Abstract
We evaluated the causal prophylactic antimalarial activity of a single oral dose of pafuramidine (DB289), an experimental prodrug of active metabolite DB75, in a randomized, double-blind, placebo-controlled, outpatient study. Sixteen healthy volunteers were dosed and challenged in a single cohort. Subjects were randomly assigned to one of three treatment arms: 100 mg pafuramidine eight days before challenge, 100 mg pafuramidine the day before challenge, or placebo. Challenge was by the bites of Plasmodium falciparum-infected Anopheles gambiae. Malaria developed in 15 persons but did not develop in one person in the day −8 pafuramidine treatment arm. Plasma levels of DB75 were lower than expected, and as intended were too low to provide suppressive prophylaxis at the earliest appearance of erythrocytic parasites. We conclude that a single dose of 100 mg pafuramidine does not adequately protect non-immune individuals against P. falciparum and shows no clinically or statistically significant evidence of causal prophylactic activity.
INTRODUCTION
Malaria in non-immune travelers is recognized as an important and increasing health care problem affecting some 25–30 million travelers from non-endemic regions who visit malaria-endemic countries each year.1 The number of malaria cases reported to the Centers for Disease Control and Prevention (CDC) in 1991–2001 was about 12,000, most acquired in Africa, and a number estimated to be only approximately 20% of the actual value.1 The CDC-recommended drugs for malaria prophylaxis include chloroquine, a fixed-dose combination of atovaquone and proguanil, mefloquine, or doxy cycline. Drug resistance, cost, and tolerability/toxicity limit the usefulness of these agents.
Itself inactive, pafuramidine (DB289) is an orally available prodrug that was developed to overcome the poor bioavailability of DB75. Both compounds are structurally related to pentamidine, a commonly used antiprotozoal agent (Figure 1). DB75 was first developed for the treatment of acquired immunodeficiency syndrome–associated Pneumocystis pneumonia and later found to have broad-spectrum antimicrobial activities2,3 including against Plasmodium falciparum and P. vivax.4,5 The mechanism of antimicrobial activity of diamidine compounds is incompletely understood. They undergo active uptake by purine transporter systems in trypanosomes,6,7 and their mechanism of action may involve interference with DNA-associated enzymes,8 – 10 inhibition of heme crystallization11 or/and collapse of the transmitochondrial membrane potential.12,13 Pafuramidine is absorbed from the gastrointestinal tract and in multiple steps is biotransformed into DB75 by cytochrome(s) P450 4F and b5 reductase.14 Plasma concentrations of both compounds show a high interindividual variability and poor dose-concentration proportionality. Both pafuramidine and DB75 have extensive tissue binding reflected by a large volume of distribution; in rats and monkeys DB75 accumulates in several tissues, particularly liver.15 After a single oral dose in humans, pafuramidine plasma concentration peaks within 1–2 hours, with a half-life of 14 hours; DB75 peaks in 3–5 hours, with a half-life of 28 hours (Immtech Pharmaceuticals, Inc., New York, NY, unpublished data). Prior to the start of this study, phase I and phase II clinical trials with pafuramidine in approximately 500 persons showed good safety profiles. The most commonly observed adverse effects included nausea, vomiting, headache, dizziness, muscle pain, drowsiness, and elevation of levels of liver enzymes. These effects were mild and self-limiting, and their frequency and severity were comparable in the pafuramidine-treated and placebo or comparison drug treatment group.
FIGURE 1.
Pafuramidine and its active metabolite DB75. These compounds are aromatic diamidines and close structural analogs of pentamidine.
Until recently, pafuramidine was in phase III trials for the treatment of early-stage human African trypanosomiasis, Pneumocystis jirovecii pneumonia in human immunodeficiency virus–infected patients, and phase II trials for treatment of uncomplicated malaria. The antimalarial efficacy of pafuramidine, dosed at 100 mg twice a day for 5 days, is more than 90% in uncomplicated P. falciparum malaria and 80% in P. vivax malaria.16 Although DB75 is active against the erythrocytic forms of P. falciparum and is expected to have suppressive prophylactic activity, its effects on exoerythrocytic parasites are unknown.
The major objective of this study was to assess the causal prophylactic activity of DB75. Causal prophylaxis is highly desirable because the number of parasites encountered in the liver is relatively small (which favors efficacy and may hinder the development of drug resistance), post-exposure prophylaxis is shorter, and there may be dual causal and suppressive activities. The expectation that DB75 may have causal activity is based on several factors. DB75 is active against multiple diverse protozoa including, but not limited to, P. jirovecii,17 Trypanosoma brucei species,18 and Giardia lamblia,19 in addition to its potent activity against P. falciparum and P. vivax.4,5 Moreover, DB75 accumulates substantially in the liver. Rats and monkeys dosed orally with [14C] pafuramidine showed accumulation of DB75 in the liver, which was sustained for weeks after dosing, at levels some 600 times those in plasma.15 Additionally, DB75 interferes with mitochondrial metabolism in Saccharomyces cerevisiae13 and T. brucei,12 and exoerythrocytic stage malaria parasite development relies heavily on mitochondrial metabolism.20,21 In Plasmodium, mitochondrial function is critical for the de novo synthesis of pyrimidines (by dihydroorotate dehydrogenase),22 and collapse of mitochondrial membrane potential is the mechanism of antimalarial action for atovaquone and primaquine, which are efficacious against tissue parasites.
In this study, we assessed the potential of pafuramidine as a causal prophylactic agent against P. falciparum in non-immune healthy volunteers. The study was designed to distinguish between causal and suppressive activity by correlating the biology of malaria with the pharmacokinetic-pharmacodynamic properties of pafuramidine and DB75.
MATERIALS AND METHODS
This study was reviewed and approved by the Johns Hopkins Medical Institutional Review Board, conducted under an Investigational New Drug Application sponsored by Immtech Pharmaceuticals, Inc., and at its inception registered with ClinicalTrials.gov (NCT00408369). The study was a single-center, randomized, double-blind, placebo-controlled clinical trial conducted entirely on an outpatient basis. Volunteers were screened, dosed, and followed as a single cohort in the outpatient clinic at the Drug Development Unit, Johns Hopkins Hospital. The malaria challenge was conducted at the insectary of the Johns Hopkins Bloomberg School of Public Health. Day 0 signified the day of challenge. Patency was defined as detection of parasitemia by quantitative buffy coat (QBC) and/or malaria blood smear. All units of pafuramidine and DB75 are expressed as base, not salt.
