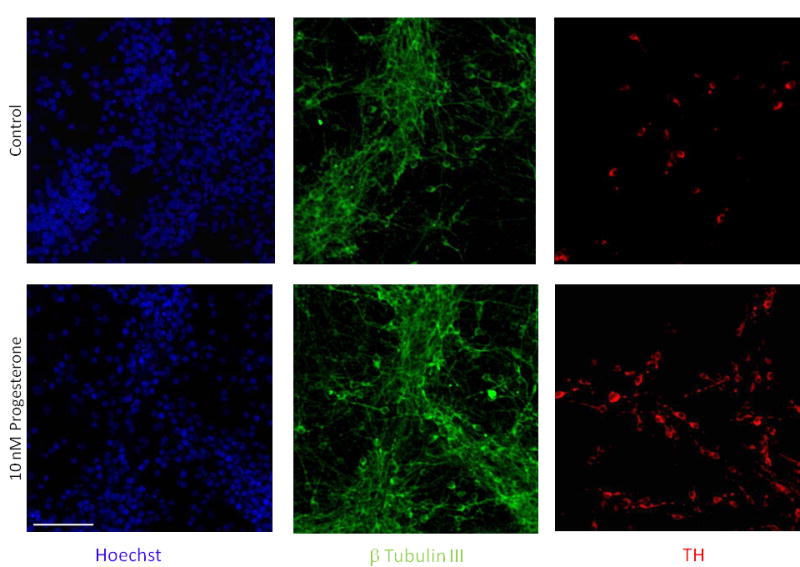
Representative confocal images of immunofluorescence detection for Hoechst (blue), β-Tubulin III (green) and TH (red). Ten nM progesterone caused an increase in the number of dopamine neurons quantified at the end of differentiation phase (stage 5). The control group was incubated only with cyclodextrin. Scale bar applies to all pictures and represents 100 μm.
Figure 2. Changes in the number of dopaminergic neurons induced by progesterone action on ESC.
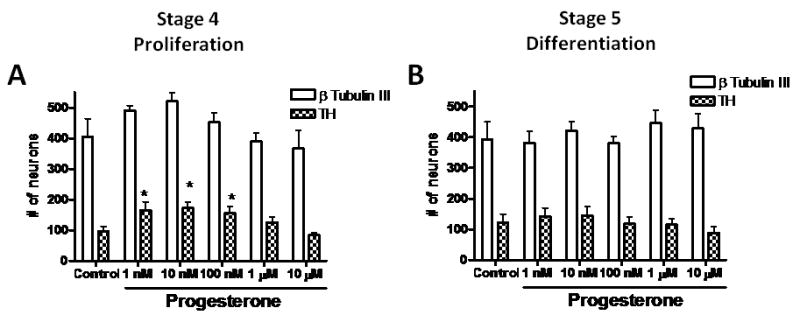
Quantification of the total number of β-Tubulin III and TH-positive cells from 8 fields from 3-6 independent experiments made in duplicate. Cells were incubated in progesterone-free N2 medium, with different progesterone concentrations during proliferation (A) or differentiation stages (B). Results are expressed as mean ± SEM. *P < 0.05 as compared with their corresponding control (cyclodextrin) group.
RU 486 effects on the increase in dopamine neurons induced by progesterone
Once we established the effects of progesterone in the number of dopamine neurons differentiated from ESC, we investigated whether such effects were mediated by intracellular PR. Co-incubations of 10 nM progesterone with RU 486 (10 μM) did not abolish the increase in the number of dopamine neurons induced by the hormone (Fig. 3A). RU 486 alone did not modify the number of TH positive cells as compared with control conditions.
Figure 3. Effects of progesterone and RU 486 on the differentiation of ESC to dopamine neurons, in progesterone-free N2 medium with or without phenol red.
