Abstract
Objective
Understanding of how female subjects learn to move accurately during a resisted weight-bearing task is limited. The purpose of this study was to examine the muscle activation patterns used by female subjects in learning a novel single-leg squat (SLS) task under visual and nonvisual conditions.
Design
Prospective training study.
Setting
University research setting.
Participants
Ten healthy young female participants.
Intervention
Subjects tracked a sinusoidal target (knee displacement) during a resisted SLS exercise during the course of 4 days, under eyes open (EO) and eyes closed (EC) conditions with the use of a custom-designed weight-bearing exercise device.
Main Outcome Measurement
The accuracy of performance in tracking the target and electromyographic activity (EMG) of 5 muscles around the knee were monitored.
Results
Subjects improved their accuracy of performance by day 2 (40% decrease in error) and retained the accuracy on day 4. Error during the EC condition was 3 times greater than EO condition. Quadriceps-to-hamstrings coactivation ratio increased with the improved accuracy from the learning. Absence of visual feedback was accompanied by a decrease in the quadriceps-to-hamstrings coactivation ratio for this task.
Conclusion
The muscle synergistic activity around the knee changes as the accuracy of the task improves during a resisted weight-bearing task. This activation pattern represents a feed forward control plan that the central nervous system adopted to optimize accurate weight-bearing knee displacement. Rehabilitation specialists should consider manipulating the visual feedback and accuracy of performance when developing weight-bearing rehabilitation training protocols to improve neuromuscular control in female patients.
INTRODUCTION
Injury to the knee joint accounts for 15–50% of all sports injuries [1]. Injury to the anterior cruciate ligament accounts for more than 20% of all knee injuries, and women have a 4- to 6-fold greater incidence of knee injury than males [2]. The increased risk of knee injury in women coincides with increased female participation in sports and, therefore, a need to focus rehabilitation programs on female athletes. One important factor contributing to injury risk is impaired neuromuscular control, which includes imbalances in muscle strength, varied muscle activation patterns, and altered timing of muscle activity, leading to injury [3,4].
Rehabilitation interventions strive to improve the nervous system’s control of muscle to optimize stiffness and prevent injury when an unexpected force is introduced [5–7]. However, increased muscle stiffness around the knee joint to prevent injury also may impair movement accuracy and, ultimately, human performance. The central nervous system (CNS) regulates muscle stiffness across the knee joint during weight-bearing activity to maintain the upright position. In the event that the knee needs to bend a precise amount, under loaded conditions, various control strategies to modulate agonists and antagonists crossing the knee come into play. Indeed, the requirement for more accurate movement may be at the expense of co-contraction of muscle and knee stability, suggesting that knee injury occurs when there is a requirement for precise movements during loaded, resisted, weight-bearing conditions.
Muscle coactivation around a joint is the degree that both the agonist and antagonist muscles are active at the same time during a movement. During the early stages of learning a non-weight-bearing motor task, the CNS uses muscle coactivation initially but, with practice, coactivation often is reduced as accurate movement is achieved [8,9]. Decreased joint stiffness, which involves a decrease in coactivation, associated with learning a non-weight-bearing movement appears appropriate for limbs that are not loaded. However, to the authors’ knowledge, few studies have examined whether this same strategy emerges during weight-bearing tasks.
Neuromuscular control of movement also relies on visual guidance with certain tasks. In upper extremity movements, vision contributes to the regulation of hand path direction and extent, whereas proprioception plays an important role in bringing the hand smoothly to rest [10]. In the lower extremity, visual information influences foot trajectory during swing phase of the leg toward a stationary target [11]. Eliminating vision during upper extremity movements increases muscle coactivation and increased joint stiffness [10,12]. Accordingly, vision appears to be an important factor determining the type of muscle synergistic strategy used. Recent research findings support that increased accuracy of performance causes a decrease in coactivation during a weight-bearing task, but the influence of visual feedback and task retention was not examined [11,12].
The single-leg squat exercise (SLS) is a dynamic weight-bearing task that represents an important part of lower-extremity rehabilitation programs [13–16]. The purpose of this investigation was to quantify performance accuracy and muscle activation strategies during the SLS exercise with and without visual feedback (eyes open and eyes closed) during the course of 4 days. Female subjects were studied because of their high predisposition to noncontact knee injuries. The authors hypothesized that knee joint stiffness (coactivation) will vary according to task accuracy and vision.
METHODS
Subjects
Ten healthy female subjects (ages 22–26 years) were recruited to participate in this study in response to a generalized research advertisement. Inclusion criteria included regular physical activity without participation in any physical training program, right leg dominance (ie, leg used to kick a ball), and ability to climb stairs without any difficulty. Exclusion criteria included body mass index greater than 29, history of neurological deficits, musculoskeletal disorders, degenerative joint diseases, cardiovascular diseases, previous knee injury or surgery, previous fractures of the lower extremity, patellar dislocations, and past or current knee pain during activity or rest. Before participation, subjects were given a brief description of the protocol and possible risks of participation and were required to sign an informed consent statement approved by the institution’s Human Subjects Review Board.