Study volunteers
Candidates were recruited by printed advertisements. Eligibility criteria included an age of 18–55 years, a body mass index of 19–31, blood type A or O that supported in vitro growth of P. falciparum, women with no reproductive potential (e.g., postmenopausal or surgically sterile), ability to score at least 80% on a written examination to test comprehension of malaria and the study, ability to comply with the follow-up schedule, and ability to provide a responsible person to assist with follow-up. Volunteers were excluded for clinically significant abnormalities on medical history, physical or laboratory examination, taking prescription or over-the-counter drugs, alcohol or drug abuse, history of malaria or significant malaria exposure, glucose-6-phosphate dehydrogenase deficiency, hemoglobin C or S, use of anti-infective drugs or quinine-containing beverages in the week preceding dosing, and allergy to mosquito bites or intolerance to antimalarial drugs. Written informed consent was obtained for the screening procedures and for human immunodeficiency virus testing. For candidates who passed the screening, informed consent for study participation was obtained at enrollment.
Dosing regimen and rationale
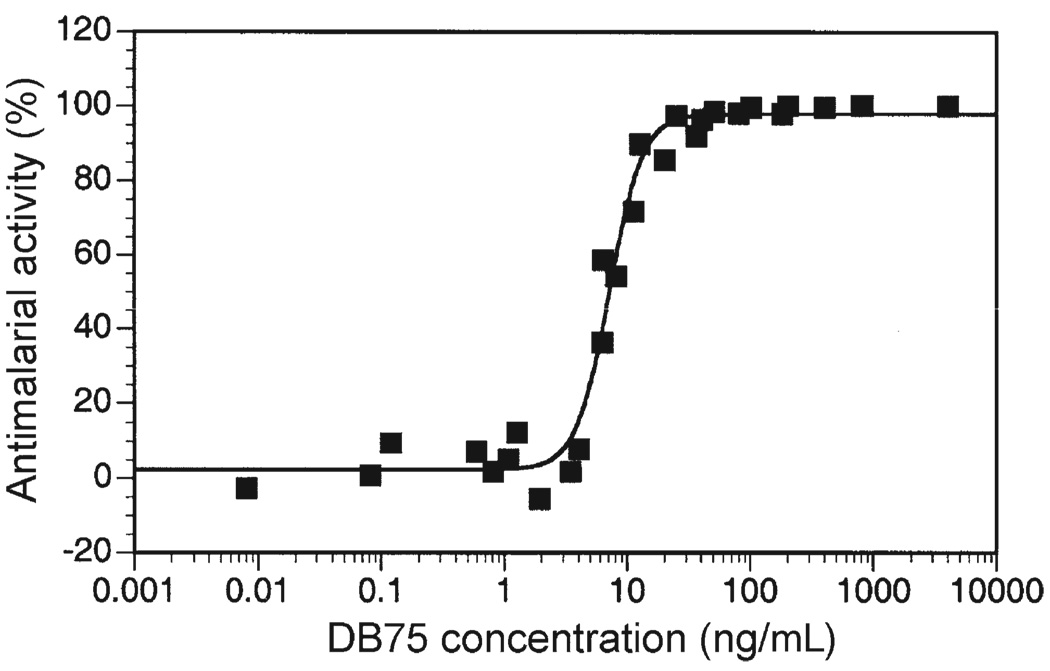
In a sporozoite challenge study, attribution of prophylactic activity to a causal mechanism requires that blood levels of DB75 be “unequivocally subtherapeutic” against erythrocytic parasites at their first possible appearance. For the NF54 strain used in the study, this is 6.5 days after challenge.23 Unequivocally subtherapeutic plasma levels of DB75 were defined as less than 0.25 ng/mL on the basis of two observations. First, DB75 consistently showed no detectable antimalarial activity in vitro at a concentration of 1 ng/mL (Figure 2), a level that is four times higher than our defined subtherapeutic level. (Of note, in vitro assays are conducted in 10% serum, which results in a higher proportion of free drug than is seen in vivo, and the possible overestimation of antimalarial activity attainable in vivo; DB75 is 77% protein-bound.) Second, in patients with acute P. falciparum malaria treated with multiple doses of pafuramidine, trough plasma concentrations of DB75 at or below 3 ng/mL were associated with an 83% failure rate, and all patients with mean trough plasma concentrations less than 2 ng/mL (a level eight times higher than our defined unequivocally subtherapeutic concentration) failed treatment (Immtech Pharmaceuticals, Inc., unpublished data). Accordingly, the designation of 0.25 ng/mL as subtherapeutic is conservative.
FIGURE 2.
In vitro activity of DB75 against asexual erythrocytic NF54 Plasmodium falciparum, as assessed by [3H] hypoxanthine incorporation.28,29 The 50% effective concentration is 7.4 ng/mL. Results of three independent assays are shown.
Conversely, for a dosing regimen of pafuramidine to be effective as a causal prophylactic, DB75 levels in the liver must be high enough to act against exoerythrocytic parasites during this period of development. Studies in rats and monkeys have shown that high levels of DB75 accumulate in the liver, and persist for weeks after dosing.15 In cynomolgus monkeys given a single oral dose of 5 mg/kg [14C] pafuramidine, 10% of the administered dose still remained in the liver in the form of DB75 at 43 days after dosing. When normalized by surface area, this dose is equivalent to 145 mg of pafuramidine for a 70 kg (1.85 m2) human (assuming monkeys weigh 5 kg and have a surface area of 0.32 m2).24 Given that human liver has a mean weight of 1.5 kg and 50% water composition,25 and if in humans 10% of a dose is associated with the liver, then after a single oral dose of 100 mg of pafuramidine, the levels of DB75 in liver will be 13,300 ng/mL. This is 1,800 times greater than the 7.4 ng/mL 50% effective concentration (EC50) for erythrocytic P. falciparum in vitro (Figure 2). Accordingly, a single oral dose of 100 mg of pafuramidine should provide substantial levels of DB75 in liver cells.
Three treatment arms were evaluated: placebo, day −1 dosing (a single tablet containing 100 mg of pafuramidine administered the day before challenge), and day −8 dosing (a single tablet containing 100 mg of pafuramidine administered 8 days before challenge). A 100-mg dose was selected because this dose appeared to be safe and likely to attain high DB75 levels in the liver. The placebo arm was essential to demonstrate infectivity of the challenge and to reduce observer bias in interpreting efficacy and toxicity data. The day −1 arm was included to provide the greatest possible drug exposure after infection (thus, greatest chance of efficacy) that has a reasonable likelihood of falling to subtherapeutic plasma levels by day 6.5. The day −8 arm almost certainly ensures subtherapeutic plasma levels by day 6.5 and was included to exploit the anticipated persistence of high liver concentrations. The two pafuramidine-treated groups were thus designed to provide maximal levels of DB75 in the liver during exoerythrocytic development and to ensure that plasma levels of DB75 were unequivocally subtherapeutic on day 6.5 after challenge. If both treatment arms were successful, this regimen would not only support a causal mechanism, but also suggest a once-weekly prophylaxis regimen.