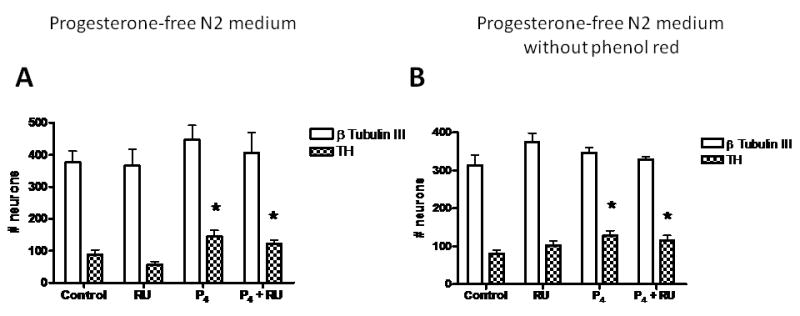
Quantification of the total number of β-Tubulin III and TH-positive cells from 8 fields from 3-5 independent experiments made in duplicate. Cells were incubated in medium with (A) or without phenol red (B), and treated with progesterone (10 nM) during proliferation stage. RU486 was added at 10 μM. Results are expressed as mean ± SEM. *P < 0.05 as compared with control (cyclodextrin) group.
Because the standard N2 medium contains phenol red and commercial preparations of this dye might contain lipophilic impurities with estrogenic activity (29, 30), we performed a series of experiments with progesterone-free N2 medium lacking phenol red. When we incubated cells in stage 4 with 10 nM progesterone, we observed again a significant increase in the number of TH positive cells at stage 5 (Fig. 3B). As we had observed in the experiment with a medium containing red phenol, co-incubation of progesterone with RU 486 did not abolish the increase in dopamine neurons induced by the hormone. Experiments to study the effects of progesterone on the expression of dopamine neuron markers were performed in medium lacking phenol red.
Progesterone-treated cells express dopaminergic molecular markers
To further characterize whether TH-positive neurons produced after progesterone treatment express dopaminergic neuron markers, semi-quantitative RT-PCR was used to evaluate the expression of genes specifically involved in dopamine neuron function at stage 5. In 2 independent experiments, we found similar expression levels of β Tubulin III, c-Ret, Lmx1b and Pitx3 in control and progesterone treated cells. In contrast, consistent up-regulation of Nurr1, En-1, AADC and DAT were observed in differentiated ESC treated with 10 nM progesterone only during stage 4 (Fig. 4).
Figure 4. Effects of progesterone on the expression of genes characteristic of midbrain dopamine neurons.
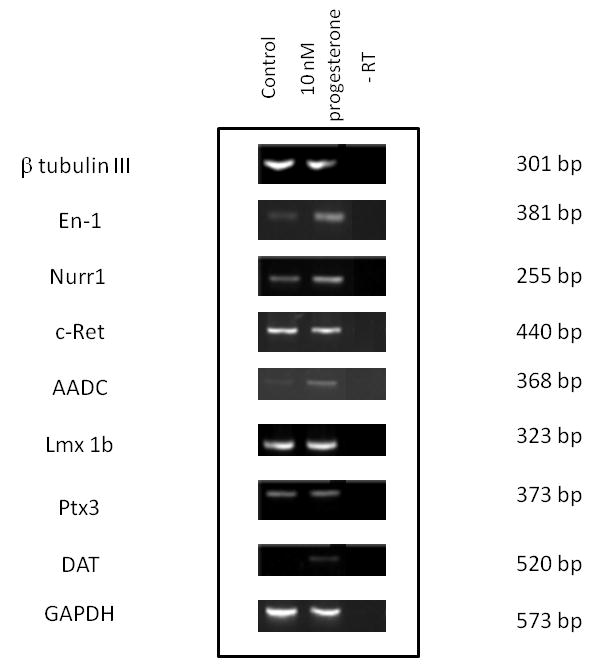
Genes involved in dopamine neuron function were analyzed by RT–PCR in stage 5. RNA without retrotranscription (-RT) produced no signal. The size of each band is indicated in base pairs (bp). Images are representative from 2 independent experiments.
Discussion
To our knowledge, this is the first report that directly tested a promoting role of progesterone in the differentiation of mouse ESC to dopamine neurons. In this paper, we demonstrated that the addition of progesterone in the range of 1 to 100 nM in the phase of proliferation promotes the induction of dopaminergic neurons. We did not observe such effects when the hormone was added at stage 5. This effect was not mediated by PR, since it was not blocked by RU 486.
