Abstract
Covalently linked layers of glucose oxidase, single-wall carbon nanotubes and poly-L-lysine on pyrolytic graphite resulted in a stable biofuel cell anode featuring direct electron transfer from the enzyme. Catalytic response observed upon addition of glucose was due to electrochemical oxidation of FADH2 under aerobic conditions. The electrode potential depended on glucose concentration. This system has essential attributes of an anode in a mediator-free biocatalytic fuel cell.
Keywords: Glucose Oxidase, Direct Electron Transfer, Biofuel Cell, Anode Stability, SWCNT
1. Introduction
By using direct, rapid electron transfer (ET) between enzyme and electrode, enzymatic biofuel cells have the potential to operate close to thermodynamic redox potentials of the enzyme active sites, thereby maximizing voltage output [1–4]. Direct ET has been achieved for many enzymes by immobilizing them within thin films of various sorts on an electrode [1,5–8]. Glucose oxidase (GOX) based glucose oxidizing electrodes have attracted attention as candidate for biocatalytic fuel cell anodes. The FAD/FADH2 redox cofactor of GOX has a sufficiently negative formal potential for a biofuel cell anode, but it is buried ~13 Å within the globular structure of GOX which greatly inhibits direct ET between enzyme and conventional electrodes [3,9]. While mediators can be used to catalyze ET [10–13], the resulting anodes operate at potentials significantly more positive than that of FAD/FADH2 and decrease cell voltage output of the biofuel Cell.
Single walled carbon nanotubes (SWCNT) with diameters ~1 nm can be used to electrically connect GOX to electrodes [14,15]. Several reports demonstrated direct ET [16] and biocatalysis using functionalized SWCNTs on gold, with FAD attached to the ends of aligned SWCNTs [17] to capture apo-GOX. CNTs on other conductive surfaces were also used to obtain direct ET for GOX [18–20]. GOX/Polyion immobilized film electrode [21,22] showed direct ET to FAD but catalysis was shown by decrease in reduction currents.
A GOX biofuel cell anode featuring direct ET should provide maximum voltage if the open circuit potential (OCP) of the electrode responds to glucose concentration and reaches the FAD/FADH2 redox potential at high glucose concentration [2]. This desirable feature has been explored minimally. The goal of the present work was to construct a stable GOX anode that facilitates direct ET and evaluate its potential response to glucose concentration under aerobic conditions. For this, we utilized poly-L-lysine (PLL) covalently linked to pyrolytic graphite (PG), an approach developed previously without CNTs to stabilize enzymes [23–25] and with CNTs to stabilize antibodies [26].
In the present work, carboxylate groups on oxidized SWCNTs were covalently linked to GOX and PLL attached to PG. This novel PLL-SWCNT-GOX assembly optimized as a fuel cell anode facilitated direct ET between GOX and the underlying electrode, provided stable catalytic response to glucose in aerobic solutions at 0.45 V vs. SCE, and its potential approached that of FAD/FADH2 as glucose concentration increased.
2. Experimental
Glucose Oxidase was from Aspergillus niger (Sigma). SWCNTs (HiPCO) from Tubes@rice (90%) were oxidized and shortened to 30–200 nm by sonication in 3:1 HNO3– H2SO4 for 4 h at 70 °C [27,28]. Poly (L-lysine) (MW 150,000–300,000), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (NHSS) and all other reagents were from Sigma-Aldrich.
The PLL-SWCNT/GOX assembly was made on basal plane pyrolytic carbon (PG) disk electrodes (A=0.16 cm2) electrochemically oxidized as described previously [23]. PG was first covered with 10 μL freshly prepared 24 mM EDC and 10 μL 4 mM poly-L-lysine (PLL) to form amide linkages between electrode carboxylates and amino groups of PLL. After 8–12 hours, electrodes were rinsed with water, followed by deposition of 10 μL carboxylated SWCNTs dispersed in DMF (0.1 mg mL−1) and 10 μL freshly prepared 24 mM EDC on the PLL-coated surface to covalently link PG-PLL with carboxylate groups on the SWCNTs. Atomic force microscopy reported previously [26] revealed a relatively flat PLL layer, but a PLL-SWCNT assembly with average protrusion height 23±5 nm and domain width 382±68 nm. Unreacted carboxylate groups on these PLL-SWCNT surfaces were now activated by 10 μL 400 mM EDC and 100 mM NHSS for 10 min. This electrode was rinsed with water, then 3 μL 15 mg mL−1 GOX was added to form amide bonds between SWCNTs carboxylates and amino groups on GOX.