Instrumentation
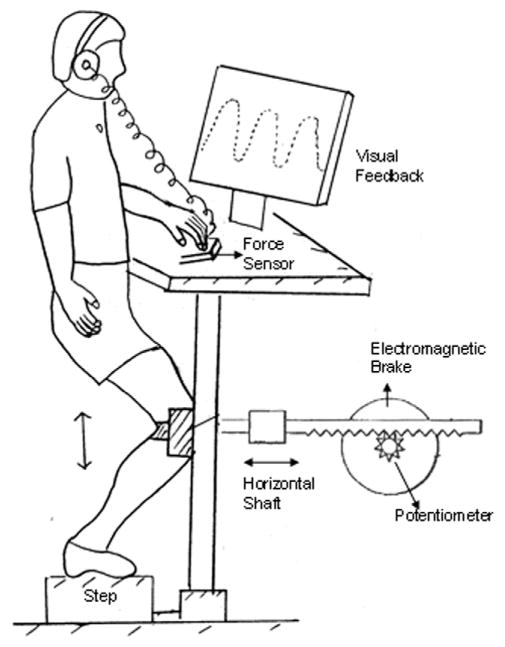
Subjects performed a resisted and controlled SLS exercise in a lower extremity perturbation device that has been described previously [16]. In brief, the device consisted of a rack-and-pinion gear system that was attached to the anterior surface of the knee joint (Figure 1). The linear displacement of the knee during the SLS was measured by a potentiometer calibrated to convert angular displacement into linear displacement (cm). Pilot studies showed that this horizontal forward and backward translation of the knee has a strong correlation to knee angular position as measured by a video motion analysis system (R2 = 0.97) [16]. Subjects went through a range of 15 cm while performing the SLS. This 15-cm displacement corresponds to approximately 30° of knee flexion.
Figure 1.
Schematic diagram of the lower extremity perturbation device.
An electromagnetic brake, under computer software control, controlled the resistance of the pinion gear. The resistance of the brake was normalized to each subject and was set at 12% body weight resistance throughout knee flexion and extension. Previous data supported that this level of resistance required moderate activity of the muscle synergists and challenged the ability to accurately use the device [16]. Linearity, repeatability, and hysteresis of the brake and potentiometer system were within 0.5% of full scale. Subjects were instructed to follow a sinusoidal tracking pattern that appeared on a computer at a frequency of 0.4 Hz. Specifically, when the subjects flexed their knee, the knee movement provided a cursor that the subjects were to superimpose over the descending line of the sinusoidal curve. When the subjects were extending the knee, they were asked to superimpose the cursor over the ascending limb of the sinusoidal curve. Thus, one complete flexion and extension cycle took approximately 2500 ms for the subjects to perform. When subjects were asked to move without vision, they were provided feedback at the start, at which time the target disappeared. The subjects were asked to move to a remembered target.
Subjects were permitted to place 2 fingers for support on a load sensor (Wafer Load Cell, Model 872, Loadstar Sensor Inc., Mountain View, CA) mounted on the left side of the device. They were instructed to put very little load through their finger, using it for light touch contact and not biomechanical support. The output from the load cell was used to provide an auditory warning if the force exceeded 5 N. Analysis of this touch force showed that subjects did not exceed 3 N of force throughout the entire testing.
Surface electromyographic (EMG) recordings were collected from 5 muscles: the rectus femoris (RF), vastus medialis oblique (VM), vastus lateralis (VL), lateral hamstrings (LH), and medial hamstrings (MH), of the exercised limb on day 1 and day 4. Before the electrodes were fixed, the skin was cleaned with alcohol to ensure adequate contact. Silver–silver chloride electrodes (8 mm in diameter) were placed by a single investigator according to the landmarks described by Cram et al [17]. The amplifier used a high-impedance circuit with a common mode rejection ratio of 87 dB at 60 Hz and a bandwidth of 15 to 4000 Hz (Model 544, Therapeutics Unlimited, Iowa City, IA).
Procedures
Each subject attended 2 preliminary training sessions (day 1 and day 2) followed by 2 testing sessions after 48 hours (day 3 and day 4). Before the start of the protocol on the first and last day, 3 maximum voluntary isometric contractions (MVICs) of each muscle were obtained. Subjects were positioned in sitting on the chair of a Kin-Com isokinetic dynamometer (Kin-Com 125E+; Chattex Corp., Chattanooga, TN) with the knee joint positioned in 45° of flexion and were asked to hold each MVIC for 3 seconds. Subjects were then placed in the experimental apparatus and their right knee strapped to the movable segment of the device. The subject was instructed to keep the noninvolved leg off the ground by flexing slightly at the knee and the left finger was allowed to make contact with the force sensor. Subjects were instructed to avoid leaning or rotating during the task and were given verbal cues if there were any deviations in the technique or form of exercise during the learning sessions. The foot placement was marked so that any change in the position could be easily detected and corrected and the same foot placement could be maintained across days.
On day 1 subjects were provided with a detailed verbal description of the protocol and instructed to follow the target as closely as possible. A 1-minute rest interval was given after every set of 10 repetitions. Subjects were asked to rate their perceived exertion on a Borg Scale after every other set of the exercise [18]. This scale is found to be sensitive to perceived levels of exertion in isolated muscle [19,20]. The rate of perceived exertion did not increase for any of the subjects throughout all the training and testing sessions, supporting that the experimental protocol was not perceived to be fatiguing. After every set of the exercise, an error score was provided to the subject. The error score gave them feedback about their spatial and temporal accuracy. Eyes closed (EC) trials were interspersed between eyes open (EO) trials. The order of trials was as follows: 2 sets EO → 1 set EC → 2 sets EO → 1 set EC → 1 set EO → 3 sets EC → 1 set EO. Subjects returned 24 hours later for day 2 of the training session, which consisted of the same protocol as day 1.