Accumulating evidence demonstrates that steroid hormones can affect fundamental process of neural developmental such as stem cell proliferation, programmed cell death, as well as neuronal migration (6, 7, 31-33). In contrast with the number of reports on oestradiol effects on brain development, there are scarce reports on progesterone effects on neural development and neuroprotection. Progesterone can play an important role in promoting and enhancing repair after traumatic brain injury and stroke (8), and also may contribute to oligodendrocyte development from oligodendrocyte progenitors (34, 35). Furthermore, progesterone may be involved in the formation of the cerebellar neuronal circuit that occurs during neonatal life by promoting neuronal growth and neuronal synaptic contact (36).
In agreement to the possible participation of progesterone in brain development and specifically in the nigrostriatal dopaminergic neurons, we found an increase in the number of TH positive cells when progesterone was added in the proliferative stage, whereas the number of β Tubulin III-positive cells remained unchanged. This result could be explained if progesterone has proliferative effects on dopamine precursors during stage 4 as reported for rat neural progenitor cells (37). However, the increase in neural cell number caused by progesterone requires other supplements: in the original description of N2 medium (20), 20 nM progesterone alone did not change the number of rat neuroblastoma cells in culture. In contrast, 20 nM, but not lower or higher concentrations of this hormone, stimulated growth in medium with insulin, transferrin, selenite and putrescine. Supplements to culture neural cells in serum-free media (i.e. N2 and B27; (38)) normally include progesterone. We have previously shown that the proportion of neurons or astrocytes in cerebrocortical neuronal cultures, initially grown in serum-containing medium and later switched to serum-free medium with B27, does not change as compared with cultures maintained in serum-containing conditions (27), supporting the notion that the progesterone effects observed here are not due to an improvement in generalized health of the cultures.
To rule out oestrogenic interference of lipophilic impurities that might contain commercial preparations of phenol red, in our experimental system, we tested whether the TH-promoting effects of progesterone during stage 4 were present in medium without phenol red. We found that the dopaminergic induction exerted by 10 nM progesterone was present in this medium.
Although the function of progesterone and its receptor in dopamine neuron differentiation is not completely understood at this time, there are reports that suggest this link: 1) PR mRNA expression has been demonstrated in developing ventral midbrain including dopamine neurons (39); 2) Immunohistochemical procedures in rats up to 2 weeks of age detected PR in midbrain dopamine cells of the substantia nigra (40); 3) ESC differentiated to dopamine neurons express PR at the protein level (3). PR expression in midbrain during fetal and neonatal development could be involved in neurodegenerative disorders such as Parkinson's disease. Taken together all these data and as a first approximation to the mechanism involved in the increase in the number of TH positive cells induced by progesterone, we used the PR antagonist RU 486. However, in progesterone-free N2 media (with or without phenol red), RU 486 did not block progesterone effects. These results suggest that progesterone actions on differentiation of ESC to dopamine neurons were not mediated through progesterone–PR binding. One possible explication of these results is that 5α-reduced neuroactive derivates of progesterone could participate in the process of differentiation. In fact, allopregnanolone is produced in both the periphery and the central nervous system via enzymatic conversion of progesterone (41). This progesterone metabolite at nM concentrations induces proliferation of neural progenitor cells in rat hippocampus and human cerebral cortex (37).
Another possibility is that the effects observed in our system could be mediated by the membrane-bound PR 25-Dx, whose participation has been proposed in the neuroprotective effects of progesterone against edema formation after traumatic brain injury (42). However, the possible role of progesterone metabolites and membrane-bound PR in differentiation of ESC might deserve further research. It would be also interesting to use progesterone conjugated to bovine serum albumin which has been reported to prevent non-genomic progesterone actions (43) as well as PR iRNA to establish whether the effects reported here are still present.
Recently, we have shown (44) that estradiol, in the presence of 20 nM progesterone, during stage 4 increases the proportion of proliferating dopamine precursors by assessing the expression of Lmx1a (a transcription factor present in committed dopamine cells) and Ki67 (an antigen present in cells with active cell cycle), resulting in higher number of dopamine neurons. In the future, intracellular effectors responsible for the up-regulation of dopamine-related transcription factors (45, 46) after progesterone treatment, might lead to a better understanding of dopamine neuron differentiation.
