Abstract
Background
Recent studies have demonstrated the expression of Toll-like receptor 3 (TLR3) in salivary glands and epithelial cell lines derived from Sjögren’s syndrome (SS) patients. Since viral infections are considered to be a trigger for SS, in this study we investigated whether in vivo engagement of TLR3 affects salivary gland function.
Methods
Female NZB/WF1 mice were repeatedly injected with polyinosinic:polycytidylic acid (poly(I:C)). TLR3 expression within submandibular glands was studied by immunohistochemistry. RNA levels of inflammatory cytokines in the submandibular glands were determined by real time PCR. Pilocarpine induced saliva volume was used as an index of glandular function.
Results
Immunohistochemical analysis of submandibular glands showed TLR3 expression in epithelium of serous and mucous acini, granular convoluted tubules and ducts. Poly(I:C) treatment rapidly upregulated the mRNA levels of type I IFN and inflammatory cytokines in the submandibular glands. By one week after treatment, the saliva volumes in poly(I:C) treated mice were significantly reduced in comparison with the PBS treated mice. Hematoxylin and eosin staining showed that salivary gland histology was normal and lymphocytic foci were not detected. Glandular function recovered after poly(I:C) treatment was stopped.
Conclusions
Our results demonstrate that engagement of TLR3 within the salivary glands results in a rapid loss of glandular function. This phenomenon is associated with the production of type I IFN and inflammatory cytokines in the salivary glands. Restoration of glandular function suggests that for viral etiology of SS, a chronic infection of salivary glands might be necessary.
Keywords: Inflammatory Cytokines, Poly(I:C), Salivary Gland, Sjögren’s Syndrome TLR3
Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disorder characterized by lymphocytic infiltration in the salivary glands (1). Xerostomia or dry mouth is a major complication of SS. Occurrence of apoptosis and glandular destruction, due to a localized autoimmune response is considered a major factor for salivary gland hypofunction (1). However, the suggestion that non apoptotic mechanisms play an equally important role in salivary gland hypofunction is emerging (2, 3). Amongst these are adverse effects of inflammatory cytokines on the secretion process. These cytokines are not only produced by the infiltrating lymphocytes but also by activated salivary gland epithelial cells (SGEC) (4).
One of the mechanisms for activation of SGEC is through the engagement of TLRs. Viral infections of the salivary glands can be a major trigger for the localized production of inflammatory cytokines. Viral nucleic acids are potent stimulators of TLR3 (dsRNA viruses), TLR7/8 (ssRNA viruses) and TLR9 (dsDNA viruses) (5) Engagement of these TLRs results in the activation of multiple signaling pathways that culminates in the production of proinflammatory cytokines such as IL-6, TNF-α, and type I IFNs (IFN-α and IFN-β). The localized and rapid induction of proinflammatory cytokines forms the first line of defense to limit the dissemination of invading virus. However, a chronic or repeated infection can create an inflammatory environment leading to organ dysfunction and development of autoimmune disease. Indeed viral etiology for SS has been experimentally demonstrated (6).
Recent studies have demonstrated the expression of different TLRs in the salivary glands from patients with SS as well as normal individuals (7, 8). Analysis of cultured salivary gland epithelial cells showed the expression of TLR2, TLR3 and TLR7. Moreover, engagement of TLR3 on these cells by poly(I:C) or Reovirus induced the production of proinflammatory cytokines (9). However, the in vivo consequences of TLR3 engagement on salivary gland function are not known. To address this question, we repeatedly injected the NZB/W F1 mice with TLR3 ligand poly(I:C).
Our study shows that TLR3 is expressed within the serous and mucous acini, granular convoluted tubules (GCT), and ductal cells. Engagement of TLR3 causes a rapid induction of type I interferons and proinflammatory cytokines within the salivary glands, resulting in a significant and rapid loss of function. However, the glandular function recovers after stopping the poly(I:C) treatment.