Voltammetry and amperometry was done using a CHI potentiostat in 0.1 M phosphate buffer pH 7.2 containing 0.137 M NaCl and 2.7 mM KCl (PBS) using a Pt counter and SCE reference electrodes. Galvanostatic operation of anode under 100 K load with a Pt cathode was performed in presence of 4 mM glucose and air. During constant current operation, solution was stirred and anode potential was monitored with SCE.
3. Results
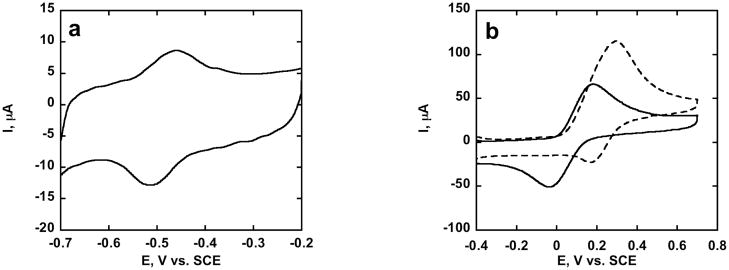
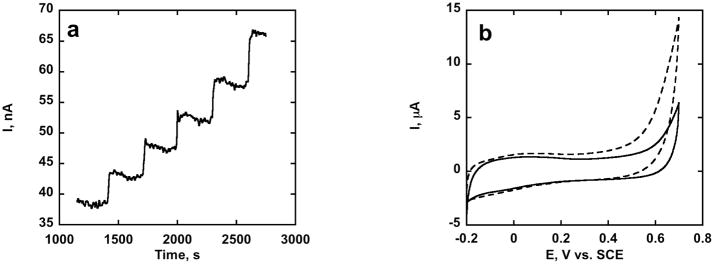
Cyclic Voltammetry (CV) of PLL-SWCNT/GOX revealed reversible direct electron transfer to the FAD cofactor in the enzyme at −0.45 V vs. SCE (Fig. 1a). Voltammetry at PLL-SWCNT/GOX in presence of hydroquinone mediator gave increased oxidation current with glucose present characteristic of biocatalytic glucose oxidation (Fig. 1b) demonstrating biocatalytic activity of immobilized GOX. GOX oxidizes glucose to gluconolactone while the FAD cofactor is reduced to FADH2, which is converted back to FAD by reducing quinone to hydroquinone. Bioelectrocatalysis was also observed via direct electrochemical oxidation of FADH2 using amperometry (Fig. 2a) and direct voltammetry under aerobic conditions (Fig. 2b). Here, FADH2 is oxidized electrochemically.
Fig. 1.
CVs of PLL-SWCNT/GOX in PBS at 0.025 V s−1: (a), anaerobic, no glucose (b) aerobic + 2 mM hydroquinone in absence (solid line) and presence of 10 mM glucose (dashed).
Fig. 2.
Biocatalytic glucose oxidation using PLL-SWCNT/GOX and direct ET in PBS: (a) aerobic amperometry at 1500 RPM, 0.45 V vs. SCE, upon multiple increases of 0.25 mM glucose. (b) aerobic CV at 0.005 V s−1 in absence (solid line) and presence of 60 mM glucose (dashed).
Oxygen is a natural co-substrate for GOX, and has a higher reduction potential. Thus it competes efficiently with the electrochemical oxidation of FADH2 [29, 30].
| (1) |
Oxidation of FADH2 by oxygen produces hydrogen peroxide that can be oxidized at about 0.7 V vs. SCE. However, no amperometric response was found at 0.45 V upon addition of hydrogen peroxide, confirming that the response in Fig. 2a is due to electrochemical oxidation of FADH2.