Finally, we also demonstrated that treatment with progesterone induced TH+ neurons that express classic molecular markers of dopaminergic cells such as Nurr1, c-Ret, Lmx1b, DAT, En1, and A9 dopamine neurons identifiers such as Pitx3. Furthermore, the expression of Nurr1 and En1, transcription factors involved in dopaminergic differentiation, as well as the dopamine related genes DAT and AADC involved in neurotransmission and synthesis of dopamine respectively were up-regulated by progesterone. In summary, we have found that progesterone induced a higher number of differentiated dopamine neurons when applied during the proliferation phase of ESC-derived neural stem cells. The present results suggest that midbrain dopamine neurons should be sensitive to progesterone and/or its metabolites during important periods of developmental and maturation.
Acknowledgments
This research was supported by grants from NINDS/FIC (NS057850) and Fundación Alemán to I.V. N.F Diaz was supported by a postdoctoral fellowship from Conacyt and by NIH funds. N. E. Díaz-Martínez received a Ph.D. fellowship from Conacyt. We acknowledge Dr. Anayansi Molina and B. Sc. Teresa Neri-Gómez for their excellent technical assistance and B. Sc. Gabriel Orozco for confocal microscope operation.
References
- 1.McKay R. Stem cells--hype and hope. Nature. 2000;406(6794):361–4. doi: 10.1038/35019186. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Diaz NF, Guerra-Arraiza C, Diaz-Martinez NE, Salazar P, Molina-Hernandez A, Camacho-Arroyo I, Velasco I. Changes in the content of estrogen alpha and progesterone receptors during differentiation of mouse embryonic stem cells to dopamine neurons. Brain Res Bull. 2007;73(1-3):75–80. doi: 10.1016/j.brainresbull.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50–6. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25(4):918–28. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–39. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 8.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Front Neuroendocrinol. 2006;27(4):376–90. doi: 10.1016/j.yfrne.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson's disease. Clin Exp Pharmacol Physiol. 2007;34(7):555–65. doi: 10.1111/j.1440-1681.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- 11.Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41(2):131–8. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- 12.Morissette M, Al Sweidi S, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol. 2008;290(1-2):60–9. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Liao PC. Estrogen and progesterone distinctively modulate methamphetamine-induced dopamine and serotonin depletions in C57BL/6J mice. J Neural Transm. 2000;107(10):1139–47. doi: 10.1007/s007020070027. [DOI] [PubMed] [Google Scholar]
- 14.Di Paolo T, Levesque D, Daigle M. A physiological dose of progesterone affects rat striatum biogenic amine metabolism. Eur J Pharmacol. 1986;125(1):11–6. doi: 10.1016/0014-2999(86)90077-4. [DOI] [PubMed] [Google Scholar]
- 15.Petitclerc M, Bedard PJ, Di Paolo T. Progesterone releases dopamine in male and female rat striatum: a behavioral and microdialysis study. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(3):491–7. doi: 10.1016/0278-5846(95)00029-u. [DOI] [PubMed] [Google Scholar]
- 16.Woolley SC, O'Malley B, Lydon J, Crews D. Genotype differences in behavior and tyrosine hydroxylase expression between wild-type and progesterone receptor knockout mice. Behav Brain Res. 2006;167(2):197–204. doi: 10.1016/j.bbr.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Guerra-Araiza C, Miranda-Martinez A, Neri-Gomez T, Camacho-Arroyo I. Sex steroids effects on the content of GAD, TH, GABA(A), and glutamate receptors in the olfactory bulb of the male rat. Neurochem Res. 2008;33(8):1568–73. doi: 10.1007/s11064-008-9665-1. [DOI] [PubMed] [Google Scholar]
- 18.Guerra-Araiza C, Villamar-Cruz O, Gonzalez-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15(10):984–90. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90(18):8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76(1):514–7. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18(6):675–9. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 22.Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59(1):89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 23.Campbell CS, Ryan KD, Schwartz NB. Estrous cycles in the mouse: relative influence of continuous light and the presence of a male. Biol Reprod. 1976;14(3):292–9. doi: 10.1095/biolreprod14.3.292. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE. Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci U S A. 2003;100(5):2951–6. doi: 10.1073/pnas.0130100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Aguero G, Gutierrez AA, Gonzalez-Espinosa D, Solano JD, Morales R, Gonzalez-Arenas A, Cabrera-Munoz E, Camacho-Arroyo I. Progesterone effects on cell growth of U373 and D54 human astrocytoma cell lines. Endocrine. 2007;32(2):129–35. doi: 10.1007/s12020-007-9023-0. [DOI] [PubMed] [Google Scholar]