The testing was done 48 hours after training on day 2. The testing session differed from the training session in that no error scores or knowledge of results was given during or after the task. The order of retention trials was as follows: 2 sets EO → 2 sets EC → 1 set EO → 1 set EC. Subjects returned on day 4 to be tested again on retention of learning. The experimental procedure was similar to the one used for the retention trials on day 3.
Data Reduction
All experimental data were collected online and subsequently analyzed by the use of Datapac 2K2 software (version 3.14; Run Technologies Inc., CA). Electromyographic activity of the quadriceps and hamstring muscles was sampled at a rate of 2000 Hz. All other signals (ie, linear potentiometer, target waveform, Schmitt trigger, brake and touch force) were digitized at a rate of 1000 Hz.
Linear displacement of the knee, determined from the potentiometer, was differentiated (time constant = 10 ms) and low pass filtered at 6 Hz using a fifth-order zero-phase lag Butterworth filter. The EMG signal, obtained on day 1 and 4, was RMS (root mean square) processed with a time constant of 10 ms. MVICs were analyzed by finding the peak RMS EMG during each of the 3 contractions and calculating the mean RMS EMG for 200 ms on either side of the peak EMG. All EMG measures were expressed as a percentage of MVIC.
Outcome Measures
Dependant variables analyzed in this study were:
Absolute Error: To obtain absolute error of performance during the learning and retention trials, the sinusoidal target was subtracted from the linear knee displacement to calculate error. The error signal was then rectified and averaged in 10% bins within each flexion and extension cycle of the SLS exercise.
Variable Error: Variable error was calculated by measuring the standard deviation about the error signal in 10% bins within each flexion and extension cycle of the SLS exercise.
Mean Electromyographic (EMG) Activity: Quadriceps and hamstrings EMG signals were RMS processed and then averaged within 10% bins of flexion and extension cycle of the SLS exercise. All values were expressed as a percentage of MVIC. For purposes of this report, data are presented as an average of the flexion and extension phases.
Coactivation Ratios: Balance of muscle activity during the task was examined by calculating vastus medialis/medial hamstrings (VM:MH) and vastus lateralis/lateral hamstrings (VL:LH) ratios within the flexion and extension phases.
Statistical Analysis
All data were analyzed by the use of a 2-factor repeated measures analysis of variance. The 2 within-subject factors were condition (EO and EC) and day (day 1–day 4). A separate analysis was performed for each of the dependent variables. The level of significance for all tests was established at α ≤ 0.05. All statistical analyses were performed with the use of SAS software (version 9; SAS Institute, Cary, NC).
RESULTS
Effect of Vision and Training on Accuracy of Performance
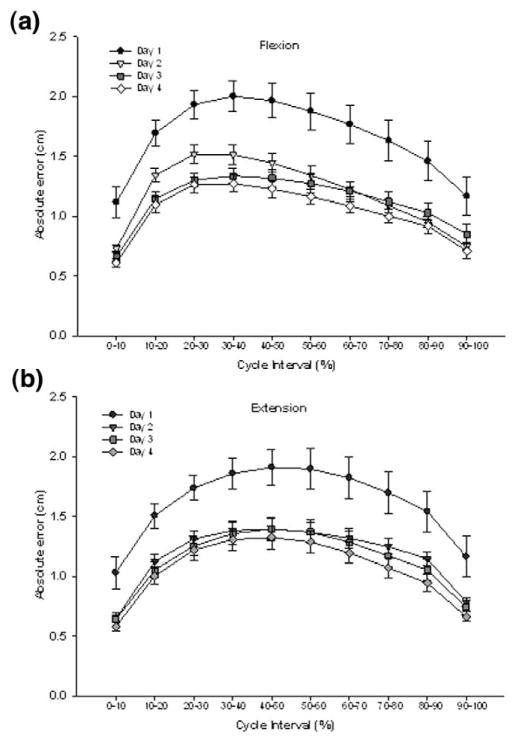
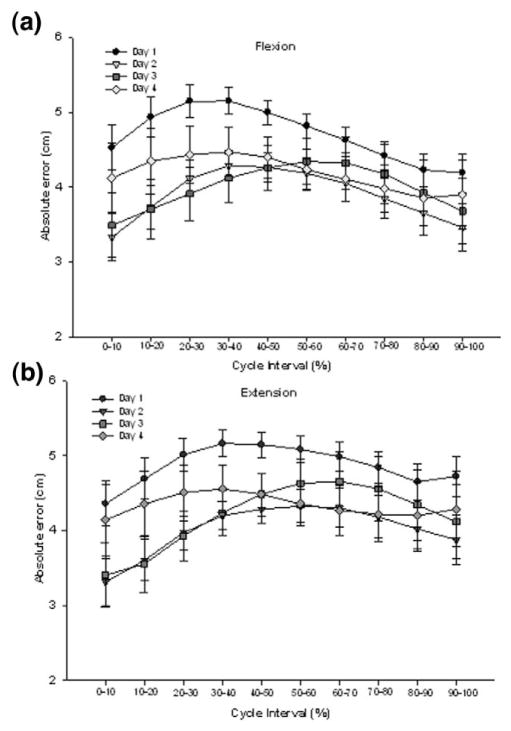
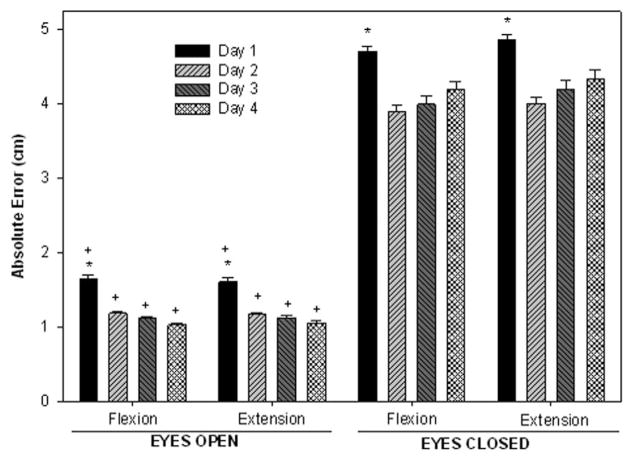