Materials and Methods
Mice
All mouse experiments were approved by the Animal Care and Use Committee of the University of Virginia. All experiments were conducted in accordance with the guidelines set by the National Institutes of Health and adequate measures were undertaken to minimize pain and discomfort to the mice. Female NZB/WF1 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and maintained under specific pathogen free conditions in a barrier facility in the University of Virginia, vivarium. Female mice (8–10 week old) were injected with 50 μg of poly(I:C) (Invivogen, San Diego, CA, USA) by intraperitoneal route every other day for a week (days 0, 2, 4, and 6). Controls were injected similarly with PBS. In an additional cohort of mice, the treatment was extended for one more week (days 8, 10, 12 and 14). Pilocarpine induced saliva was collected at different time points. Mice were anesthetized with Ketamine/Xylazine and injected with a freshly prepared solution of pilocarpine hydrochloride (MP Biomedicals, Solon, OH, USA) by intraperitoneal route (0.5μg/gm body weight). After 2 minute interval, saliva was collected for 20 minutes in a microcentrifuge tube on ice. The tubes were briefly centrifuged and volume of saliva determined. Saliva was frozen at −70°C till use. Mice were bled through the tail vein and blood was collected in heparinized tubes on ice. Plasma was separated immediately and stored in aliquots at −70°C till use.
Real Time PCR
After euthanasia, pieces of submandibular gland were rapidly frozen in liquid N2. For RNA preparation, frozen glands were resuspended in RLT buffer (Qiagen Inc, Valencia, CA) and homogenized using TissueLyser system (Qiagen Inc, Valencia, CA), following manufacturers instructions. Total RNA from tissue homogenate was purified using RNAeasy mini kit (Qiagen Inc, Valencia, CA) followed by first strand cDNA synthesis using the QuantiTect Rev. Transcription kit (Qiagen Inc, Valencia, CA). Expression levels of IFN-β1, Mx1, PRKR, IFI44, IL-6, TNF-α, IL-1β, and CCL5 were determined by Real Time PCR on MyiQ machine (Bio-Rad laboratories, Hercules, CA, USA) using the Taqman gene expression assays (Applied Biosystems, Foster City, CA, USA). The relative gene expression was calculated using the 2 (−Delta Delta C(T)) method (10). A pooled RNA sample (Ambion, Austin, TX, USA) was used as reference calibrator and house keeping gene GAPDH was used as a control.
Immunohistochemistry
Submandibular glands were collected in 10% buffered formalin, processed for histology using standard techniques, and embedded in paraffin. Hematoxylin and eosin stained sections were evaluated for histopathology as described earlier (6). Immunohistochemical staining was carried out as previously described (11). Five micron sections of paraffin embedded salivary gland were used for detection of TLR3 and Aquaporin 5 (AQP5). After deparaffinization and rehydration, the slides were rinsed in PBS and antigen retrieval was accomplished by boiling in antigen unmasking solution (Vector Laboratories, Burlingame, CA, USA) for 20 min in a microwave (1.6 kW at 80% power). Endogenous peroxidase activity was quenched with H2O2 in methanol and non specific interaction was blocked by incubation with normal goat serum and Avidin Biotin blocking solutions (Vector Laboratories, Burlingame, CA, USA). The slides were then incubated with antibodies to either rabbit anti-aquaporin-5 (EMD, Calbiochem, San Diego, CA, USA) or rabbit anti-TLR-3 (Imgenex Corp, San diego, CA) at 4°C overnight in a humid chamber. Sections incubated with purified rabbit polyclonal IgG at equal concentrations were used as negative controls. After washing, the sections were incubated with biotinylated goat anti-rabbit IgG (Southern Biotech, Birmingham, AL, USA) for 30 min at room temperature. Bound antibody was detected using the ABC system (Vector Laboratories, Burlingame, CA, USA) and the sections developed by DAB substrate. The slides were counterstained with hematoxylin and mounted in Cytoseal 60.
Statistical analysis
Statistical significance of variance was determined by the unpaired Students t-test using GraphPad Prism software.
Results
Mouse Submandibular glands express TLR3
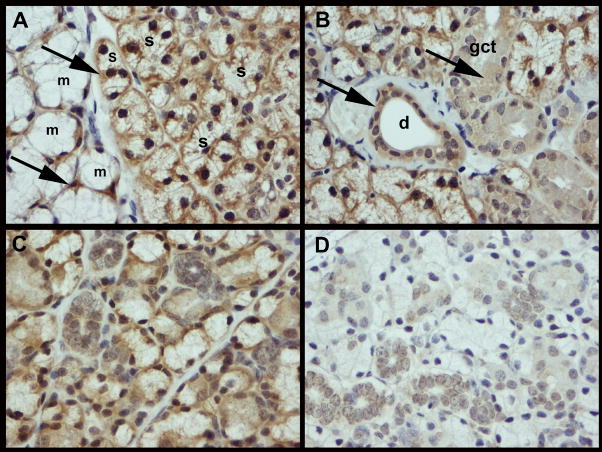
To determine the expression of TLR3 in SS prone NZB/WF1 female mice, sections of submandibular glands were stained with anti-TLR3 antibody. As shown in figure 1A, TLR3 expression was seen on the base of epithelial cells of serous and mucus acini. However, in granular convoluted tubules and ducts, TLR3 staining was seen in the epithelial cell cytoplasm (1B). This was not unique to NZB/WF1 mice that are susceptible to salivary gland disease. Analysis of non autoimmune C57BL/6 females also showed a similar pattern of TLR3 distribution (figure 1C). There was no significant difference in the TLR3 staining pattern following treatment with poly(I:C) described below.