Sample size
A total of 10 volunteers was required, assuming one-sided significance α = 0.05, to detect at least 20% difference in infection rate between placebo and either of the pafuramidine-treated groups, with a statistical power of 80%. With these criteria, a placebo:drug ratio of 2:3 does not alter the required number. Therefore, four placebo recipients and six in each of two treatment groups (total of 16 volunteers) were selected. This number of volunteers provided a study small enough to be managed safely and large enough to provide an estimate of efficacy that has an exact binomial 95% confidence interval of 54–100% if six of six in a treatment arm are protected and four of four placebo recipients are not. To ensure the availability of 16 volunteers at the time of challenge, 19 were recruited and randomly assigned to three treatment arms: placebo (five persons), day −1 (seven persons), or day −8 (seven persons).
Randomization and drug dosing
Pafuramidine (coated tablets of pafuramidine maleate containing 100 mg of pafuramidine base) and matching placebo were provided by Immtech Pharmaceuticals, Inc. Preparation of unit doses and randomization were performed by the Johns Hopkins Hospital Investigational Pharmacy. Unit doses were packaged, labeled with the study day and subject number, and provided to study personnel. At the time the randomization scheme was developed, one person from each arm was randomly selected for exclusion from challenge. These persons were followed for safety and pharmacokinetics. The code and the identity of those selected for exclusion were available only to the investigational pharmacy.
Volunteers were asked to fast after midnight before and four hours after dosing. All treatments were administered with high-fat breakfast (900 calories, 50% fat) and under direct observation. The placebo group received a placebo tablet on days −8 and −1. The day −8 treatment group received a pafuramidine tablet on day −8 and a placebo tablet on day −1. The day −1 group received a placebo tablet on day −8 and a pafuramidine tablet on day −1. All study personnel and the volunteers were blinded to the treatment assignment throughout the study.
Mosquito infection and malaria challenge
Anopheles gambiae were propagated and maintained in the environmentally controlled insectary of the Johns Hopkins Bloomberg School of Public Health by standard method.26 They were infected with 16–18-day-old gametocyte cultures of the NF54 strain of P. falciparum27 and used for challenge 17 days after infection. Sensitivity to chloroquine (EC50 = 3.0 ng/mL) and DB75 (EC50 = 7.3 ng/mL) were confirmed28,29 before challenge.
After pafuramidine/placebo dosing, 16 volunteers (four placebo, six in each treatment arm) were selected for challenge from the 19 who were dosed. Each person was bitten by five infected mosquitoes using a previously described method.26 After feeding, the mosquitoes were dissected to ascertain the presence of blood in the abdomen and of sporozoites in the salivary glands. Additional mosquitoes were allowed to feed until the volunteer was bitten by five infected mosquitoes. Each volunteer was then given follow-up instructions, a pager and wallet card listing names and contact number of investigators who were available at all times, and was fitted with a medical alert bracelet.
Assessment of efficacy and safety
Clinic visits were scheduled for all subjects on days −8 to 0. Those challenged returned daily on days 1–21, every other day on days 23–35, and weekly until 12 weeks after challenge, unless parasitemia was detected (below). Those not challenged were seen on days 7 and 14.
Efficacy evaluations were conducted on the 16 persons who were challenged. Efficacy was defined as the absence of parasitemia by all five diagnostic methods (Giemsa-stained thick and thin blood smears, QBC, PCR, and blood culture). For real-time evaluation and treatment decisions, blood for thick and thin blood smears and QBC was drawn and examined daily from day 5 to day 21, every other day from day 23 to day 35, and weekly until 12 weeks after challenge. Baseline QBC was evaluated on day −1. All microscopy methods were conducted in accordance with the Clinical Laboratory Improvement Amendments. Blood for PCR was collected on day −1, day 1, daily from day 5 to day 21, and at 6 weeks after challenge; these were not assayed in real-time. Blood was cultured on days 7 and 8.
Assessment of safety for all 19 persons included a subjective clinical interview conducted in a non-directed fashion on each visit, and objective laboratory evaluation on days −8, −1, 7, and 14. Additional laboratory evaluations were done at patency and when deemed necessary. Those who developed symptomatic or laboratory abnormalities were evaluated every 1–3 days until the abnormalities resolved. Safety laboratory evaluation included hematocrit, hemoglobin, leukocyte count and differential count, platelet count, reticulocyte count, prothrombin time, international normalized ratio, activated partial thromboplastin time, Na, K, Cl, HCO3, creatinine, glucose, albumin, total bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, magnesium, calcium, amylase, and lipase. Severity of adverse events was graded on the basis of the World Health Organization Toxicity Criteria (2000) (http://www.accessdata.fda.gov/scripts/cder/onctools/whotox.cfm).
Before the study blind was broken, all clinical symptoms, signs, and laboratory findings were reviewed and evaluated for severity and judged for their relationship to pafuramidine, malaria, and/or other factors.
Polymerase chain reaction
Blood was collected in sodium citrate and stored at −80°C. DNA was extracted from 200 µL of whole blood using the GenElute™ Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO) per the manufacturer’s instructions and eluted into a final volume of 200 µL. DNA was amplified using subtelomeric variable open reading frame (STEVOR) primers and nested PCR conditions as previously described.30 A 20-µL aliquot of DNA was amplified in a 100-µL reaction in the first round using primers P5, P18, P19, and P20, and 2 µL of the reaction product was then amplified in a 50-µL reaction in the second round using primers P17 and P24. Five microliters of the final reaction was used for electrophoresis in a 3% agarose gel containing 0.5 µg/mL of ethidium bromide.
Patency and treatment of patent infection
Either positive blood smear (as determined by two independent examiners) or QBC (as determined by two independent examiners) was considered prophylactic failure and treated as patent infection. At patency, blood was drawn for smears, QBC, PCR, parasite culture and drug sensitivity testing, and pafuramidine and DB75 levels. Volunteers then received chloroquine phosphate (1 g salt followed by 500 mg salt at 6, 24, and 48 hr after diagnosis), and had the option to take acetaminophen, up to 1.3 g every 8 hour until symptoms resolved. Volunteers were instructed to return for clinical assessment and parasitologic examination (blood smears, QBC, and PCR), daily until three consecutive negative QBCs were obtained, and at six weeks after diagnosis. Blood was obtained for safety monitoring at diagnosis, when indicated during malaria illness, at the time of third negative QBC, and six weeks after diagnosis.