The mean absolute errors (AE) of performance observed during the SLS task through days 1–4 performed with EO and EC are shown in Figures 2 and 3, respectively. The greatest errors were in the midportion of the flexion and extension phases of the task. A 2-way repeated measures analysis of variance (vision × day) on the absolute errors showed no significant interactions. A significant main effect of condition was seen during both the flexion (F1,9 = 316.20; P < .0001) and extension phases (F1,9 = 328.36; P <.0001). As hypothesized, absolute error during EC was found to be greater (by almost 200%) than when visual feedback was available (mean error: flexion, EC 4.22 cm, EO 1.28 cm; extension, EC 4.37 cm, EO 1.29 cm]. A significant main effect of day was also noted for both flexion (F1,27 = 8.55; P = .0004) and extension (F1,27 = 8.86; P = .0003) phases. In summary, absolute error decreased by 44% from day 1 to day 4 in the EO condition and decreased by 12% in the EC condition (Figure 4). No difference was seen between day 2, day 3, and day 4, suggesting that subjects were able to reach proficiency in one session and retain that proficiency over several days.
Figure 2.
Absolute errors of performance in the EO condition within the flexion (a) and extension (b) phases of the single leg squat task. Mean error values are presented for day 1 (circles), day 2 (triangles), day 3 (squares), and day 4 (diamonds). Values are means ± SE of all 10 subjects.
Figure 3.
Absolute errors of performance in the EC condition within the flexion (a) and extension (b) phases of the single leg squat task. Mean error values are presented for day 1 (circles), day 2 (triangles), day 3 (squares), and day 4 (diamonds). Values are means ± SE of all 10 subjects.
Figure 4.
Mean absolute error during the flexion and extension phases of the SLS task in the EO and EC conditions. The vertical bars represent the average errors over days 1, 2, 3, and 4. The y axis represents absolute error of performance in centimeters. *Indicates significant difference from other days when conditions are combined. +Indicates significant difference from EC when days are combined.
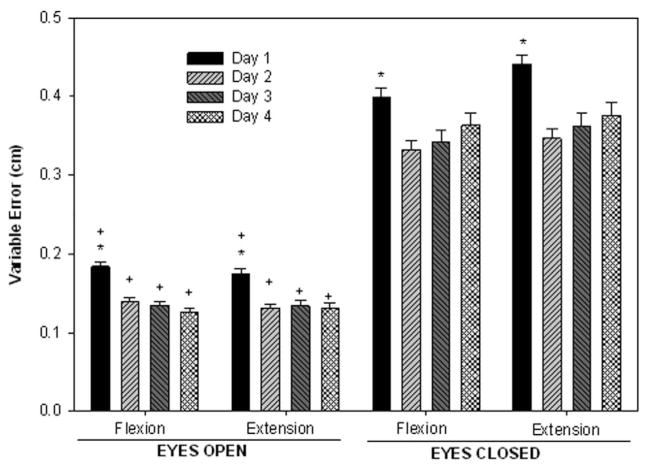
Variable errors showed that subjects were very consistent in the performance of the SLS task, with the greatest variability in errors occurring during the transitions between flexion and extension. A significant effect of vision during both flexion (F1,9 = 13.86; P <.0001) and extension (F1,9 = 4.88; P < .001) was found. Overall variable error was ~ 140% greater in the EC condition than the EO. A significant main effect of day was present for both the flexion (F9,81 = 8.48; P = .004) and extension (F9,81 = 10.40; P = .001) phases. In summary, variable error declined by 24% with training from day 1 to day 4 (Figure 5).
Figure 5.
Mean variable error during the flexion and extension phases of the SLS task in the EO and EC conditions. The vertical bars represent the average errors over days 1, 2, 3, and 4. The y axis represents variable error of performance in centimeters. *Indicates significant difference from other days when conditions are combined. +Indicates significant difference from EC when days are combined.