Figure 1. Epithelial cells of murine salivary glands express TLR3.
Representative photomicrographs of immunohistochemical staining of salivary glands from NZB/WF1 (A, B) and C57BL/6 (C) female mouse for TLR3 expression. (A) TLR3 is detected on the base of secretory cells of mucus (m) and serous (s) acini in the salivary glands and is indicated with arrows. (B) In addition, TLR3 staining was observed in the cytoplasm of the ductal (d) and granular convoluted tubular (gct) epithelium. (C) A similar distribution of TLR3 expression was seen in a non-autoimmune C57BL/6 female. (D) Sections incubated with rabbit polyclonal serum antibody served as negative controls (magnification 40X).
Poly(I:C) induces pro-inflammatory cytokines within the salivary glands
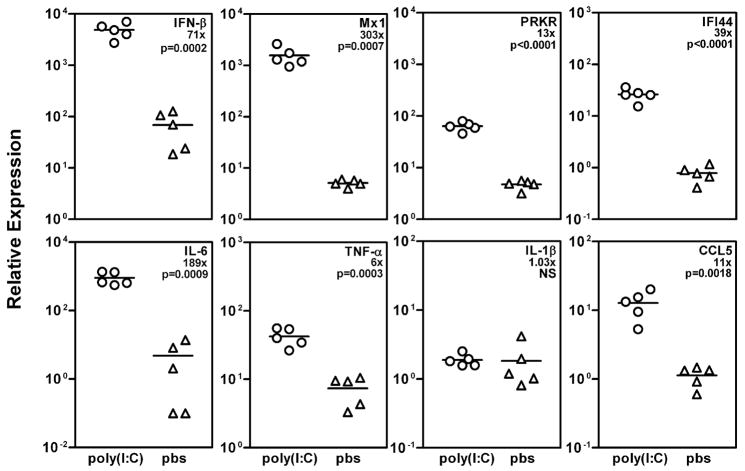
NZB/WF1 mice were treated with 50μg of poly(I:C), and submandibular glands were harvested for RNA extraction after 3.5h. Preliminary studies had shown a peak expression of type I interferon responsive genes at this time point followed by a drop by 6 hours after treatment with polyI:C. We investigated the expression of IFN-β1, type I IFN responsive genes (Mx1, PRKR, and IFI44), inflammatory cytokines (IL-6, TNF-α and IL-1β) and CCL5 by real time PCR (figure 2). In comparison with PBS treated mice, submandibular glands of poly(I:C) treated mice showed significant upregulation of IFN-β1 as well as type I IFN responsive genes Mx1, PRKR and IFI44. Similarly, levels of IL-6, TNF-α and CCL5 were also significantly upregulated. However, at this time point no difference in the expression levels of IL-1β were observed between the 2 groups of mice.
Figure 2. Expression of type I IFN, IFN responsive genes and proinflammatory cytokine genes is upregulated in the submandibular glands of poly(I:C) treated mice.
Expression levels of IFN-β1, Mx1, PRKR, IFI44, IL-6, TNF-α, IL-1β, and CCL5 in submandibular glands of poly(I:C) (n=5) and PBS (n=5) treated mice were determined by real time PCR using Taqman gene expression assays. Each data point represents RNA expression in one mouse and data are represented as mean duplicate relative expression calculated by the 2(−Delta Delta C(T)) method. Numbers denoted with ‘x’ represent fold increase in RNA expression in poly(I:C) treated mice in comparison with PBS treated mice. Also shown are p values determined using non parametric student’s t-test. NS; not significant.