Drug assay and pharmacokinetic analyses
Blood for pafuramidine and DB75 determinations was collected from all persons who were dosed, immediately before dosing (time 0) and at 4 hours after dosing on both dosing days (day −8 and day −1), daily from day −7 to day 9, inclusive, and at patency. Plasma was collected and stored at −70°C prior to shipping for liquid chromatography/mass spectrometry analyses at Tandem Laboratory (Salt Lake City, UT).15 Lower limits of quantitation for both pafuramidine and DB75 are 0.25 ng/mL. Concentration-time plots for pafuramidine and DB75 were visually examined at all sampling periods. Pharmacokinetic parameters and frequent dosing simulation were obtained by a non-compartmental analytical method using WinNonlin software (Professional Version 4.1; Pharsight Corporation, Mountain View, CA). The maximal plasma concentration (Cmax) was identified by linear trapezoidal rule, and single dose pharmacokinetic parameters were calculated for the area under the concentration-time curve (AUC0–144 hours from time zero to 144 hours after dosing), terminal elimination rate constant and elimination half-life (t1/2) (by linear regression of log-plasma concentration-time curves), total apparent oral clearance (Cl/F), and apparent volume of distribution (V/F).
Urine was assayed for other antimalarial drugs using a previously described method.31 This test, which does not detect pafuramidine or DB75, was performed once before challenge and on day 7. An additional assay was conducted on day 21 for the challenged volunteer who did not develop patent parasitemia.
Statistical analyses
Descriptive and statistical analyses were performed using Stata (Intercooled Stata 9.2;StataCorp, College Station, TX) and SPSS version 9.0.1 (SPSS Inc., Chicago, IL). The median and interquartile ranges (IQR) were used to describe Cmax, AUC0–144 hours, elimination t1/2, V/F, and Cl/F of pafuramidine and DB75. The proportion of volunteers diagnosed with malaria in the placebo arm was compared using Fisher’s exact test with that of the day −1 or day −8 treatment arms. Time to parasitemia in each treatment arm and by each diagnostic method was plotted and analyzed using Kaplan-Meier survival curves with the log rank test and Wilcoxon signed rank analytical methods.
RESULTS
Volunteers
Some 450 candidates responded to our advertisements. By phone interview, the two most frequent reasons for exclusion were women of reproductive potential, and inability to comply with the long follow-up. A total of 50 candidates entered the screening phase and 31 were excluded (21 failed laboratory criteria, 4 could not comply with follow-up, 3 did not complete screening, 2 admitted the use of recreational drugs, and 1 failed serum support for malaria growth in vitro). Nineteen volunteers fulfilled the eligibility requirements and were enrolled. All 19 completed the study. Sixteen volunteers were selected and challenged.
Eighteen volunteers were male
There were 11 Caucasians, 6 African-Americans, and 2 Hispanic persons. Average age was 38 years (range = 23–54 years; average body mass index was 25.7 (range = 19.8–30.2). There was no significant difference in age, race, or body mass index between the placebo and drug-treated groups. The follow-up rate was 100% for a total of 451 person-visits.
Challenge
All persons tolerated the mosquito challenge well. A total of 122 mosquitoes were required to obtain 5 infectious bites per volunteer, and the procedure was completed in 3.8 hours. The volunteer who did not develop parasitemia was the eighth of 16 to be challenged and was completed within 1.5 hours of initiation of his challenge procedure. There was no correlation between challenge order and days to patency, and for all three arms there was no significant difference in the interval between dosing and challenge (data not shown). The number of mosquitoes required for each subject ranged from 6 to 9, 6 to 12, and 5 to 9 in the placebo, day −8, and day −1 arms, respectively. Salivary gland parasite indices ranged from 8 to 20, 5 to 12, and 7 to 15 in the placebo, day −8, and day −1 arms, respectively.
Efficacy
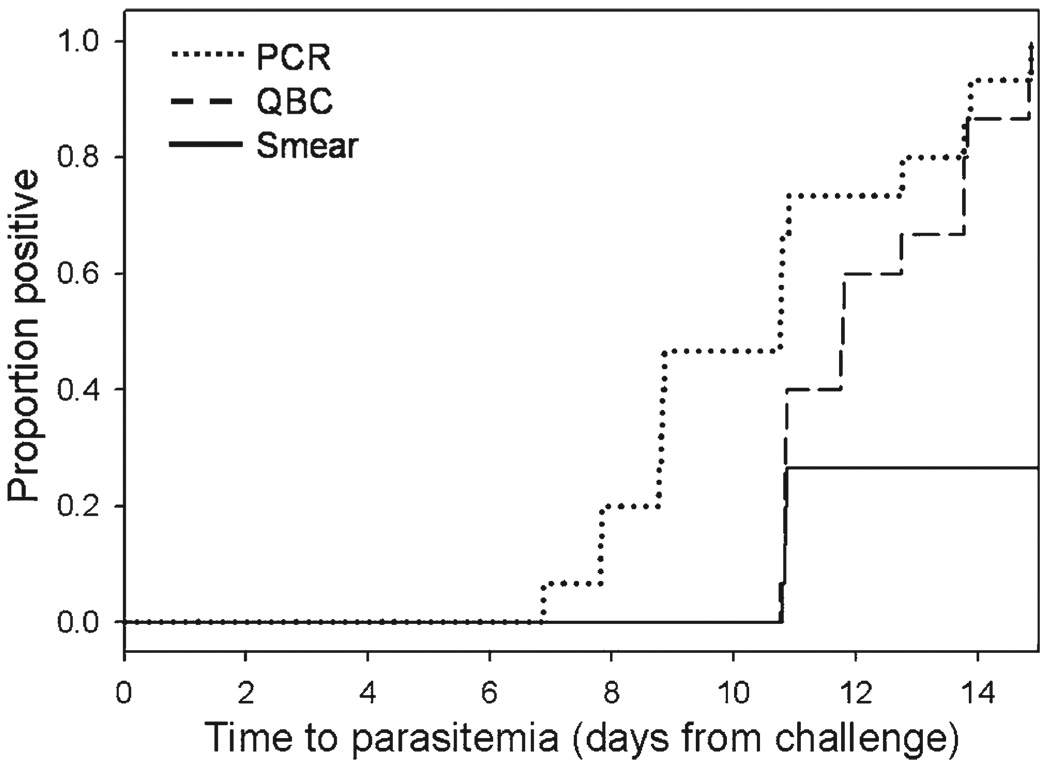
Fifteen of the 16 challenged volunteers developed patent parasitemia: 4 of 4 volunteers in the placebo arm, 6 of 6 in the day −1 arm, and 5 of 6 in the day −8 arm. One volunteer in the day −8 group did not develop parasitemia. All positive cases occurred 11–15 days after challenge: 6 on day 11, 3 on day 12, 1 on day 13, 3 on day 14, and 2 on day 15. The mean prepatent period was 12.5 days (range = 11–15 days), and there was no significant difference in the time-to-patency between the placebo and either of the treatment arms. All 15 cases were identified by QBC. At patency, a simultaneous thick blood smear was also positive for 4 of the 15 (3 on day 11 and 1 on day 12). None of the 15 persons was positive by thin blood smear. All positive cases were later confirmed by PCR, and the PCR result was negative for the person who did not develop malaria. As in previous reports and consistent with its greater sensitivity,32–35 PCR identified parasitemia up to 4 days earlier than QBC (Figure 3). None of the blood cultures collected on days 7 and 8 was positive. Eight samples cultured at patency were positive and the parasites remained fully sensitive to DB75 (EC50 = 7.0 ng/mL).