- 26.Molina-Hernandez A, Velasco I. Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J Neurochem. 2008;106(2):706–17. doi: 10.1111/j.1471-4159.2008.05424.x. [DOI] [PubMed] [Google Scholar]
- 27.Velasco I, Velasco-Velazquez MA, Salazar P, Lajud N, Tapia R. Influence of serum-free medium on the expression of glutamate transporters and the susceptibility to glutamate toxicity in cultured cortical neurons. J Neurosci Res. 2003;71(6):811–8. doi: 10.1002/jnr.10538. [DOI] [PubMed] [Google Scholar]
- 28.Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21(10):1200–7. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 29.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83(8):2496–500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bindal RD, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA. Lipophilic impurities, not phenolsulfonphthalein, account for the estrogenic activity in commercial preparations of phenol red. J Steroid Biochem. 1988;31(3):287–93. doi: 10.1016/0022-4731(88)90352-4. [DOI] [PubMed] [Google Scholar]
- 31.Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21(3):512–20. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 32.Han HJ, Heo JS, Lee YJ. Estradiol-17beta stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol. 2006;290(4):C1067–75. doi: 10.1152/ajpcell.00222.2005. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Cerdeno V, Noctor SC, Kriegstein AR. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur J Neurosci. 2006;24(12):3475–88. doi: 10.1111/j.1460-9568.2006.05239.x. [DOI] [PubMed] [Google Scholar]
- 34.Gago N, Akwa Y, Sananes N, Guennoun R, Baulieu EE, El-Etr M, Schumacher M. Progesterone and the oligodendroglial lineage: stage-dependent biosynthesis and metabolism. Glia. 2001;36(3):295–308. doi: 10.1002/glia.1117. [DOI] [PubMed] [Google Scholar]
- 35.Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135(1):47–58. doi: 10.1016/j.neuroscience.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsui K. Progesterone biosynthesis and action in the developing neuron. Endocrinology. 2008;149(6):2757–61. doi: 10.1210/en.2007-1592. [DOI] [PubMed] [Google Scholar]
- 37.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25(19):4706–18. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35(5):567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 39.Beyer C, Damm N, Brito V, Kuppers E. Developmental expression of progesterone receptor isoforms in the mouse midbrain. Neuroreport. 2002;13(6):877–80. doi: 10.1097/00001756-200205070-00028. [DOI] [PubMed] [Google Scholar]
- 40.Quadros PS, Schlueter LJ, Wagner CK. Distribution of progesterone receptor immunoreactivity in the midbrain and hindbrain of postnatal rats. Dev Neurobiol. 2008;68(12):1378–90. doi: 10.1002/dneu.20664. [DOI] [PubMed] [Google Scholar]
- 41.Charalampopoulos I, Remboutsika E, Margioris AN, Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol Metab. 2008;19(8):300–7. doi: 10.1016/j.tem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Guennoun R, Meffre D, Labombarda F, Gonzalez SL, Deniselle MC, Stein DG, De Nicola AF, Schumacher M. The membrane-associated progesterone-binding protein 25-Dx: expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res Rev. 2008;57(2):493–505. doi: 10.1016/j.brainresrev.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Ali A, Ramirez VD. Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. J Psychiatry Neurosci. 1996;21(3):187–97. [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz NF, Diaz-Martinez NE, Camacho-Arroyo I, Velasco I. Estradiol promotes proliferation of dopaminergic precursors resulting in a higher proportion of dopamine neurons derived from mouse embryonic stem cells. Int J Dev Neurosci. 2009 doi: 10.1016/j.ijdevneu.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klafke R, Wurst W, Prakash N. Genetic control of rodent midbrain dopaminergic neuron development in the light of human disease. Pharmacopsychiatry. 2008;41 1:S44–50. doi: 10.1055/s-2008-1080902. [DOI] [PubMed] [Google Scholar]
- 46.Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8(1):21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]