Loss of salivary gland function in poly(I:C) treated mice
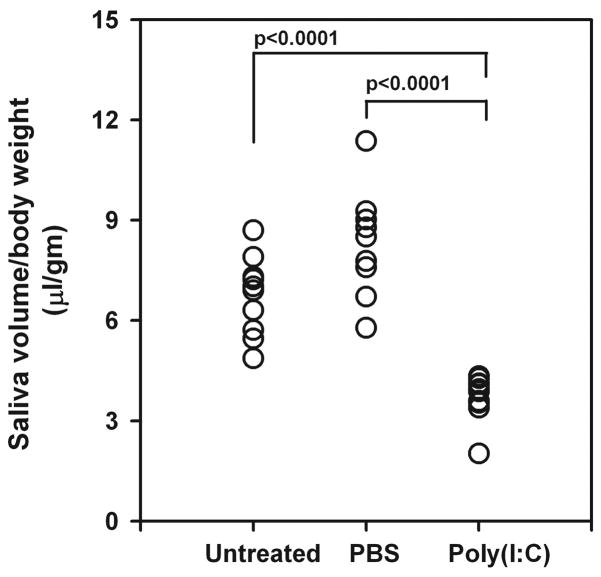
To determine the effect of TLR3 engagement and inflammatory cytokine production on salivary gland function, mice were injected 4 times (days 0, 2, 4 and 6) with poly(I:C). Pilocarpine induced saliva volume was determined, 1 day after the last injection. Figure 3 shows that poly(I:C) treated mice have a significant reduction (p<0.0001) in the amount of saliva produced in comparison with the PBS treated mice.
Figure 3. Poly(I:C) treatment causes rapid loss of salivary gland function.
NZB/WF1 mice were treated either with poly(I:C) (n=10) or PBS (n=9) for one week (days 0, 2, 4 and 6). Pilocarpine induced saliva volume was determined on day 7. Data are represented as saliva volume/body weight (μl/gm). Note poly(I:C) treatment causes a significant (p<0.0001) drop in the saliva produced compared to untreated and PBS treated mice.
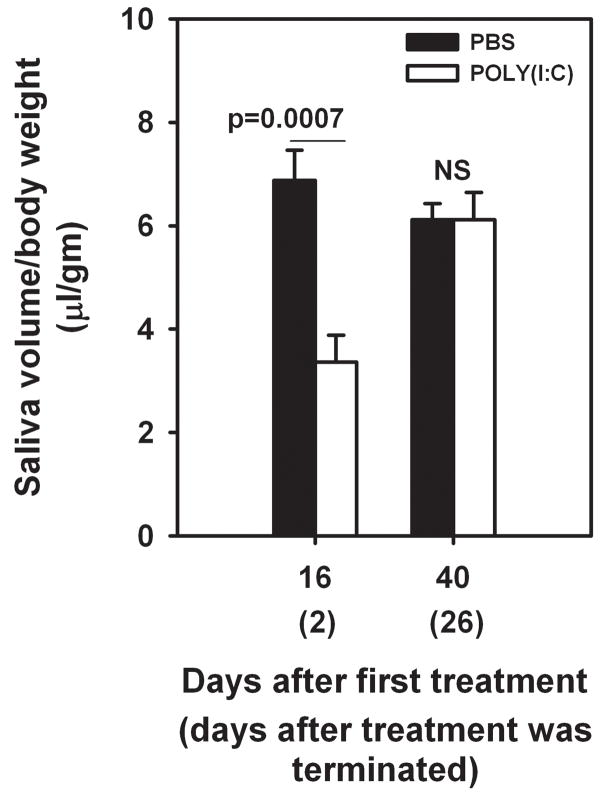
Additional cohort of mice (5 per group) were treated either with poly(I:C) or PBS every other day for 2 weeks. Salivary gland function was determined on days 2 and 26, after the last injection. The volume of saliva was significantly lower in poly(I:C) treated mice 2 days after last injection. However, by 26 days after the treatment was stopped, the poly(I:C) treated mice regained normal salivary gland function comparable to PBS treated mice. These data suggest that persistent stimulation was required for sustained loss of function.
Discussion
In this study, we have demonstrated that treatment of mice with poly(I:C), results in the production of inflammatory cytokines within the submandibular gland and a significant loss of glandular function. Poly(I:C) activates innate immune responses through the engagement of TLR3 (12). Thus, this study is the first report showing in vivo effects of TLR3 engagement on salivary gland function.
TLR3 expression is localized predominantly in the intracellular compartments. However, surface expression is also detected on certain cell types (reviewed in 13). Spachidou and colleagues have demonstrated surface expression of TLR3 on salivary gland epithelial cell lines (8). Our mouse studies provide further evidence for this and also show intracellular staining. The pattern of TLR3 expression within the SS prone NZB/WF1 and a non autoimmune B6 mouse was identical. Thus, expression of TLR3 within the salivary gland is independent of genetic susceptibility to SS.