FIGURE 3.
Time to initial parasitemia detected by polymerase chain reaction (PCR), quantitative buffy coat (QBC) test, or thick blood smear. Parasitemia was detected by PCR (median = 10.8 days, interquartile range = 8.8–11.8 days) earlier than by QBC (median = 11.8 days, inter-quartile range = 10.8–13.8 days) (P = 0.0009, by Wilcoxon signed ranks test).
Results of all urine tests for surreptitious use of antimalarial drugs were negative.
Safety
Prior to patency, mild and reversible subjective symptoms (headache, nausea, sinus congestion) were noted in five volunteers in the day −1 arm and 2 in the day −8 arm and were judged possibly or probably related to pafuramidine. No symptoms were noted in the placebo group before the onset of malaria. At patency, 4 of 15 volunteers were asymptomatic; the other 11 had myalgias (73%), fever (60%), headache (53%), and gastrointestinal upset (7%). As expected, symptoms worsened after treatment with chloroquine.36,37 Fourteen of 15 persons with malaria were treated symptomatically with acetaminophen (range = 1–3 g/day) for several days (range = 1–10 days) for total doses of 0 to 17.5 g (one person was not treated).
The person in the day −8 treatment arm who did not develop patent parasitemia had a facial and periorbital rash secondary to exposure to poison ivy on day 6, and was treated with topical corticosteroid cream and oral diphenhydramine (total dose = 450 mg over days 7–12) Signs and symptoms resolved completely within three weeks.
All laboratory abnormalities developed after patency (Supplemental Table 1). Elevations of hepatic aminotransferase levels were seen in 10 of 15 persons with malaria (Table 1,Supplemental Figure 1). There were no significant differences in the pattern, frequency, or severity of laboratory changes between the placebo and pafuramidine-treated groups.
TABLE 1.
Relationship between liver toxicity and various risk factors
| No. of persons affected | |||
|---|---|---|---|
| No. of risks | Risk factors | Toxicity (10) | No toxicity (9) |
| 0 | No malaria, no acetaminophen, no pafuramidine | 0 | 1 |
| 1 | Malaria alone* | 0 | 0 |
| Acetaminophen alone* | 0 | 0 | |
| Pafuramidine alone | 0 | 3 | |
| 2 | Malaria plus acetaminophen | 3 | 1 |
| Malaria plus pafuramidine | 1 | 0 | |
| Acetaminophen plus pafuramidine* | 0 | 0 | |
| 3 | Malaria plus acetaminophen plus pafuramidine | 6 | 4 |
| N/A | All malaria | 10 | 5 |
| All acetaminophen | 9 | 5 | |
| All pafuramidine | 7 | 7 | |
No person in the study had these combinations of risk factors.; N/A = not applicable.
There were no clinically or statistically significant changes in results of renal function tests.
Pharmacokinetics
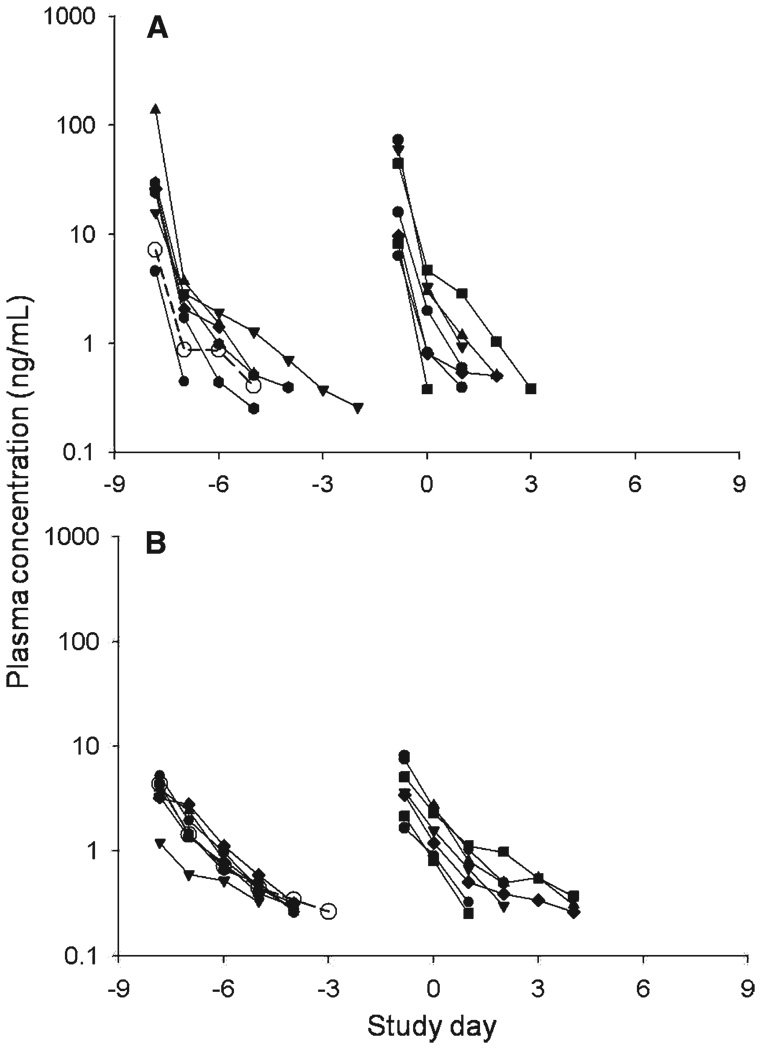
Pharmacokinetic parameters for pafuramidine and DB75 are shown in Table 2, and the plasma concentration-time plots are shown in Figure 4. Compared with previous studies, the pafuramidine plasma concentrations were higher than expected, with AUC0–144 hours almost three times the previous findings in healthy volunteers (Immtech Pharmaceuticals, Inc., unpublished data). Curiously, however, plasma concentrations of DB75 were lower than expected, with Cmax one-third and AUC0–144 hours 40% less than the previous observations. By day 6.5 and thereafter, plasma concentrations of both compounds were undetectable (i.e., < 0.25 ng/mL). Most other findings were similar to those reported previously: the median terminal half-lives were 17.2 hours (IQR =10.8–24.0 hours) and 30.9 hours (IQR = 20.2–36.2 hours) for pafuramidine and DB75, respectively, with large volumes of distribution, consistent with extensive tissue accumulation. The Cmax and AUC0–144 hours of DB75 were not significantly different in malaria-positive and malaria-negative persons.