Effect of Training and Vision on Muscle Activation Patterns
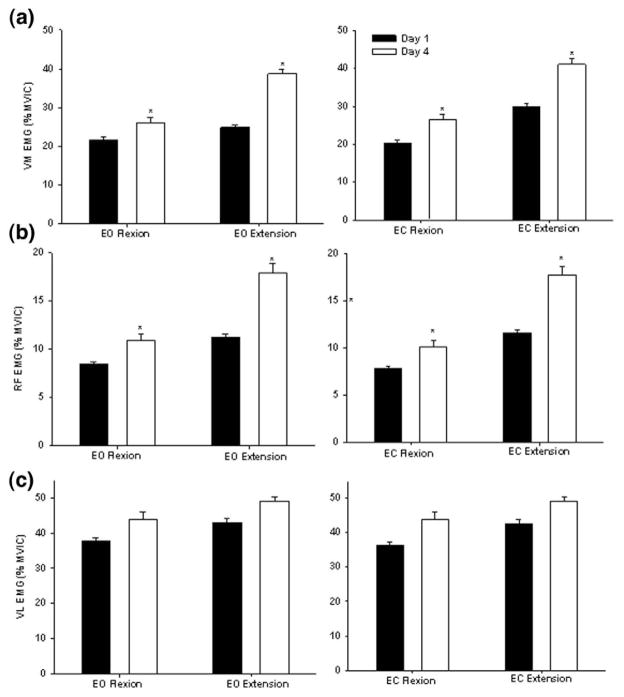
Analysis of VM muscle activity revealed a significant main effect of day in flexion (F1,9 = 1.79; P = .0427) and extension (F1,9 = 3.06; P = .031). VM activity during day 4 was 30% greater than day 1 during both the EO and EC conditions (Figure 6a). In the flexion phase, RF activity showed a significant effect of day (F1,9 = 3.15; P .023) and condition (F1,9 = 5.4; P = .0162). The authors found that RF activity was increased by 37% from day 1 to day 4 and RF activity was found to be 6% greater during the EO condition than EC condition. In the extension phase, a significant main effect of day was observed (F1,9 = 6.59; P = .0170). The RF activity was increased by ~54% from day 1 to day 4 during extension (Figure 6b). Overall, VL was the most active in both the flexion and extension phases of the task in both the EO and EC conditions across all days. However, this muscle was the least modulated by vision or training. Activity of VL remained relatively uniform throughout the exercise (Figure 6c).
Figure 6.
Mean EMG activity of the vastus medialis (a), rectus femoris (b), and vastus lateralis (c) during the flexion and extension phases of the SLS task in the EO and EC conditions. The vertical bars represent the average activity on days 1 (dark bar) and 4 (white bar). The y-axis represents muscle activity as a percent of maximum voluntary isometric contraction (MVIC). *Indicates significant difference from day 1 when conditions are combined.
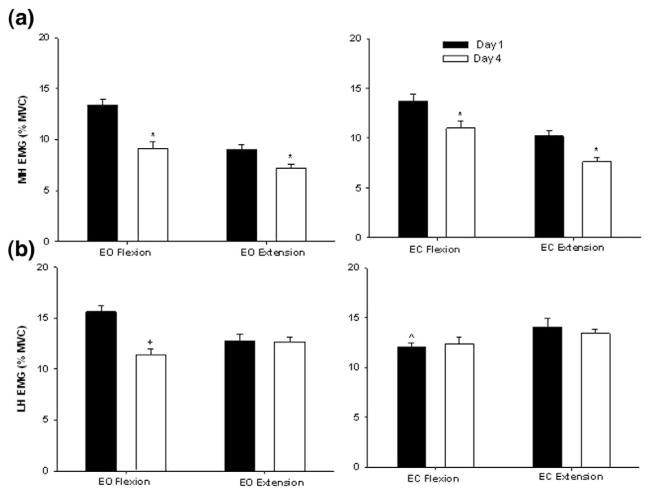
The MH activity was reduced as the individual improved accuracy by day 4 (Figure 7a). A significant main effect of day was observed for both flexion (F1,9= 7.53; P = .036) and extension (F1,9= 4.28; P = .045). MH activity was 35% less on day 4 when compared with day 1. The LH activity during the EO condition was also 24% lower on day 4 than day 1. A significant 2-way interaction of vision × day (F1,9 = 5.81; P =.021) was identified in the flexion phase for the LH. Simple effects analysis showed that LH activity in EO was 27% greater than EC. None of the main effects or interactions reached significance in the extension phase (Figure 7b).
Figure 7.
Mean EMG activity of the medial hamstrings (a) and lateral hamstrings (b) during the flexion and extension phases of the SLS task in the EO and EC conditions. The vertical bars represent the average activity on days 1 (dark bar) and 4 (white bar). The y axis represents muscle activity as a percent of maximum voluntary isometric contraction (MVIC). *Indicates significant difference from day 1 when conditions are combined. +Indicates significant difference from day 1 only during EO flexion. ^Indicates significant difference from EO only on day 1.