Binding of TLR3 by poly(I:C) results in the activation of signaling pathways involving interferon regulatory factor 3 (IFR3) and NF-kB, leading to predominant production of type I IFNs (particularly IFN-β) (13, 14). Microarray analysis of gene expression within the salivary glands of patients with SS have demonstrated an upregulation of type I IFN responsive genes (15, 16). The present study suggests that salivary gland cells activated by TLR3 ligand are the major source of type I IFNs within the gland. At time points, when the glandular function was significantly compromised, we did not observe any inflammatory cell infiltrates within the salivary gland (Supplementary figure 1). This finding is highly significant as it reinforces the glandular tissue as an active participant in disease pathogenesis (4). Whether patients with SS have a genetic predisposition for overproduction of type I IFNs in salivary glands, following innate stimuli needs to be investigated. A study reporting development of SS in a multiple sclerosis patient treated with IFN-β demonstrates an important role for this cytokine in the disease process (17).
Although the precise mechanism for glandular dysfunction following poly(I:C) treatment is not clear, we hypothesize that functional loss is through a cumulative effect of localized production of type I IFNs and other inflammatory cytokines. This is supported by the finding that stopping poly(I:C) treatment restored the pilocarpine induced saliva production. Inflammatory cytokines can affect salivary gland function in different ways. Zoukhri et al have demonstrated that injection of IL-1β within the lacrimal gland caused extensive tissue damage and loss of acinar cells (18). However, the H&E stained sections of salivary glands from poly(I:C) treated mice did not show any evidence for tissue destruction (supplementary figure 1).
In patients with SS, resdistribution of aquaporin 5 (AQP5) has been considered as one of the mechanisms contributing towards hypofunction (19). This pathway does not seem to be operative in our model system as staining for AQP5 did not show any changes between the poly(I:C) and PBS treated mice (supplementary figure 2). To investigate the role of autoantibodies, sera obtained at different time points were analyzed for reactivity to cell extracts by western blots. Compared to PBS treated mice, poly(I:C) treated mice had higher levels of autoantibodies (supplementary figure 3). However, at day 44 post treatment, despite higher circulating levels of autoantibodies, glandular function had recovered. These data suggest a minimal role for autoantibodies in inducing glandular dysfunction in this model.
Recently Dawson et al have reviewed the possible mechanisms involved in the non-apoptotic model of salivary gland hypofunction (3). Whether some of these; such as breakdown and inhibition of neurotransmitter release, effects of NO and perturbed Ca2+ metabolism are involved in our model needs to be determined. The possibility that these mechanisms might be important is indirectly suggested by studies involving TLR4 stimulation in salivary glands. Treatment of mice with LPS was shown to induce the expression of IL-1β, IL-6 and TNF-α (20). Amongst these cytokines, IL-1β protein was localized in the cells of GCT. Although effects on glandular function were not investigated, other studies have suggested a role for IL-1β in inhibiting neurotransmitter release (21). In another study, treatment of rats with LPS caused salivary gland hypofunction (22). It was suggested that increased NO metabolism and prostaglandin synthesis within the salivary gland was responsible for the observed hypofunction.
Regardless of the mechanisms involved, these studies and our findings, clearly establish that innate stimuli through TLRs expressed within salivary glands directly affect the glandular function. Thus, chronic infections of salivary gland hold the potential for initiating salivary gland dysfunction and development of SS.
Supplementary Material
Figure 4. Glandular function recovers after stopping poly(I:C) treatment.
Mice (n=5 per group) were either treated with poly(I:C) or PBS every other day for 2 weeks. Pilocarpine induced saliva was determined on days 16, and 40 (2 and 26 days after stopping treatment). Data are represented as mean±SEM saliva volume/body weight (μl/gm). Note that mean saliva volume in poly(I:C) treated mice was significantly lower than the PBS treated mice 2 days after stopping the treatment. However, on day 26 after stopping the treatment, the difference in mean saliva volume between the 2 groups of mice was not significant.
Acknowledgments
This work was supported by grants from the National Institutes of Health, USA, K01AR051391 (USD); K01DK063065 and R01DK069769 (HB).
Footnotes
Conflict of Interest Statement
None of the authors have any conflict of interest.