TABLE 2.
Pharmacokinetics of pafuramidine and active metabolite DB75 after a 100-mg single oral dose*
| Median values for 14 treated persons (interquartile range) |
||
|---|---|---|
| Parameter | Pafuramidine | DB75 |
| Cmax (ng/mL) | 15.9 (7.0–33.6) | 3.6 (2.6–4.6) |
| Elimination half-life (hours) | 17.2 (10.8–24.0) | 30.9 (20.2–36.2) |
| AUC0–144hr (ng·hours/mL) | 292 (102–529) | 112 (85.3–144) |
Cmax = maximal plasma concentration; AUC = area under the concentration-time curve.
FIGURE 4.
Plasma concentration-time profiles of pafuramidine (A) and its active metabolite DB75 (B). For each panel, curves on the left represent the day −8 treatment arm and those on the right represent the day −1 treatment arm. Samples with plasma levels < 0.25 ng/mL are not plotted. Open symbols = subject in day −8 treatment group in whom malaria did not develop.
DISCUSSION
In this study, a single oral dose of 100 mg of pafuramidine, given either one or eight days before sporozoite challenge, did not reliably prevent the development of parasitemia in non-immune persons challenged with P. falciparum transmitted by A. gambiae mosquitoes. Thus, pafuramidine in these regimens had no useful prophylactic activity, either causal or suppressive.
Failure of suppressive prophylaxis was expected because the dosing regimens were designed to eliminate this mechanism, and in all pafuramidine-treated volunteers, DB75 levels on day 6.5 were < 0.25 ng/mL, thus unequivocally subtherapeutic against erythrocytic parasites. Several factors may have led to inadequate causal prophylaxis including insufficient drug levels in the liver or lack of intrinsic activity against tissue parasites.
In animals, levels of the active metabolite DB75 in liver are correlated with plasma levels of DB75. In this study, DB75 plasma levels were uniformly less than half of expected values, despite the higher than expected concentrations of precursor pafuramidine. Infrequent pharmacokinetic samplings and missed Cmax values could account for this finding. However, in a simulation of our pharmacokinetic sampling schedule using data from the comparator study, Cmax and AUC0–144 hours of DB75 were not meaningfully different from the observed values, making this an unlikely possibility. The lower exposure of DB75 in plasma could be explained by a decrease in the conversion of pafuramidine to DB75 and/or an increase in DB75 elimination. Because the elimination half-life of DB75 is comparable to that in previous reports, the latter seems unlikely. More likely is the conversion to DB75, which requires oxidative demethylation by cytochrome(s) P450 4F and subsequent reduction by cytochrome b5 reductase.15 Involvement of these enzymes in drug metabolism is uncommon and little is known about their genetic polymorphism.38 Despite the lower than expected plasma concentrations of DB75, levels in the liver should still be substantial. Based on studies in two animal species, the observed plasma levels of DB75 would result in hepatic accumulation to levels some 1,000-fold higher than the in vitro EC50 values for erythrocytic parasites. Therefore, the observed lack of causal activity could be attributed to a less than expected accumulation of DB75 in the human liver or to the possibility that DB75 does not act against exoerythrocytic stage parasites. These two alternatives cannot be distinguished in this study.
One volunteer who received 100 mg of pafuramidine on day −8 did not develop parasitemia detectable by any of the five assay methods for up to 12 weeks after challenge. The baseline characteristics of this volunteer and the plasma levels of pafuramidine and DB75 were not notably different from those of the other persons (Figure 4). His challenge period was midway through the process and overlapped with those of nine other persons. However, there are several possibilities for why he did not develop malaria. First, pafuramidine in this regimen may provide causal prophylactic activity in a small number of persons. Although interesting, such efficacy is clearly insufficient to be practical. Second, this volunteer developed a periorbital reaction to poison ivy and was treated with a relatively high dose of diphenhydramine on days 7–12 after challenge, an interval that overlaps the emergence of erythrocytic parasites. Antimalarial activity of histamine-1 antagonists including chlorpheniramine39,40 and astemizole41 has been reported, and diphenhydramine or its metabolites may have provided suppressive prophylaxis.
Pafuramidine was well-tolerated in this study. The major complaints and laboratory changes were those typically experi enced by healthy volunteers who develop patent malaria, and were concurrent with the onset of parasitemia.36 Of some interest are the changes in liver function test results. The protocol was not specifically designed to assess hepatotoxicity, and the interpretation of these changes is limited by the small sample size and also by three possible causes of liver toxicity: malaria, acetaminophen, and pafuramidine. Of these three causes, liver toxicity appears to correlate best with patent malaria. This suggestion is supported by the fact that 1) all persons with elevations of enzyme levels had malaria, 2) no elevations were found in persons who did not have malaria, regardless of exposure to pafuramidine or acetaminophen, and 3) the onset of elevation of enzyme levels was closely associated with the advent of patency (Supplemental Figure 1). However, it is important to note the higher-than expected frequency and degree of elevations of aminotransferase levels in those with both malaria and acetaminophen exposure (Table 1). In these persons, the degree of abnormality combined with increasing exposure to acetaminophen, and grade 3 toxicity (uncommon in acute malaria alone)35 was seen only in those who took acetaminophen. In this setting, the possible contribution of pafuramidine to hepatotoxicity is difficult to assess but it appears to be less than the contributions of patent malaria and acetaminophen.
In the interval since this study was completed, new findings of renal and hepatic toxicity led Immtech Pharmaceuticals, Inc. to discontinue the development program for pafuramidine. Although we saw no evidence of renal toxicity, the sample size may have been too small to detect this effect or the design may have excluded important contributing factors.