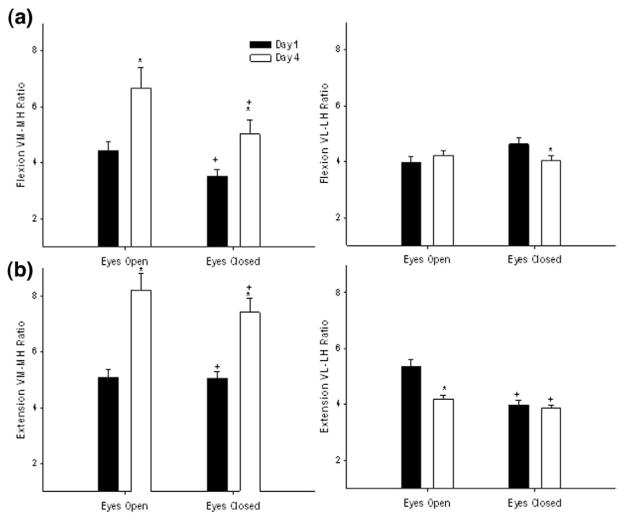
During flexion, the VM:MH coactivation ratio showed a significant main effect of day (F2,18 = 13.81; P = .0025) and vision (F1,9 = 5.75; P = .0475) (Figure 8). On average, subjects increased their coactivation ratio by ~40% from day 1 to day 4 by increasing their VM activity. The VM:MH coactivation ratio was 32% greater during EO than EC. The VL:LH ratio during flexion showed a significant interaction of vision × day (F2,18 = 6.41; P = .0218). Follow-up tests showed that the VL:LH ratio differed between the EO and EC conditions on day 1 (P = .0341), but no difference was found by day 4. On day 1, there was a 21% decreased coactivation ratio during the EC condition because of decreased LH activity.
Figure 8.
Effect of training and visual feedback on the pattern of medial and lateral quadriceps hamstrings coactivation during flexion (a) and extension phases (b). VM:MH and VL:LH coactivation ratios are presented for day 1 (black bars), and day 4 (white bars). Values are means ± SE. *Indicates significant difference from day 1 when conditions are combined. +Indicates significant difference from EO when days are combined.
During the extension phase of the SLS exercise, a significant main effect of day (F2,18 = 9.72; P =.0072) and (F1,9 = 17.98; P = .0133) was seen for the VM:MH ratio (Figure 8). When both the conditions were combined, subjects increased their VM:MH coactivation ratio on day 4 by 42% through increased recruitment of the VM and decreased activation of the MH. With all days combined, subjects showed ~ 25% greater VM:MH coactivation ratios with EO when compared to EC. A significant main effect of vision (F1,9 = 5.32; P = .045) was seen for VL:LH ratio during extension. On average, the subjects had 17% greater VL:LH coactivation ratio during EO than EC.
DISCUSSION
The purpose of this study was to examine accuracy of performance and muscle activation strategies of a lower extremity weight-bearing task performed under different conditions of visual feedback across a 4-day training session in female subjects. Weight-bearing tasks, like the SLS exercise used in this study, form an integral part of rehabilitation after injury. Yet, information about the learning patterns of such exercises are lacking. The novelty of this study was to incorporate a sinusoidal target-tracking task of the knee during the performance of the weight-bearing exercise, thereby controlling the rate and amplitude of the exercise and providing feedback about motor performance. In support of the hypothesis, the results of the study showed that subjects improved accuracy of performance from day 1 to day 4. Learning of the task was accompanied by a significant increase in the activity of the vastus medialis and rectus femoris and a decrease in medial hamstrings activity, leading to increased VM:MH coactivation ratio with training.
Muscle coactivation ratios decreased when the task was performed in the absence of visual feedback because of increased activity of the hamstring muscles. These findings indicate that improved task performance (accuracy) changed the balance of agonist-antagonist coactivation. This finding suggests that preventive rehabilitation programs should consider a metric for accuracy of task performance during weight-bearing tasks.
Trajectory tracking tasks have evolved as a tool for evaluating motor control in healthy people and those with coordination deficits [21–23]. The target tracking task involves a constant method of self-correction with visual feedback or knowledge of results during which the individual’s motor planning capabilities are continually challenged.
During the past decade, training with a target pattern has emerged as a motor learning tool for functional rehabilitation, especially in the paretic limb of the upper extremity in patients with stroke. Trajectory tracking tasks, which are used in individuals with stroke for training hand function, showed improved upper extremity function accompanied by increased cortical excitability [24]. Most studies that examine trajectory tracking have focused on the upper extremity. However, Maffiuletti et al [25] examined a lower limb trajectory tracking task on a leg press machine and found that healthy subjects significantly improved their tracking error within the second day of learning. No differences in performance emerged when the task was performed concentrically or eccentrically. Tracking accuracy also was not different whether the task was performed by the dominant or non-dominant lower limb. Chung and colleagues [22] used a knee joint tracking training task, which involved tracking sine waves of changing frequencies in partial weight bearing.
Training with target tracking increased motor skill performance which was associated with cortical excitability [24]. Perez et al [26] showed that during the acquisition of a visuomotor skill task, selective presynaptic inhibition of group Ia afferents occurs, which contributes to the modulation of sensory inputs during the learning process. More recently, it was also shown that visuomotor skill learning in humans is accompanied by changed corticospinal excitability [27].
The present study used a 4-day training protocol under different conditions of visual feedback. Subjects showed a significant improvement in accuracy and consistency of performance on day 2 and maintained this on days 3 and 4. Because just 1 day of learning brought about significant decrease in tracking errors, future studies assessing the acquisition of motor skill during this weight-bearing task can be tested on day 2. Because subjects improved in performance with training, muscle activity was modulated similarly under both conditions of visual feedback for all muscles except the lateral hamstrings. Specifically, there was an increase in the VM:MH coactivation ratios with learning although the magnitude of this coactivation ratio was less in the eyes closed condition. Although all the muscles recorded were active during the weight-bearing exercise, the vastus medialis and rectus femoris were modulated the most with training. Consistent with the authors’ hypothesis, an increase in quadriceps activity occurred with training. It is not clear why the vastus lateralis did not modulate in a consistent manner as the vastus medialis and rectus femoris, but suggests a differential control mechanism.