References
- 1.JONSSON R, BOLSTAD AI, BROKSTAD KA, et al. Sjogren’s syndrome--a plethora of clinical and immunological phenotypes with a complex genetic background. Ann N Y Acad Sci. 2007;1108:433–47. doi: 10.1196/annals.1422.046. [DOI] [PubMed] [Google Scholar]
- 2.DAWSON LJ, CAULFIELD VL, STANBURY JB, et al. Hydroxychloroquine therapy in patients with primary Sjogren’s syndrome may improve salivary gland hypofunction by inhibition of glandular cholinesterase. Rheumatology (Oxford) 2005;44:449–55. doi: 10.1093/rheumatology/keh506. [DOI] [PubMed] [Google Scholar]
- 3.DAWSON LJ, FOX PC, SMITH PM. Sjogrens syndrome--the non-apoptotic model of glandular hypofunction. Rheumatology (Oxford) 2006;45:792–8. doi: 10.1093/rheumatology/kel067. [DOI] [PubMed] [Google Scholar]
- 4.MANOUSSAKIS MN, KAPSOGEORGOU EK. The role of epithelial cells in the pathogenesis of Sjogren’s syndrome. Clin Rev Allergy Immunol. 2007;32:225–30. doi: 10.1007/s12016-007-8007-4. [DOI] [PubMed] [Google Scholar]
- 5.VERCAMMEN E, STAAL J, BEYAERT R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.OHYAMA Y, CARROLL VA, DESHMUKH U, et al. Severe focal sialadenitis and dacryoadenitis in NZM2328 mice induced by MCMV: a novel model for human Sjogren’s syndrome. J Immunol. 2006;177:7391–7. doi: 10.4049/jimmunol.177.10.7391. [DOI] [PubMed] [Google Scholar]
- 7.KAWAKAMI A, NAKASHIMA K, TAMAI M, et al. Toll-like receptor in salivary glands from patients with Sjogren’s syndrome: functional analysis by human salivary gland cell line. J Rheumatol. 2007;34:1019–26. [PubMed] [Google Scholar]
- 8.SPACHIDOU MP, BOURAZOPOULOU E, MARATHEFTIS CI, et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: Increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2007;147:497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ITTAH M, MICELI-RICHARD C, GOTTENBERG JE, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur J Immunol. 2008;38:1–7. doi: 10.1002/eji.200738013. [DOI] [PubMed] [Google Scholar]
- 10.LIVAK KJ, SCHMITTGEN TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.BAGAVANT H, SHARP C, KURTH B, et al. Induction and immunohistology of autoimmune ovarian disease in cynomolgus macaques (Macaca fascicularis) Am J Pathol. 2002;160:141–9. doi: 10.1016/S0002-9440(10)64358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ALEXOPOULOU L, HOLT AC, MEDZHITOV R, et al. Recognition of double-stranded RNA and activation of NF-kB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 13.MATSUMOTO M, SEYA T. TLR3: Interferon induction by double-stranded RNA including poly (I:C) Advanced Drug Delivery Rev. 2008;60:805–12. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.KAWAI T, AKIRA S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–9. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 15.HJELMERVIK TOR, PETERSEN K, JONASSEN I, et al. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–44. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 16.GOTTENBERG JE, CAGNARD N, LUCCHESI C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–5. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DE SANTI L, COSTANTINI MC, ANNUNZIATA P. Long time interval between multiple sclerosis onset and occurrence of primary Sjögren’s syndrome in a woman treated with interferon-beta. Acta Neurol Scand. 2005;112:194–6. doi: 10.1111/j.1600-0404.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 18.ZOUKHRI D, MACARI E, KUBLIN CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.STEINFELD S, COGAN E, KING LS, et al. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjogren’s syndrome patients. Lab Invest. 2001;81:143–8. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 20.YAO C, LI X, MURDIASTUTI K, et al. Lipopolysaccharide-induced elevation and secretion of interleukin-1beta in the submandibular gland of male mice. Immunology. 2005;116:213–22. doi: 10.1111/j.1365-2567.2005.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ZOUKHRI D, KUBLIN CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2001;42:925–32. [PMC free article] [PubMed] [Google Scholar]
- 22.LOMNICZI A, MOHN C, FALETTI A, et al. Inhibition of salivary secretion by lipopolysaccharide: Possible role of prostaglandins. Am J Physiol Endocrinol Metab. 2001;281:405–11. doi: 10.1152/ajpendo.2001.281.2.E405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.