This study involving 16 healthy volunteers challenged in a single cohort and followed entirely an outpatient basis was safely and efficiently conducted. Careful selection and education of volunteers helped to ensure 100% compliance with some 450 clinic visits. The study design also provides reasonable statistical significance within the limits of practicality. Furthermore, for this indication, even one treatment failure would largely if not entirely rule out pafuramidine as a viable prophylactic agent. The study design also minimizes the recognized confounding effect of biologic variations in different batches of infected mosquitoes,42 enables the enrollment of responsible and fully informed volunteers (who can participate without missing time from work), and permits a long follow-up period to detect late parasitemia that may result from partially treated tissue parasites.43
In summary, a single oral dose of 100 mg of pafuramidine administered before exposure to P. falciparum malaria infection does not provide meaningful causal prophylaxis in non-immune healthy volunteers. Good clinical efficacy of pafuramidine in the treatment of acute malaria in malariaendemic settings indicates its potential for suppressive prophylaxis. However, the now-recognized possibilities of hepatic and renal toxicity likely preclude this application.
Supplementary Material
Acknowledgments
We thank the volunteers for their interest in and persistent commitment to the study; the study team members of the Drug Development Unit and the staffs at the Investigational Pharmacy and the General Clinical Research Center for efficient, reliable, and cheerful participation; Jeannette Fanning for outstanding administrative support; Drs. Patricia Charache and Richard Moore for their support and advice; Tom Spahr, Amelia Maters, Yessika Vasquez, and the entomology team for their superb technical assistance; Dr. Paul Lietman for his service and advice as safety monitor; and Dr. Carol Olson for steadfast support and for reading the manuscript.
Financial support: This work was supported by the General Clinical Research Center, funded by the National Center for Research Resources (grant M01RR000052) and by Immtech Pharmaceuticals, Inc. (Vernon Hills, IL). Myaing M. Nyunt, for whom this study served as the major PhD thesis project, was supported by a National Institutes of Health Clinical Research Scholar Awards (K12) and the Clinical Pharmacology Training Program (T32GM066691).
Footnotes
Note: Supplemental Figure 1 (Relationship between alanine aminotransaminase (ALT) level and diagnosis of patent malaria) and Supplemental Table 1 (Laboratory abnormalities) appear online at http://www.ajtmh.org.
REFERENCES
- 1.Leder K, Black J, O’Brien D, Greenwood Z, Kain KC, Schwartz E, Brown G, Torresi J. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin Infect Dis. 2004;39:1104–1112. doi: 10.1086/424510. [DOI] [PubMed] [Google Scholar]
- 2.Werbovetz K. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr Opin Investig Drugs. 2006;7:147–157. [PubMed] [Google Scholar]
- 3.Yeates C. DB-289 Immtech International. IDrugs. 2003;6:1086–1093. [PubMed] [Google Scholar]
- 4.Bell CA, Hall JE, Kyle DE, Grogl M, Ohemeng KA, Allen MA, Tidwell RR. Structure-activity relationships of analogs of pentamidine against Plasmodium falciparum and Leishmania mexicana amazonensis. Antimicrob Agents Chemother. 1990;34:1381–1386. doi: 10.1128/aac.34.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocken CH, van der Wel A, Arbe-Barnes S, Brun R, Matile H, Scheurer C, Wittlin S, Thomas AW. Plasmodium vivax: in vitro susceptibility of blood stages to synthetic trioxolane compounds and the diamidine DB75. Exp Parasitol. 2006;113:197–200. doi: 10.1016/j.exppara.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Carter NS, Berger BJ, Fairlamb AH. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J Biol Chem. 1995;270:28153–28157. doi: 10.1074/jbc.270.47.28153. [DOI] [PubMed] [Google Scholar]
- 7.Damper D, Patton CL. Pentamidine transport and sensitivity in brucei-group trypanosomes. J Protozool. 1976;23:349–356. doi: 10.1111/j.1550-7408.1976.tb03787.x. [DOI] [PubMed] [Google Scholar]
- 8.Bell CA, Cory M, Fairley TA, Hall JE, Tidwell RR. Structure-activity relationships of pentamidine analogs against Giardia lamblia and correlation of antigiardial activity with DNA-binding affinity. Antimicrob Agents Chemother. 1991;35:1099–1107. doi: 10.1128/aac.35.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dykstra CC, Tidwell RR. Inhibition of topoisomerases from Pneumocystis carinii by aromatic dicationic molecules. J Protozool. 1991;38:78S–81S. [PubMed] [Google Scholar]
- 10.Shapiro TA, Englund PT. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc Natl Acad Sci USA. 1990;87:950–954. doi: 10.1073/pnas.87.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stead AM, Bray PG, Edwards IG, DeKoning HP, Elford BC, Stocks PA, Ward SA. Diamidine compounds: selective uptake and targeting in Plasmodium falciparum. Mol Pharmacol. 2001;59:1298–1306. doi: 10.1124/mol.59.5.1298. [DOI] [PubMed] [Google Scholar]
- 12.Lanteri CA, Tidwell RR, Meshnick SR. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob Agents Chemother. 2008;52:875–882. doi: 10.1128/AAC.00642-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanteri CA, Trumpower BL, Tidwell RR, Meshnick SR. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2004;48:3968–3974. doi: 10.1128/AAC.48.10.3968-3974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saulter JY, Kurian JR, Trepanier LA, Tidwell RR, Bridges AS, Boykin DW, Stephens CE, Anbazhagan M, Hall JE. Unusual dehydroxylation of antimicrobial amidoxime prodrugsby cytochrome b5 and NADH cytochrome b5 reductase. Drug Metab Dispos. 2005;33:1886–1893. doi: 10.1124/dmd.105.005017. [DOI] [PubMed] [Google Scholar]
- 15.Midgley I, Fitzpatrick K, Taylor LM, Houchen TL, Henderson SJ, Wright SJ, Cybulski ZR, John BA, McBurney A, Boykin DW, Trendler KL. Pharmacokinetics and metabolism of the prodrug DB289 (2,5-bis[4-(N-methoxyamidino)phenyl]furan monomaleate) in rat and monkey and its conversion to the antiprotozoal/antifungal drug DB75 (2,5-bis(4-guanylphenyl)furan dihydrochloride) Drug Metab Dispos. 2007;35:955–967. doi: 10.1124/dmd.106.013391. [DOI] [PubMed] [Google Scholar]