A decrease in the coactivation ratio (increased activation of antagonist muscles around a joint) after motor skill training was reported by others [9,28]. It has been suggested that the CNS increases limb stiffness early in the learning phase to compensate for absence of an internal model, but once the dynamics of the task are understood, there is an increase in coactivation ratio [28]. A recent study showed there is an increase in quadriceps activity accompanied by a decrease in hamstrings activity during the flexion phase of a weight-bearing exercise [15]. This strategy of increased quadriceps activity and associated increased coactivation ratio is retained after 4 days as demonstrated in this study.
Neuromuscular control of movement includes integration of afferent information from the visual, somatosensory, and vestibular systems. As a major functional role of these sensory systems is control of posture and balance, the absence of one or more systems has been largely explored during quiet standing. However, this remains a less-explored area in the modulation of muscle activity during movement. Because vision plays an important role in daily functional tasks, we explored the role of vision during weight bearing was explored by having subjects perform the knee joint tracking task under 2 conditions: visual feedback (during EO) and no visual feedback (during EC). Under both conditions, subjects showed improvements in performance with training with the greatest learning observed between day 1 and day 2. Absolute error during EC was almost 3 times greater than the EO condition. Learning was greater when visual feedback was available to detect errors online and correct the movement trajectory immediately. Without vision, a large positional error accumulated despite reasonable ability to follow the remembered target waveform. In addition, as expected, a decrease in muscle coactivation ratios was observed when the task was performed with eyes closed. This finding is consistent with studies that have demonstrated that reduced feedback increased limb stiffness [12]. An interesting finding to be noted here was the absence of change in the lateral hamstrings activity with learning when vision was not available. Lateral hamstrings activity, during the EC condition, was decreased on day 1 when compared with the EO condition, and this magnitude did not change as a result of learning on day 4 in the EC condition, suggesting that absence of visual feedback influences lateral hamstrings activity.
An important limitation of this study is that the coactivation ratio is just one part of the entire neuromuscular control puzzle that determines coordinated movement. Several factors are instrumental in training controlled movement, including the hip abductor strength, frontal plane alignment, and overall trunk control. In addition, the nonweight-bearing hip angle likely changes the mechanics of this task and therefore should be considered when prescribing this task.
CONCLUSIONS
During the past decade, weight-bearing exercises like the SLS have been increasingly used for lower extremity rehabilitation. Several studies have done kinematic and EMG of the SLS exercise [29,30]. However, these previous reports did not include accuracy of knee control and performance during the task and the degree of resistance imposed was not adjusted. The findings of this study support the notion that weight-bearing exercise with visual feedback about task accuracy influences the muscle activation strategies used during the task. The results of the present study demonstrated that subjects improved their performance on this target-tracking SLS task within 2 days of training. However, the authors did not test the ability of subjects to transfer what they learned to a novel situation. The target-matching SLS paradigm used in this study is a novel task. Accordingly, this study verified that the assessment was sensitive in female subjects without known pathology. Given the findings of this study, the authors believe this protocol would be sensitive to new motor control strategies that develop because of central nervous system lesion, ligamentous laxity, or surgical repair of ligaments.
Neuromuscular training programs aim at improving muscle strength, motor coordination, and overall proprioceptive ability. The strategy that accompanied performance accuracy in this task was increased quadriceps activity with concurrent reduction in hamstrings activity. Accordingly, this study verified the important “trade-off” that exists between precision of movement and joint stiffness through muscle activation. As evident from this study, greater quadriceps activity developed at the expense of hamstring activity when the subjects developed greater precision with the task. It is likely that patients with neuromuscular control deficits experience similarly decrease joint stiffness through muscles as precise movement control is learned. Future studies will directly assess the extent to which these control strategies change as a result CNS compromise and/or anterior cruciate ligament injury and reconstruction. On the basis of this report, rehabilitation specialists should be aware that “accuracy of performance” under visual and nonvisual conditions might be important components to incorporate into various rehabilitation strategies.