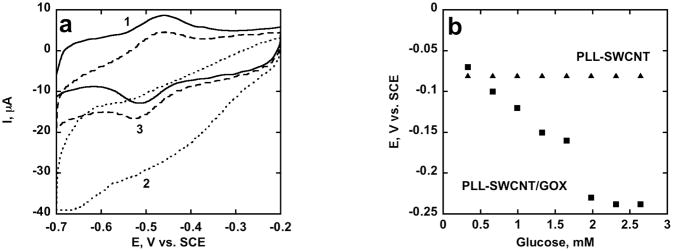
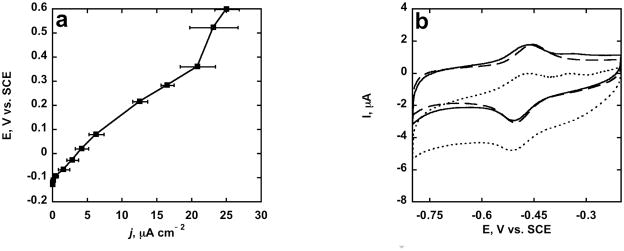
Voltammetry (Fig. 3a) under aerobic conditions also confirmed bioelectrocatalysis via direct ET at PLL-SWCNT/GOX. In absence of glucose, GOX catalyzes oxygen reduction to peroxide evident from the FAD reduction peak with concurrent loss of the FADH2 oxidation peak. But in the presence of glucose, the electrocatalytic reduction peak was smaller with the reappearance of the FADH2 oxidation peak. These results are consistent with those obtained previously on other GOX electrodes [29,30]. Also, under aerobic conditions, the OCP of the PLL/SWCNT/GOX anode reached a steady value of −0.23 V vs. SCE at >2 mM glucose (Fig. 3b). Galvanostatic operation of PLL-SWCNT/GOX anode with 100 K load produced limiting current density of ≈23 μA cm−2 at 4 mM glucose concentration under aerobic conditions (Fig. 4a).
Fig 3.
(a) CV at PLL-SWCNT/GOX electrode in PBS at 0.025 V s−1 – 1: under anaerobic conditions in absence of glucose, 2: under aerobic conditions in absence of glucose. 3: under aerobic conditions with 10 mM glucose (b) influence of glucose concentration on Open Circuit Potential of PLL-SWCNT/GOX electrode in PBS under aerobic conditions.
Fig. 4.
(a) Polarization curve for PLL-SWCNT/GOX anode in aerobic PBS + 4 mM Glucose (b) Anode Stability: CVs of PLL-SWCNT/GOX in PBS at 0.005 V s−1: anaerobic, in absence of glucose – at 0 hrs (dashed line) and after incubating the anode in PBS containing 1 mM glucose for 140 hrs (solid); aerobic, in presence of 1 mM glucose (dotted).
Heterogeneous electron transfer rate constant (ks) for PLL-SWCNT/GOX assembly was estimated at 114±21 s−1 using Laviron's peak separation method [31] after correcting for the constant non-kinetic peak separation [32].
A PLL-SWCNT/GOX electrode was incubated in aerobic PBS buffer containing 1 mM glucose for a week to drive glucose oxidation enzymatically with oxygen as co-substrate. This produces H2O2 which can damage unstabilized proteins [23]. CVs of this electrode in presence and absence of substrate and air suggested good stability (Fig. 4b).
4. Discussion
Current emphasis on biofuel cell applications to in vivo implantable energy generation requires a small size, semi-permanent biocatalytic electrode. Fig 1a demonstrates direct ET for our PLL-SWCNT/GOX electrode. Intimate contact of the SWCNT with the GOX immobilized on the PLL-SWCNT composite layer facilitated direct ET. Bioelectrocatalysis featuring direct ET at GOX electrodes has been observed voltammetrically for FADH2 oxidation (eqns 2,3) or for FAD reduction (eqns 2,4,5).
| (2) |
| (3) |
| (4) |
| (5) |
However, bioelectrocatalysis via FADH2 oxidation has been demonstrated at glucose concentration higher than physiological conditions [10,12,17] or under anaerobic conditions [20, 33]. Bioelectrocatalysis via FAD reduction doesn’t represent the function of a biofuel cell anode. Also, the presence of oxygen in physiological glucose-containing media is inevitable, but can be disadvantageous as it can scavenge electrons from FADH2 and lower current output. The enzyme reaction also forms hydrogen peroxide that may damage enzymes in the biofuel cell.
We have evaluated above the performance of the PLL-SWCNT/GOX anode under aerobic conditions at glucose concentrations comparable to physiological conditions. This bioanode with direct ET from the enzyme redox group produced a good catalytic response to glucose due to electrochemical oxidation of FADH2 under aerobic conditions (Fig. 2).