- 16.Yeramian P, Meshnick SR, Krudsood S, Chalermrut K, Silachamroon U, Tangpukdee N, Allen J, Brun R, Kwiek JJ, Tidwell R, Looareesuwan S. Efficacy of DB289 in Thai patients with Plasmodium vivax or acute, uncomplicated Plasmodium falciparum infections. J Infect Dis. 2005;192:319–322. doi: 10.1086/430928. [DOI] [PubMed] [Google Scholar]
- 17.Tidwell RR, Jones SK, Geratz JD, Ohemeng KA, Bell CA, Berger BJ, Hall JE. Development of pentamidine analogues as new agents for the treatment of Pneumocystis carinii pneumonia. Ann NY Acad Sci. 1990;616:421–441. doi: 10.1111/j.1749-6632.1990.tb17862.x. [DOI] [PubMed] [Google Scholar]
- 18.Das BP, Boykin DW. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J Med Chem. 1977;20:531–536. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 19.Bell CA, Dykstra CC, Naiman NA, Cory M, Fairley TA, Tidwell RR. Structure-activity studies of dicationically substituted bis-benzimidazoles against Giardia lamblia: correlation of antigiardial activity with DNA binding affinity and giardial topoisomerase II inhibition. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meis JF, Verhave JP, Meuwissen JH, Jap PH, Princen HM, Yap SH. Fine structure of Plasmodium berghei exoerythrocytic forms in cultured primary rat hepatocytes. Cell Biol Int Rep. 1984;8:755–765. doi: 10.1016/0309-1651(84)90114-0. [DOI] [PubMed] [Google Scholar]
- 21.Meis JF, Verhave JP, Wirtz P, Meuwissen JH. Histochemical observations on the exoerythrocytic malaria parasite Plasmodium berghei in rat liver. Histochemistry. 1984;81:417–425. doi: 10.1007/BF00489744. [DOI] [PubMed] [Google Scholar]
- 22.Gutteridge WE, Dave D, Richards WH. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582:390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- 23.Murphy JR, Baqar S, Davis JR, Herrington DA, Clyde DF. Evidence for a 6.5-day minimum exoerythrocytic cycle for Plasmodium falciparum in humans and confirmation that immunization with a synthetic peptide representative of a region of the circumsporozoite protein retards infection. J Clin Microbiol. 1989;27:1434–1437. doi: 10.1128/jcm.27.7.1434-1437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chappell WR, Mordenti J. Extrapolation of toxicological and pharmacological data from animals to humans. In: Testa B, editor. Advances in Drug Research. Volume 20. New York: Academic Press; 1991. pp. 244–254. [Google Scholar]
- 25.Gray H. Splanchnology. In: Warwick R, Williams PL, editors. Gray’s Anato my. 35th edition. Edinburgh, UK: Longman Group Ltd.; 1973. pp. 1344–1363. [Google Scholar]
- 26.Davis JR. Laboratory methods for the conduct of experimental malaria challenge of volunteers. Vaccine. 1994;12:321–327. doi: 10.1016/0264-410x(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 27.Ponnudurai T, Leeuwenberg AD, Meuwissen JH. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop Geogr Med. 1981;33:50–54. [PubMed] [Google Scholar]
- 28.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posner G, Gonzalez L, Cumming J, Klinedinst D, Shapiro T. Synthesis and antimalarial activity of heteroatom-containing bicyclic endoperoxides. Tetrahedron. 1997;53:37–50. [Google Scholar]
- 30.Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, Carter R, Saul A. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57:495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 31.Mount DL, Nahlen BL, Patchen LC, Churchill FC. Adaptations of the Saker-Solomons test: simple, reliable colorimetric field assays for chloroquine and its metabolites in urine. Bull World Health Organ. 1989;67:295–300. [PMC free article] [PubMed] [Google Scholar]
- 32.Anthony RL, Bangs MJ, Anthony JM, Purnomo On-site diagnosis of Plasmodium falciparum, P. vivax, and P. malariae by using the quantitative buffy coat system. J Parasitol. 1992;78:994–998. [PubMed] [Google Scholar]
- 33.Pinto MJ, Rodrigues SR, Desouza R, Verenkar MP. Usefulness of quantitative buffy coat blood parasite detection system in diagnosis of malaria. I ndian J Med Microbiol. 2001;19:219–221. [PubMed] [Google Scholar]
- 34.Rickman LS, Long GW, Oberst R, Cabanban A, Sangalang R, Smith JI, Chulay JD, Hoffman SL. Rapid diagnosis of malaria by acridine orange staining of centrifuged parasites. Lancet. 1989;1:68–71. doi: 10.1016/s0140-6736(89)91428-1. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro TA, Ranasinha CD, Kumar N, Barditch-Crovo P. Prophylactic activity of atovaquone against Plasmodium falciparum in humans. Am J Trop Med Hyg. 1999;60:831–836. doi: 10.4269/ajtmh.1999.60.831. [DOI] [PubMed] [Google Scholar]
- 36.Church LW, Le TP, Bryan JP, Gordon DM, Edelman R, Fries L, Davis JR, Herrington DA, Clyde DF, Shmuklarsky MJ, Schneider I, McGovern TW, Chulay JD, Ballou WR, Hoffman SL. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis. 1997;175:915–920. doi: 10.1086/513990. [DOI] [PubMed] [Google Scholar]
- 37.Epstein JE, Rao S, Williams F, Freilich D, Luke T, Sedegah M, de la Vega P, Sacci J, Richie TL, Hoffman SL. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. 2007;196:145–154. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins MM, Prachal JT. A high-frequency polymorphism of NADH-cytochrome b5 reductase in African-Americans. Hum Genet. 1997;99:248–250. doi: 10.1007/s004390050347. [DOI] [PubMed] [Google Scholar]
- 39.Nakornchai S, Konthiang P. Potentiation of antimalarial drug action by chlorpheniramine against multidrug-resistant Plasmodium falciparum in vitro. Parasitol Int. 2006;55:195–199. doi: 10.1016/j.parint.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 40.Sowunmi A, Fehintola FA, Ogundahunsi OA, Oduola AM. Comparative efficacy of chloroquine plus chlorpheniramine and halofantrine in acute uncomplicated falciparum malaria in Nigerian children. Trans R Soc Trop Med Hyg. 1998;92:441–445. doi: 10.1016/s0035-9203(98)91084-7. [DOI] [PubMed] [Google Scholar]
- 41.Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ., Jr A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol. 2006;2:415–416. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- 42.Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, Quakyi IA, Carter R, Trosper JH, Hockmeyer WT. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg. 1986;35:66–68. doi: 10.4269/ajtmh.1986.35.66. [DOI] [PubMed] [Google Scholar]
- 43.Coatney G, Copper W, Young M, McLendon S. Studies in human malaria I. The protective action of sulfadiazine and sulfapyrazine against sporozoite-induced falciparum malaria. Am J Hyg. 1947;46:84–104. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.