Acknowledgments
Funded in part by NIH NR0213478
Footnotes
Disclosure: 8B NIH
Disclosure: 8B NIH
Disclosure Key can be found on the Table of Contents and at www.pmrjournal.org
References
- 1.de Loes M, Dahlstedt LJ, Thomee R. A 7-year study on risks and costs of knee injuries in male and female youth participants in 12 sports. Scand J Med Sci Sports. 2000;10:90–97. doi: 10.1034/j.1600-0838.2000.010002090.x. [DOI] [PubMed] [Google Scholar]
- 2.Griffin LY, Agel J, Albohm MJ. Noncontact anterior cruciate ligament injuries: Risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24:765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 4.Huston LJ, Greenfield M, Wojtys EM. Anterior cruciate ligament injuries in the female athlete—Potential risk factors. Clin Orthop Rel Res. 2000;372:50–63. doi: 10.1097/00003086-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Etty Griffin LY. Neuromuscular training and injury prevention in sports. Clin Orthop Rel Res. 2003;409:53–60. doi: 10.1097/01.blo.0000057788.10364.aa. [DOI] [PubMed] [Google Scholar]
- 6.Kvist J. Rehabilitation following anterior cruciate ligament injury: Current recommendations for sports participation. Sports Med. 2004;34:269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- 7.Williams GN, Chmielewski T, Rudholph K, Buchanan TS, Snyder-Mackler L. Dynamic knee stability: Current theory and implications for clinicians and scientists. J Orthop Sports Phys Ther. 2001;31:546–566. doi: 10.2519/jospt.2001.31.10.546. [DOI] [PubMed] [Google Scholar]
- 8.Osu R, Franklin DW, Kato H, et al. Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. J Neurophysiol. 2002;88:991–1004. doi: 10.1152/jn.2002.88.2.991. [DOI] [PubMed] [Google Scholar]
- 9.Gribble PL, Mullin LI, Cothros N, Mattar A. Role of cocontraction in arm movement accuracy. J Neurophysiol. 2003;89:2396–2405. doi: 10.1152/jn.01020.2002. [DOI] [PubMed] [Google Scholar]
- 10.Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol. 2005;93:3200–3213. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds RF, Day BL. Visual guidance of the human foot during a step. J Physiol. 2005;569:677–684. doi: 10.1113/jphysiol.2005.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroeve S. Learning combined feedback and feedforward control of a musculoskeletal system. Biol Cybern. 1996;75:73–83. doi: 10.1007/BF00238741. [DOI] [PubMed] [Google Scholar]
- 13.Livengood AL, DiMattia MA, Uhl TL. “Dynamic Trendelenburg”: Single-leg-squat test for gluteus medius strength. Athletic Therapy Today. 2004;9:24–25. [Google Scholar]
- 14.Wilk KE, Reinold MM, Hooks TR. Recent advances in the rehabilitation of isolated and combined anterior cruciate ligament injuries. Orthop Clin North Am. 2003;34:107–137. doi: 10.1016/s0030-5898(02)00064-0. [DOI] [PubMed] [Google Scholar]
- 15.Madhavan S, Shields RK. Weight-bearing exercise accuracy influence muscle activation strategies of the knee. J Neurol Phys Ther. 2007;31:12–19. doi: 10.1097/01.npt.0000260569.69863.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields RK, Madhavan S, Gregg E, et al. Neuromuscular control of the knee during a resisted single-limb squat exercise. Am J Sports Med. 2005;33:1520–1526. doi: 10.1177/0363546504274150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cram JR, Kasman GS, Holtz J. Introduction to Surface Electromyography. Gaithersburg: Aspen Publishers Inc; 1998. pp. 360–375. [Google Scholar]
- 18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 19.Hunter SK, Critchlow A, Enoka RM. Influence of aging on sex differences in muscle fatigability. J Appl Physiol. 2004;97:1723–1732. doi: 10.1152/japplphysiol.00460.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hunter SK, Lepers R, MacGillis CJ, Enoka RM. Activation among the elbow flexor muscles differs when maintaining arm position during a fatiguing contraction. J Appl Physiol. 2003;94:2439–2447. doi: 10.1152/japplphysiol.01038.2002. [DOI] [PubMed] [Google Scholar]
- 21.Carey JR, Patterson R, Hollenstein PJ. Sensitivity and reliability of force tracking and joint-movement tracking scores in healthy subjects. Phys Ther. 1988;68:1087–1091. doi: 10.1093/ptj/68.7.1087. [DOI] [PubMed] [Google Scholar]
- 22.Chung YJ, Cho SH, Lee YH. Effect of the knee joint tracking training in closed kinetic chain condition for stroke patients. Restor Neurol Neurosci. 2006;24:173–180. [PubMed] [Google Scholar]
- 23.Winstein CJ. Knowledge of results and motor learning—implications for physical therapy. Phys Ther. 1991;71:140–149. doi: 10.1093/ptj/71.2.140. [DOI] [PubMed] [Google Scholar]
- 24.Carey JR, Kimberley TJ, Lewis SM. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 25.Maffiuletti NA, Bizzini M, Schatt S, Munzinger U. A multi-joint lower-limb tracking-trajectory test for the assessment of motor coordination. Neurosci Lett. 2005;384:106–111. doi: 10.1016/j.neulet.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 26.Perez MA, Lungholt BK, Nielsen JB. Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol. 2005;568:343–354. doi: 10.1113/jphysiol.2005.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol. 2006;573:843–855. doi: 10.1113/jphysiol.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoroughman KA, Shadmehr R. Electromyographic correlates of learning an internal model of reaching movements. J Neurosci. 1999;19:8573–8588. doi: 10.1523/JNEUROSCI.19-19-08573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beutler AT, Cooper LW, Kirkendall DT, Garrett WE., Jr Electromyographic analysis of single-leg, closed chain exercises. J Athl Train. 2002;37:13. [PMC free article] [PubMed] [Google Scholar]
- 30.Zeller BL, McCrory JL, Kibler WB, Uhl TL. Differences in kinematics and electromyographic activity between men and women during the single-legged squat. Am J Sports Med. 2003;31:449–456. doi: 10.1177/03635465030310032101. [DOI] [PubMed] [Google Scholar]