Bioelectrocatalysis relied on the applied anodic potential. The reduction potential of the electrode competes with the oxygen reduction potential to withdraw electrons from FADH2. At sufficiently positive electrode potential, electrons from FADH2 can tunnel efficiently to the electrode and not to the freely diffusing oxygen [17]. Thus, the amperometric response at 0.45V vs. SCE upon addition of glucose is due to the oxidation current from FADH2 set up when electrode has sufficiently positive overpotential to compete with the oxygen reduction potential. It is the interplay between electrode overpotential and oxygen reduction potential that controls the flow of electron from FADH2 to the electrode. At low overpotentials, e.g. <0.45 V vs. SCE, FADH2 electrons are scavenged by oxygen and the amperometric oxidation current decreases.
Catalytic voltammetry at PLL-SWCNT/GOX electrode (Fig. 3a) under aerobic conditions in presence of glucose represents two reactions – glucose and the electrode are both competing for FAD reduction. Glucose reduces FAD enzymatically and the electrode electrochemically. The enzymatic reduction rate is much faster than electrochemical rate of FAD reduction, evident from the difference in the FAD reduction peak in presence and in absence of glucose. More importantly, electrochemical FADH2 oxidation strongly competes with the enzymatic pathway featuring oxygen reduction as seen from the increased FADH2 oxidation peak in presence of glucose confirming effective electron transfer between the GOX redox group and the electrode during biocatalysis.
Electron transfer from FADH2 in the enzyme to the electrode surface suggests that SWCNTs are within tunneling distance, but that’s insufficient for the electrode to be responsive to the glucose concentration at open circuit potential. The redox group in GOX is buried in the protein globular structure and the direct electrical contact of the FADH2 redox group to the electrode surface is essential for the electrode to show Nernstian behavior. This aspect of the enzyme electrode is very important in mediator-less biofuel cell if the biofuel cell anode is to operates close to the FAD/FADH2 redox potential in the presence of glucose to produce maximum cell voltage output [2,10]. PLL-SWCNT/GOX had open circuit potential responsive to the glucose concentration (Fig. 3b) that makes it suitable for applications as biofuel cell anode.
Immobilization of GOX on CNT via adsorption or on polyion layer by electrostatic attraction produced ks values about 2 s−1 [2, 14] where as direct linkage of SWCNTs and GOX/FAD [17, 30] resulted in ks values in the range 70–100 s−1. FAD is located deep in the protein (13 Å) from the surface and such high transfer rate may be possible if there is a direct contact of FAD with the nanotubes to eliminate electron hopping or tunneling limitations [14,17,30]. The large ks of PLL-SWCNT/GOX suggests that SWCNTs may approach FAD in the protein to electrically linking it to the underlying PG surface [14].
Under galvanostatic operation, anodic current from PLL-SWCNT/GOX oxidation developed at potential–0.1 V vs. SCE and above. A limiting current density plateau ~23 μA cm−2 began at 0.3 V vs. SCE (Fig. 4a). Previous studies with GOX anodes based on direct ET reported large currents only at much higher glucose concentrations (20–60 mM) and in anaerobic solutions.
PLL-SWCNT/GOX anode showed excellent physical and biochemical stability evident from the retention of CV peak currents and promotion of bioelectrochemical catalysis when stored with catalytic turnover for 6 days (Fig. 4b).
Conclusions
Above we describe construction and performance of a GOX anode suitable for a mediator-free biofuel cell operating under aerobic conditions. Electrically connecting GOX to the electrode with a PLL-SWCNT composite enabled direct ET with anode potential under control of the glucose concentration. Further work on incorporating this anode into a biofuel cell with high power density is underway.
Acknowledgments
This research was supported in part by a Science Foundation Ireland Walton Research Fellowship to JFR and in part by PHS grant ES013557 from NIEHS/NIH. Nighat Hussain sincerely acknowledges support from NSF/URAP program at University of Connecticut, Department of Chemistry. JFR is grateful for a Walton Research Fellowship from Science Foundation Ireland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cracknell JA, Vincent KA, Armstrong FA. Chem Rev. 2008;108:2439. doi: 10.1021/cr0680639. [DOI] [PubMed] [Google Scholar]
- 2.Ivnitski D, Branch B, Atanassov P, Apblett C. Electrochem Commun. 2006;8:1204. [Google Scholar]
- 3.Barton SC, Gallaway J, Atanassov P. Chem Rev. 2004;104:4867. doi: 10.1021/cr020719k. [DOI] [PubMed] [Google Scholar]
- 4.Willner I, Baron R, Willner B. Biosens Bioelectro. 2007;22:1841. doi: 10.1016/j.bios.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Rusling JF, Zhang Z. In: Biomolecular Films. Rusling JF, editor. Marcel Dekker; N. Y: 2003. p. 1. [Google Scholar]
- 6.Rusling JF, Wang B, Yun S. In: Handbook of Bioelectrochemistry. Bartlett PN, editor. John Wiley; N. Y: 2008. p. 39. [Google Scholar]
- 7.Rusling JF, Zhang Z. Handbook Of Surfaces And Interfaces Of Materials. In: Nalwa RW, editor. Biomolecules, Biointerfaces, and Applications. Vol. 5. Academic Press; 2001. Ch 2.10. [Google Scholar]
- 8.Rusling JF, Hvastkovs EG, Hull DO, Schenkman JB. Chem Commun. 2008:141. doi: 10.1039/b709121b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi Q, Zhang J, Dong S, Wang E. Electrochim Acta. 1994;39:2431. [Google Scholar]
- 10.Degani Y, Heller A. J Phys Chem. 1987;91:1285. [Google Scholar]
- 11.Degani Y, Heller A. J Am Chem Soc. 1988;110:2615. [Google Scholar]
- 12.Barriere F, Kavanagh P, Leech D. Electrochim Acta. 2006;51:5187. [Google Scholar]
- 13.Pellissier M, Barriere F, Downard AJ, Leech D. Electrochem Commun. 2008;10:835. [Google Scholar]
- 14.Guiseppi-Elie A, Lei C, Baughman RH. Nanotechnol. 2002;13:559. [Google Scholar]
- 15.Willner B, Katz E, Willner I. Curr Opin in Biotechnol. 2006;17:589. doi: 10.1016/j.copbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Chou A, Rahmat W, Paddon-Row MN, Gooding JJ. Electroanal. 2005;17:38. [Google Scholar]
- 17.Patolsky F, Weizmann Y, Willner I. Angew Chemie Int Ed. 2004;43:2113. doi: 10.1002/anie.200353275. [DOI] [PubMed] [Google Scholar]
- 18.Liu SN, Yin YJ, Cai CX. Chin J Chem. 2007;25:439. [Google Scholar]
- 19.Cai C, Chen J. Anal Biochem. 2004;332:75. doi: 10.1016/j.ab.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 20.Tominaga M, Nomura S, Taniguchi I. Electrochem Commun. 2008;10:888. [Google Scholar]
- 21.Wang D, Chen L. Electrochim Acta. 2009;54:4316. [Google Scholar]
- 22.Fu C, Yang W, Evans D. Electrochem Commun. 2009;11:997. [Google Scholar]
- 23.Vaze A, Parizo M, Rusling JF. Langmuir. 2004;20:10943. doi: 10.1021/la048712g. [DOI] [PubMed] [Google Scholar]
- 24.Vaze A, Rusling JF. Langmuir. 2006;22:10788. doi: 10.1021/la061138j. [DOI] [PubMed] [Google Scholar]
- 25.Guto PM, Kumar CV, Rusling JF. Thermostable Poly(lysine)-Enzyme Films for Biocatalysis at 90 °C. J Phys Chem B. 2007;111:9125. doi: 10.1021/jp071525h. [DOI] [PubMed] [Google Scholar]
- 26.Cataldo V, Vaze A, Rusling JF. Improved Detection Limit and Stability of Amperometric Carbon Nanotube-based Immunosensors by Crosslinking Antibodies with Polylysine. Electroanalysis. 2008;20:115. doi: 10.1002/elan.200704040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X, Munge B, Patel V, Jensen G, Bhirde A, Gong JD, Kim SN, Gillespie J, Gutkind JS, Papadimitrakopoulos F, Rusling JF. J Amer Chem Soc. 2006;128:11199. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Kim SN, Papadimitrakopoulos F, Rusling JF. Molec Biosyst. 2005;1:70. doi: 10.1039/b502124c. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Killard AJ, Smyth MR. Electroanalysis. 2006;18:1131. [Google Scholar]
- 30.Liu G, Paddon-Row MN, Gooding JJ. Electrochem Commun. 2007;9:2218. [Google Scholar]
- 31.Laviron E. J Electroanal Chem. 1979;101:19. [Google Scholar]
- 32.Hirst J, Armstrong FA. Anal Chem. 1998;70:5062. doi: 10.1021/ac980557l. [DOI] [PubMed] [Google Scholar]
- 33.Rosalba Rincon DI, Pylypenko S, Atanassov P. 213th ECS Conf; Phoenix, AZ. 18–22 May, 2008. [Google Scholar]